Abstract
Purpose
To develop and validate modified Look-Locker (MOLLI) protocols to generate myocardial T1 maps within clinically acceptable breath-hold durations and to compare partition coefficients (\g=l\) of gadolinium (Gd)-DTPA determined from either bolus injection (BI) or continuous infusion (CI) techniques.
Materials and Methods
T1 mapping was performed in phantoms and in 10 volunteers on a 1.5T scanner using the standard (3-3-5) MOLLI technique and two MOLLI schemes with shorter breath-hold durations. Imaging was performed precontrast and every 5 minutes following a bolus of 0.1 mmol/kg Gd-DTPA and a 15-minute delayed continuous infusion of 0.001 mmol/kg Gd-DTPA until equilibrium T1 in the myocardium was achieved to enable direct comparison of T1 relaxation times between techniques and λ's between the BI and CI methods.
Results
There was good agreement of T1 values between the 3-3-5 standard MOLLI protocol and the modified 3-5 MOLLI protocol in both phantom studies over a range of heart rates and in human subjects. Both MOLLI protocols produced similar measurements of λ using both the BI and CI methods.
Conclusion
A reduced breath-hold MOLLI T1 mapping protocol combined with the BI method can accurately characterize T1 and λ in clinically applicable breath-hold durations without requiring a long equilibrium phase infusion.
Keywords: T1 mapping, cardiac MRI, partition coefficient, modified Look-Locker
WHILE LATE GADOLINIUM-ENHANCED (LGE) cardiac magnetic resonance (CMR) is the gold standard for evaluating focal myocardial fibrosis, LGE cannot adequately evaluate pathologies characterized by diffuse myocardial fibrosis, as there is no reference area of normal myocardium. T1 mapping after injection of gadolinium (Gd) has demonstrated promise in evaluating diffuse fibrosis and correlates with the degree of fibrosis from cardiac biopsy specimens (1). However, the T1 of the myocardium is also a function of the Gd dose, Gd clearance rate, and time after injection of Gd. T1 mapping precontrast and following continuous infusion (CI) of Gd can be used to determine the partition coefficient (λ) and volume of distribution (vd) of Gd, which correlates with the histological severity of myocardial fibrosis in hypertrophic cardiomyopathy and aortic stenosis (2). An alternative technique measures the T1 of Gd before and at multiple time-points after bolus injection (BI) of contrast and determines (vd) from a linear fit of 1/T1 of the myocardium versus 1/T1 of the blood pool. This method has been applied to evaluating diffuse fibrosis in cardiomyopathy and in congenital heart disease (3,4).
While multiple strategies have been used to quantify myocardial T1 values, they all have some limitations (1–6). Traditional Look-Locker pulse sequences are efficient, but the heart is in a different phase of the cardiac cycle on each source image requiring segmentation of each image and precluding the generation of T1 maps (7). Performing multiple inversion recovery pulse sequences enables imaging in a specific phase of the cardiac cycle but is time-consuming since a separate breath-hold is required for each source image needed to determine the T1 map. The modified Look-Locker technique (MOLLI) obtains the source images during a single breath-hold in the same phase of the cardiac cycle and is a good candidate for clinical T1 mapping (8,9). A limitation for robust clinical utility of the standard MOLLI pulse sequence is that it requires 17 heartbeats for data acquisition, which results in a breath-hold duration that may be too long many cardiac patients. A sequence similar to the standard MOLLI but with fewer timepoints resulting in a shorter breath-hold duration may be preferable provided that such a sequence would produce T1 maps of equivalent quality (10).
Several different protocols of contrast administration for determining λ and vd include a bolus method, CI, and a bolus followed by CI (2,3,6,11). In the CI method Gd is slowly infused until the T1 in the myocardium and blood pool are constant and then λ and vd can be directly calculated from the precontrast and equilibrium T1 values (2,11). The BI method assumes that the exchange between the blood pool and myocardium is fast with respect to Gd clearance from the blood such that there is equilibrium between the concentration of the Gd in the blood pool and the myocardium at each timepoint following Gd administration (3,6). In this regime, the Gd concentration in the blood pool and myocardium are in dynamic equilibrium where λ is determined from the slope of a plot of R1 values from the myocardium and LV blood pool. There have been limited studies directly comparing these approaches (12).
