Abstract
Objectives
Endogenous dentin collagenolytic enzymes, matrix metalloproteinases (MMPs) and cysteine cathepsins, are responsible for the time-related hydrolysis of collagen matrix of the hybrid layers. As the integrity of the collagen matrix is essential for the preservation of long-term dentin bond strength, inhibition or inactivation of endogenous dentin proteases is necessary for durable resin-bonded composite resin restorations.
Methods
Dentin contains collagenolytic enzymes, matrix metalloproteinases (MMPs) and cysteine cathepsins, which are responsible for the hydrolytic degradation of collagen matrix in the bonded interface. Several tentative approaches to prevent enzyme function either directly or indirectly have been proposed in the literature.
Results
Chlorhexidine, a general inhibitor of both MMPs and cysteine cathepsins, applied before primer/adhesive application is the most tested method. In general, these experiments have shown that enzyme inhibition is a promising scheme to improve hybrid layer preservation and bond strength durability. Other enzyme inhibitors, e.g. enzyme-inhibiting monomers and antimicrobial compounds, may be considered promising alternatives that would allow more simple clinical application than chlorhexidine. Cross-linking collagen and/or dentin organic matrix-bound enzymes could render hybrid layer organic matrix resistant to degradation, and complete removal of water from the hybrid layer with ethanol wet bonding or biomimetic remineralization should eliminate hydrolysis of both collagen and resin components.
Significance
Identification of the enzymes responsible for the hydrolysis of hybrid layer collagen and understanding their function has prompted several innovative approaches to retain the hybrid layer integrity and strong dentin bonding. The ultimate goal, prevention of collagen matrix degradation with techniques and commercially available materials that are simple and effective in clinical settings may be achievable in several ways, and will likely become reality in the near future.
Introduction
In 1999, a new method was developed to accelerate the effects of aging [1] on resin-dentin bonds. The resin-bonded teeth were cut vertically in the × and y directions to form 1 × 1 × 8 mm sticks for storage in water. That study revealed that resin-dentin bond strength fell significantly in 3 months of storage. Thus, although initial bond strengths were high, they fell rapidly over time. The mechanism causing this was not known. Others demonstrated that resin-bonded sticks stored in oil were stable over time, but unstable when stored in aqueous solutions. This lead to the widespread use of the term “hydrolysis”, although such terms begged the question of how water lowered bond strength. This problem was only seen in resin-dentin bonds, because resin-enamel bonds are very stable over time [2].
About 50 vol.% of dentin is composed of minerals, the rest being type I collagen and non-collagenous proteins (30 vol.%) and water (20 vol.%) (reviewed in [3]). During the bonding of composite restorations, the surface and subsurface mineral component is removed either totally by acid etching in etch-and rinse (E&R) adhesives or partially with acidic primers or adhesives in self-etch (SE) adhesives. The exposed collagen matrix is then infiltrated with solvated adhesive resin comonomers, ideally encapsulating the entire matrix with resin, forming the so-called hybrid layer that after monomer polymerization, firmly anchors the adhesive and the overlaying restoration to dentin (Figure 1).
Figure 1.
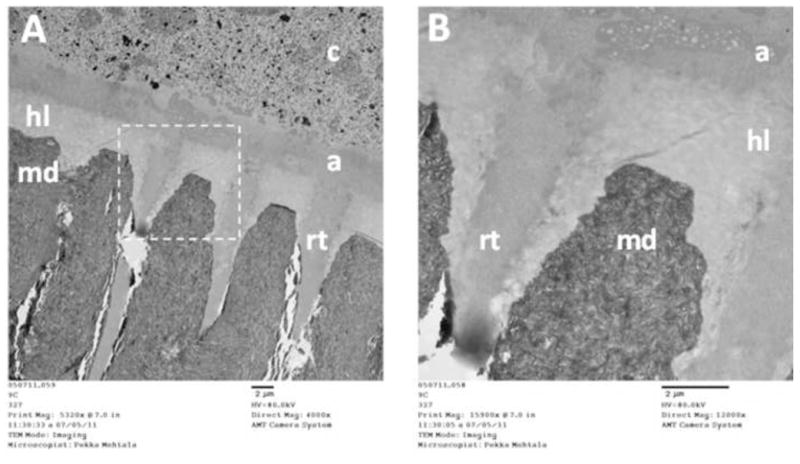
Transmission electron microscope (TEM) image of undemineralized, unstained human tooth showing the dentin-adhesive interface created with 2-step E&R adhesive (Scotchbond 1XT, 3M ESPE).
A) On the top of the mineralized dentin (md) is the hybrid layer (hl), where the exposed dentin collagen mesh is infiltrated with adhesive monomers, creating a mechanical interlock between dentin-bound collagen and polymerized adhesive. On top of the hybrid layer, adhesive (a) forms a chemical bond with the restorative resin composite (c). Adhesive resin tags (rt) penetrate into dentinal tubules, sealing them and providing additional retention.
B) Higher magnification image from the area marked with dashed box in A, with collagen matrix readily seen in the hybrid layer, even in unstained sections. (Images courtesy of BDs Pekka Mehtälä and Dr. Saulo Geraldeli).
In reality, however, adhesive monomers are not able to fully encapsulate the exposed collagen matrix, leaving totally or partially exposed collagen fibrils at the bottom of the hybrid layer, lacking the protection of polymerized resin. This poorly infiltrated zone is subjected to nanoleakage and is present especially when E&R adhesives are used [4]. Even though SE adhesives should theoretically encapsulate the collagen fibrils completely simultaneously with demineralization, many studies have also shown some nanoleakage within SE adhesives [5,6], even in the absence of a detectable exposed demineralized collagen zone [7–15]. Moreover, residual solvents in the hybrid layer contribute to incomplete infiltration of resin monomers into water-filled collagen fibril matrices, and more water may enter the hybrid layer not only during the bonding procedure, but also later [4,12]. As a result, portions of hybrid layers always contain areas filled with water instead or resin, as has been demonstrated with transmission electron microscopy (TEM) using water-soluble tracers [6,16]. Even though these water-rich