The goals of this study were two-fold: 1) to develop a MOLLI pulse sequence using fewer timepoints to reduce breath-hold duration, and validate the technique against the standard MOLLI pulse sequence in phantoms and in normal subjects both pre- and postcontrast to determine λ and vd, and 2) to directly compare λ and vd values obtained with these pulse sequences using either a bolus or continuous infusion protocol.
MATERIALS AND METHODS
Pulse Sequence Protocol Development
The standard MOLLI pulse sequence (Fig. 1a) consists of a series of three Look-Locker experiments with images obtained in diastole with three heartbeats between acquisitions to allow for relaxation of longitudinal magnetization, and the nominal TI is incremented for each successive IR train to sample more points along the T1-recovery curve. The standard sequence acquires three images in the first two Look-Locker segments and five images for the third inversion (3-3-5 standard protocol), which collects 11 images over 17 heartbeats. We developed two shorter protocols (10). In the first protocol (Fig. 1b) we collect three images following the first Look-Locker experiment followed by three relaxation beats, followed by a second Look-Locker experiment with five images (3-5 protocol), which collects eight images over 11 heartbeats to evaluate the effects of removing three points from the T1 recovery curve without changing the time allowed for relaxation. In the second protocol (Fig. 1c), which we designed for postcontrast imaging where the T1s are shorter, there are three Look-Locker segments followed by two, two, or four images, but only allowing two beats for recovery between inversions (2-2-4 protocol). The pulse sequence parameters for the steady-state free precession (SSFP) readout were kept constant between these protocols and included: TE 1.1 msec, TR 2.5 msec, flip angle 35°, field of view (FOV) 340 × 260 mm, resolution 1.8 mm × 1.8 mm, thickness 8 mm. The starting TI (to the first image readout) was chosen to be 110 msec, with an increment of 80 msec for the subsequent Look-Locker experiments.
Figure 1.
Schematics of MOLLI pulse sequence variants. Bloch simulation of the longitudinal magnetization and acquisition scheme of (a) the standard MOLLI-3-3-5 which collects 11 source image over 17 heartbeats from three Look-Locker modules with three recovery beats between modules, (b) the modified 3-5 MOLLI which collects eight source images over 11 heartbeats from two Look-Locker modules with three recovery beats between modules, and (c) the 2-2-4 modified MOLLI which collects eight source images over 12 heartbeats from three Look-Locker modules with over 11 heartbeats, and (c) the 2-2-4 modified MOLLI which collects eight source images from three Look-Locker modules with two recovery beats between modules.
Phantom Experiments
To validate the T1 values obtained from these shortened protocols, experiments were performed in phantoms consisting of multiple tubes filled with 2% aga-rose gel that were doped with different concentrations of CuSO4 (0.25, 0.5, 0.75, 1, 1.25, 1.5,1.75, and 2 mmol) to have a range of T1 values (250–1000 msec) with a T2 similar to that of myocardium (~50 msec). An additional tube consisting of 0.5% agarose with 0.225 mmol CuS04 was used to simulate the precontrast T1 and T2 of blood (~1500 msec T1, and 200 msec T2). The T1 of each tube was determined using a standard inversion recover gradient-echo pulse sequence repeated for 25 different TI times ranging from 100 msec to 2500 msec with a two-parameter fit of the equation shown below using a nonlinear least squares algorithm implemented in MatLab (Math-Works, Natick, MA):
| [1] |
The T2 was quantified using standard spin-echo pulse sequences with 10 different echo times. This served as the gold standard measurement. The three MOLLI protocols were performed with simulated RR intervals ranging from 600–1200 msec corresponding to heart rates (HRs) of 60–100 beats per minute. T1 was determined from both two-parameter and three-parameter fits and compared to the IR-gradient echo reference.
Human Experiments
MRI Protocol
Ten normal subjects (age 34 ± 11) underwent CMR imaging using a 1.5T Siemens Avanto scanner (Erlangen, Germany) between July and September 2010 under an Institutional Review Board (IRB)-approved protocol. All subjects signed informed consent. Seven of the 10 subjects had their hematocrit (Hct) measured so that the vd of Gd could be determined from λ as described below. The imaging and Gd injection protocols were designed to perform both BI and CI methods in each subject during the same imaging experiment (Fig. 2). At a single short axis location, the T1 of the myocardium and blood pool were determined using all three of the MOLLI protocols described above (3-3-5 standard, 3-5, and 2-2-4). The subjects then received a bolus intravenous injection of 0.1 mmol/kg Gd-DTPA. Following Gd injection, T1 maps were obtained using the three MOLLI protocols described above every 5 minutes. At 15 minutes a continuous infusion of 0.001 mmol/kg/min of Gd was administered until the T1 in the myocardium and left ventricle (LV) cavity reached equilibrium. Once equilibrium was achieved an additional final set of T1 mapping data was obtained using each of the MOLLI protocols.
Figure 2.

Schematic of the T1 mapping protocol. T1 maps were obtained precontrast with all three pulse sequences. T1 maps were obtained with all sequences at 5, 10, and 15 minutes after the bolus injection of Gd-DTPA. At 15 minutes the continuous infusion was started and T1 maps were obtained with all three sequences every 5 minutes until equilibrium signal intensity was established in the myocardium and blood-pool.
Data Analysis
The endocardial and epicardial borders of the myocardium were manually segmented from the T1 maps and the mean T1 in a region of interest (ROI) in the ventricular cavity was taken as the blood T1. T1 maps were calculated offline using a two-parameter nonlinear least squares fit of Eq. [1] using a MatLab script. In the phantom experiments and in a subset of the human experiments the data were reconstructed using both a two-parameter fit and the previously described three-parameter fit to the equations shown below (9):
| [2] |
To determine λ using the CI method, we used the mean myocardial and LV T1 values from the precontrast T1 (T1pre) images and from the average of the last three acquired T1 maps corresponding to equilibrium (T1post) using the following Equation (13):
| [3] |
To determine λ using the bolus method, the mean myocardial and LV T1 values from the precontrast images and the data points at 5, 10, and 15 minutes postbolus (prior to the start of the continuous infusion) were used. The λ was determined as the slope of 1/T1myocardium versus 1/T1blood.
For the seven subjects where Hct was drawn, vd was determined using the following equation:
| [4] |
Statistical Analysis
All continuous variables are expressed as their mean and standard deviation. The mean T1 values in the blood pool and myocardium from the precontrast, and equilibrium T1 maps, as well as vd and λ determined from the CI and BI methods, were compared for the three MOLLI protocols using a repeated-measures analysis of variance (ANOVA) to separate the effects of variability in T1 between subjects from the variation resulting from differences in the individual pulse sequences. When the null hypothesis could be rejected, post-hoc comparisons of the data from the individual pulse sequences were compared using the Bonferroni method to adjust for multiple comparisons. Comparisons between two means were performed using two-sided paired t-tests with P < 0.05 considered significant in all analyses.
RESULTS
Phantom Experiments
Table 1 shows the T1s of the phantoms as determined by the IR gradient echo, and each of the three MOLLI variants at low HR (60 bpm) and a high HR (100 bpm) using the two-parameter fit. The average percent error in T1 measurements over the range of T1 values and HRs evaluated (60–100 bpm in 10-bpm intervals) for the 3-3-5 standard MOLLI, 3-5 MOLLI, and 2-2-4 MOLLI were 4.7%, 5.3%, 7.5%, and 4.1%, 4.8%, and 8.1% for the two-parameter fit and three-parameter fits, respectively. For the longest T1 and highest HR evaluated (1459 msec, HR 100 bpm, 600 msec RR interval) the percent error in T1 measurements for the 3-3-5 standard MOLLI, 3-5 MOLLI, and 2-2-4 MOLLI were 6.9%, 5.5%,23.6%, and 7.8%, 5.9%, and 33.2% for the two-parameter and three-parameter fits, respectively. As very similar results were found with both a two-parameter and three-parameter fit, we used a simplified two-parameter fit of the data for the clinical study.
Table 1.
T1 Phantom Measurements for MOLLI Pulse Sequences at HR of 60 or 100 BPM
| Reference | 3-3-5 HR 60 | 3-5 HR 60 | 2-2-4 HR 60 | 3-3-5 HR 100 | 3-5 HR100 | 2-2-4 HR 100 |
|---|---|---|---|---|---|---|
| 1459.1 | 1487 (1.8%) | 1492 (2.2%) | 1384 (5.1%) | 1345 (7.8%) | 1373 (5.8%) | 975 (33%) |
| 1150.3 | 1088 (5.5%) | 1084 (5.7%) | 1054 (8.4%) | 1033 (10%) | 1042 (9.5%) | 925 (20%) |
| 855.6 | 819 (4.3%) | 809 (5.4%) | 807 (5.7%) | 801 (6.4%) | 797 (6.8%) | 756 (12%) |
| 699.3 | 680 (2.7%) | 674 (3.7%) | 678 (3.1%) | 672 (3.9%) | 669 (4.4%) | 651 (6.7%) |
| 630.9 | 629 (2.7%) | 626 (0.8%) | 629 (3.3%) | 624 (1.1%) | 622 (1.4%) | 611 (3.2%) |
| 568.4 | 538 (5.3%) | 535 (5.9%) | 539 (5.2%) | 535 (5.8%) | 534 (6.1%) | 531 (6.7%) |
| 521.1 | 483 (7.1%) | 479 (8.0%) | 484 (7.1%) | 480 (7.8%) | 476 (8.7%) | 476 (8.5%) |
| 479.5 | 447 (6.7%) | 444 (7.5%) | 448 (6.6%) | 444 (7.4%) | 441 (6.6%) | 443 (7.7%) |
| 447.0 | 422 (5.4%) | 420 (6.1%) | 423 (5.5%) | 419 (6.2%) | 417 (5.0%) | 419 (6.2%) |
Percent error from IR gradient echo reference in parentheses.
Human Experiments
All volunteers successfully completed the imaging protocol. The mean HR during the imaging session was 62 ± 10 BPM. Figure 3 shows precontrast and post-contrast T1 maps obtained from the standard 3-3-5 MOLLI, 3-5 MOLLI, and 2-2-4 MOLLI pulse sequences in one volunteer. The average time after the bolus injection to achieve a T1 within 10 msec of the equilibrium T1 in the myocardium (average of final three timepoints) was 30 ± 10 minutes. The subjects underwent postcontrast T1 mapping for an average of 48 ± 15 minutes.
Figure 3.
T1 maps from the three MOLLI variants: T1 maps precontrast from the (a) the standard 3-3-5, (b) 3-5, and (c) 2-2-4 MOLLI pulse sequences and postcontrast from the (d) the standard 3-3-5 (e) 3-5, and (f) 2-2-4 MOLLI pulse sequences demonstrate similar image quality.
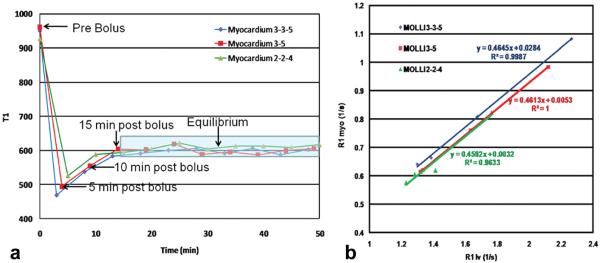
Figure 4a shows T1 as a function of time for the three pulse sequences. During the approach to equilibrium the images are obtained about 1 minute apart, which accounts for the small differences in T1 between the three pulse sequences during this time. Table 2 shows the T1 values in the myocardium precontrast and at equilibrium for the three MOLLI protocols. ANOVA analysis demonstrated that the precontrast T1 value in the blood pool as determined from the 2-2-4 protocol was significantly different from that of the 3-3-5 standard MOLLI, and 3-5 MOLLI protocols (P < 0.01). At equilibrium the T1 values from the myocardium and LV cavity were not different. Figure 4b shows a plot of the relaxation rates of the myocardium versus the blood pool. The slope of these curves, which is λ, is similar.
Figure 4.
Time course of T1 following contrast injection and determination of partition coefficient. a: Plot of T1 as a function of time during the imaging protocol for the three MOLLI variants. During the approach to equilibrium the images are obtained about 1 minute apart which accounts for the small differences in T1 between the three pulse sequences during this time. b: The slope of the linear fit of 1/T1 myocardium versus 1/T1 blood is the partition coefficient of Gd. Note that all points fall along the same line, demonstrating that the equilibrium assumption of the bolus method is appropriate. In this subject all three MOLLI pulse sequences yielded similar values for the partition coefficient.
Table 2.
Comparison of Precontrast and Equilibrium T1, Values
| 3-3-5 Standard | 3-5 MOLLI | 2-2-4 MOLLI | ANOVA | |
|---|---|---|---|---|
| Precontrast T1 blood | 1482.8±88.3 msec | 1489.9±104.6 msec | 1379.3±102.1 msec* | P < 0.01 |
| Precontrast T1 myo | 974.1±22.7 msec | 966.1±31.5 msec | 917.4±84.3 msec | P = 0.10 |
| Equilibrium T1 blood | 490.8±35.7 msec | 485.8±39.0 msec | 485.6±40.8 msec | P = 0.07 |
| Equilibrium T1 myo | 605.6±30.7 | 599.9±30.4 | 601.6±32.2 msec | P = 0.11 |
P < 0.01 compared to standard MOLLI.
For the standard 3-3-5 MOLLI there was a small but statistically significant bias between the two-parameter and three-parameter fit for precontrast LV cavity and myocardial T1 relaxation times (3 msec [0.21%], P = 0.04 and 27 msec [2.9%], P < 0.01, respectively). At the 20-minute timepoint only the myocardial fits had a small bias (11 msec [1.8%] P < 0.01). For the 3-5 MOLLI there was no bias in the LV T1 relaxation times between the two-parameter and three-parameter fits precontrast or 20 minutes postcontrast. There was a very small bias for the myocardial T1 precontrast (12.7 msec [1.3%] P < 0.01) at the 20 minutes postcontrast (4.5 msec [0.8%] P < 0.01) timepoint. These biases were smaller than the standard deviation of the individual T1 fits and are thus not likely to be clinically significant and justify the use of a two-parameter fit in the clinical studies.
Figure 5 shows the determination of λ by both the bolus and continuous infusion methods in one subject. In the bolus method (Fig. 5a), λ is determined from the slope of a fit of 1/T1myocardium versus 1/T1blood. The continuous infusion method (Fig. 5b) can also be thought of as determining a slope between the pre- and post-1/T1 values in the myocardium and blood pool. These curves illustrate that the bolus method represents equilibrium of the ratio between the 1/T1 in the myocardium and the 1/T1 in the blood pool, and thus provides the same λ as the continuous infusion method.
Figure 5.

Determination of partition coefficient by bolus and infusion methods. a: In the bolus method the partition coefficient is determined from the slope of a fit of 1/T1 myocardium versus 1/T1 blood. b: The continuous infusion method can also be thought of as determining a slope between the pre- and post-1/T1 values in the myocardium and blood pool. Note that the slope (partition coefficient) is nearly identical between these two methods. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table 3 shows λ from all MOLLI sequences using either the continuous infusion method or the bolus method. By ANOVA analysis the 2-2-4 protocol was different from the 3-3-5 standard MOLLI technique (P = 0.02). The λ obtained using the standard MOLLI and the 3-5 MOLLI protocols were similar (P = NS), as were the partition coefficients determined by the BI or CI methods for any of the pulse sequences. Bland–Altman analysis between the 3-3-5 standard and 3-5 MOLLI demonstrated no bias in the determination of λ by the CI method (mean bias 0.002 ± 0.015, P = 0.75) or the BI methods (0.002 ± 0.010, P = 0.60). Table 4 shows the vd from the seven subjects who consented to have Hct drawn. Similar vd was found for both the 3-3-5 standard, and 3-5 MOLLI pulse sequence for both the continuous infusion and bolus methods. The 2-2-4 MOLLI pulse sequence resulted in a vd for the continuous infusion that was statistically different from the 3-3-5 MOLLI pulse sequence.
Table 3.
Comparison of Bolus and Continuous Infusion Partition Coefficients
| 3-3-5 Standard | 3-5 MOLLI | 2-2-4 MOLLI | ANOVA | |
|---|---|---|---|---|
| Continuous infusion λ | 0.457±0.039 | 0.455±0.036 | 0.440±0.032* | P = 0.03 |
| Bolus-slope λ | 0.457±0.043 | 0.458±0.038 | 0.449±0.034 | P = 0.11 |
| T-test | P = 0.77 | P = 0.47 | P = 0.16 |
P = 0.02 compared to standard MOLLI.
Table 4.
Comparison of Bolus and Continuous Infusion Volumes of Distribution
| 3-3-5 Standard | 3-5 MOLLI | 2-2-4 MOLLI | ANOVA | |
|---|---|---|---|---|
| Continuous infusion Vd | 0.285±0.018 | 0.281±0.014 | 0.275±0.018* | P = 0.04 |
| Bolus-slope Vd | 0.285±0.018 | 0.285±0.017 | 0.281±0.015 | P = 0.04 |
| T-test | P = 0.83 | P = 0.31 | P = 0.21 |
P < 0.01 compared to standard MOLLI.
DISCUSSION
In this study we demonstrate in both phantom measurements and in human studies that a single shortened MOLLI scheme (3-5 MOLLI) could be used to reliably determine T1 values for the calculation of λ and vd. As expected, prior to contrast administration when the T1s of the blood and myocardium are long, there was a significant bias in the 2-2-4 MOLLI scheme, as only two beats are used to allow for relaxation. Following equilibrium infusion, where the three sequences could be evaluated postcontrast without the confounding factors of the clearance of Gd, all three sequences yielded similar T1 values for the blood pool and myocardium. Thus, ensuring adequate relaxation between Look-Locker modules is essential particularly precontrast where the T1s of the blood pool and myocardium are long. However, postcontrast the time for relaxation can be reduced to two heartbeats without significantly affecting the calculated T1s.
Both quantification of the vd and measurement of T1 at a fixed point after Gd enhancement have been proposed to quantify diffuse fibrosis. Measurement of T1 at a “fixed” time after contrast administration is faster to perform, but has multiple important limitations. This technique assumes that patients will have similar kinetics of Gd accumulation and renal clearance. Furthermore, T1 data can only be compared between studies when the same dose of contrast and imaging time is used. Determination of vd or λ should provide an index of the extracellular volume, which is largely independent of contrast dose, the timing of imaging postcontrast, or the kinetics of Gd.
Accurate measurement of T1 is important for the correct determination of λ, and vd as the error from each of the T1 measurements propagates into the uncertainty of this derived parameter. By analysis of propagation of error for a two-point determination of λ, a 5% uncertainty in each of the T1 measurements would result in a 17% uncertainty in λ and vd for an individual subject. However, between subjects the variation in postcontrast T1 measurements in our study was 5% and the variation of vd was only 6%.
Recently, shMOLLI has been introduced, which overcomes the long breath-hold duration of the conventional MOLLI sequence (14). This pulse sequence consists of a MOLLI acquisition with a 5-1-1 pattern with only a single rest heartbeat between Look-Locker acquisitions. This results in 5–7 source images over a nine-heartbeat acquisition. As there is insufficient magnetization recovery with only a single rest beat for longer T1 times, shMOLLI only uses five inversion times to fit T1's longer than the RR interval, which is the case for most precontrast values in the myocardium. For T1's shorter than certain percentages of the RR interval, the additional two source images (six and seven) are used in a conditional manner to improve the fit for short T1 values. In a clinical study, there was a 15% increase in the uncertainty of T1 values in the myocardium as compared to MOLLI, likely due to the reduced number of images used for the fit. Recently, a different “hybrid” MOLLI sequence was developed that uses a shortened sequence of MOLLI images. In that article a different imaging scheme was used pre- or postcontrast to reduce some of the known HR dependence of T1 measurements with conventional (3-3-5) MOLLI (12). In our experiments the uncertainty was similar for the 2-2-4 and 3-5 MOLLI sequences demonstrating that a single sequence (3-5 MOLLI) could reliably be used for both precontrast and postcontrast imaging without requiring complicated analysis based on HR and T1 to determine which data points to use.
We also demonstrate the equivalence of a BI method or CI method for determining the partition coefficient and volume of distribution of the myocardium from normal volunteers. Both the BI and CI methods were performed in a single imaging session in the same subjects, thus minimizing any variability in slice location or other factors between studies. The infusion protocol was identical to that of Flett et al (2), who validated their results with histological fibrosis from biopsy samples. The delay from the injection to infusion of 15 minutes allowed us to perform measurements following the initial bolus of Gd within the same experimental protocol. Our results are in agreement with the results of Schelbert et al (12), in which subjects were imaged during two different imaging sessions.
Our phantom experiments demonstrate a bias in T1 determination particularly for high HRs and long T1 values, which has been previously demonstrated with MOLLI. Our results are similar, showing a 9% difference for an HR of 100, and a T1 of ~1500 msec. Furthermore, we showed similar results using either a two-parameter or three-parameter fit. There are multiple factors that affect the signal recovery in the MOLLI pulse sequence. As the time between inversion pulses varies, so does the Mz following each inversion, this effect is typically not considered in the fitting of the data, and is a major contributor to the HR dependence of the MOLLI pulse sequence. There is a saturation effect during the SSFP readout which is a function of the T1, T2, and the flip angle, the number of readout lines, and the number of readouts following each inversion pulse. Finally, the SSFP signal response is affected by the off-resonance frequency. Given these multiple features confounding the fit of the data, it is not surprising that a two-parameter or three-parameter fit results in similar determination of T1. These issues need to be explored further. In our experience in the clinical studies, more reliable T1 maps with less uncertainty were obtained using the two-parameter fit.
Only normal subjects were included in this study, thus results may differ in patients with myocardial fibrosis. However, it has been demonstrated that the 3-5 MOLLI technique can detect significant differences in vd and λ in subjects with LV hypertrophy as compared to normal volunteers (15). In subjects with normal perfusion, the assumption of “equilibrium” following bolus injection is adequate after 5 minutes postcontrast (6). Thus, bolus timepoints at 5, 10, and 15 minutes postcontrast as used in this study were reasonable. The 5-minute postcontrast relaxation rates fell along the same regression line as both the precontrast and equilibrium data points, thus demonstrating that the assumption of dynamic equilibrium between the blood pool and myocardium was reasonable. In patients who may have abnormal perfusion or increased volume of distribution of Gd, data points at longer times from the bolus injection may be required for this equilibrium assumption to hold. However, the validity of this assumption is easily verified by plotting the T1 data and visualizing the regression line.
While there are some potential theoretical issues with using standard MOLLI as the reference technique, the phantom results demonstrate adequate determination of T1 at least at lower HRs. Given that the average HR in this study was 62 ± 10, the HR dependence of MOLLI was unlikely to significantly bias the results. From our phantom studies, at this HR we would only expect a 2%–3% error in the determination of T1 over the range of T1s evaluated. The HR dependence may be further decreased by increasing the number of relaxation beats between inversion trains to 4 or 5 (instead of 3) allowing more complete recovery of magnetization before the second inversion pulse. Improvement in accuracy of fitting the data may be possible by fitting the data to a Bloch simulation model which takes into account the relevant sequence parameters and HR. These issues should be explored in future studies.
In conclusion, this study demonstrates that determination of the partition coefficient of Gd is equivalent using either a BI method or CI. As the BI method is easier to perform and more easily implemented into a typical clinical protocol, it will likely become the preferred method for quantifying the partition coefficient of Gd and volume of distribution of Gd. Furthermore, we demonstrate that a protocol with reduced acquisition duration produces results similar to the conventional MOLLI technique. These are important findings, as patients with heart failure often have difficulty holding their breath and cannot tolerate lying supine in the scanner for long imaging sessions. Further research on improving methods for accurate T1 mapping will be clinically important in the quantification of diffuse myocardial fibrosis in multiple cardiac pathologies. This is particularly relevant to the accurate determination of the partition coefficient of Gd and volume of distribution of Gd, which rely on multiple T1 measurements.
ACKNOWLEDGMENT
The authors thank John Christopher RT-(R))(MR) and Jennifer Hunter, RN, for help in performance of the human studies.
DISCLOSURES Drs. Salerno, Kramer, and Epstein receive support from Siemens Healthcare; Dr. Kramer is a consultant to St. Jude Medical; Drs. Salerno and Kramer receive research support from Astra-Zeneca; Dr. Salerno is supported by an AHA Scientist Development Grant (10SDG2650038); Drs. Janardhanan and Jiji are supported by an NIH training grant (NIH 5T32EB003841).
REFERENCES
- 1.Iles L, Pfluger H, Phrommintikul A, et al. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J Am Coll Cardiol. 2008;52:1574–1580. doi: 10.1016/j.jacc.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 2.Flett AS, Hayward MP, Ashworth MT, et al. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010;122:138–144. doi: 10.1161/CIRCULATIONAHA.109.930636. [DOI] [PubMed] [Google Scholar]
- 3.Jerosch-Herold M, Sheridan DC, Kushner JD, et al. Cardiac magnetic resonance imaging of myocardial contrast uptake and blood flow in patients affected with idiopathic or familial dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2008;295:H1234–H1242. doi: 10.1152/ajpheart.00429.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broberg CS, Chugh SS, Conklin C, Sahn DJ, Jerosch-Herold M. Quantification of diffuse myocardial fibrosis and its association with myocardial dysfunction in congenital heart disease. Circ Cardiovasc Imaging. 2010;3:727–734. doi: 10.1161/CIRCIMAGING.108.842096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flacke S, Allen JS, Chia JM, et al. Characterization of viable and nonviable myocardium at MR imaging: comparison of gadolinium-based extracellular and blood pool contrast materials versus manganese-based contrast materials in a rat myocardial infarction model. Radiology. 2003;226:731–738. doi: 10.1148/radiol.2263020151. [DOI] [PubMed] [Google Scholar]
- 6.Klein C, Nekolla SG, Balbach T, et al. The influence of myocardial blood flow and volume of distribution on late Gd-DTPA kinetics in ischemic heart failure. J Magn Reson Imaging. 2004;20:588–593. doi: 10.1002/jmri.20164. [DOI] [PubMed] [Google Scholar]
- 7.Nacif MS, Turkbey EB, Gai N, et al. Myocardial T1 mapping with MRI: comparison of Look-Locker and MOLLI sequences. J Magn Reson Imaging. 2011;34:1367–1373. doi: 10.1002/jmri.22753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Messroghli DR, Greiser A, Frohlich M, Dietz R, Schulz-Menger J. Optimization and validation of a fully-integrated pulse sequence for modified Look-Locker inversion-recovery (MOLLI) T1 mapping of the heart. J Magn Reson Imaging. 2007;26:1081–1086. doi: 10.1002/jmri.21119. [DOI] [PubMed] [Google Scholar]
- 9.Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivanan-than MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med. 2004;52:141–146. doi: 10.1002/mrm.20110. [DOI] [PubMed] [Google Scholar]
- 10.Janardhanan R, Jiji R, Brooks J, Epstein F, Kramer CM, Salerno M. A comparison of methods for determining the partition coefficient of gadolinium in the myocardium using T1 mapping. J Cardiovasc Magn Reson Imaging. 2011;13(Suppl 1):O81. doi: 10.1002/jmri.23875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flacke SJ, Fischer SE, Lorenz CH. Measurement of the gadopentetate dimeglumine partition coefficient in human myocardium in vivo: normal distribution and elevation in acute and chronic infarction. Radiology. 2001;218:703–710. doi: 10.1148/radiology.218.3.r01fe18703. [DOI] [PubMed] [Google Scholar]
- 12.Schelbert EB, Testa SM, Meier CG, et al. Myocardial extravascular extracellular volume fraction measurement by gadolinium cardiovascular magnetic resonance in humans: slow infusion versus bolus. J Cardiovasc Magn Reson. 2011;13:16. doi: 10.1186/1532-429X-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diesbourg LD, Prato FS, Wisenberg G, et al. Quantification of myocardial blood flow and extracellular volumes using a bolus injection of Gd-DTPA: kinetic modeling in canine ischemic disease. Magn Reson Med. 1992;23:239–253. doi: 10.1002/mrm.1910230205. [DOI] [PubMed] [Google Scholar]
- 14.Piechnik SK, Ferreira VM, Dall'Armellina E, et al. Shortened modified Look-Locker inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson. 2010;12:69. doi: 10.1186/1532-429X-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janardhanan R, Adenaw N, Jiji R, et al. Quantifying myocardial fibrosis in hypertensive left ventricular hypertrophy using T1 mapping. J Cardiovasc Magn Reson Imaging. 2012;14(Suppl 1):P172. [Google Scholar]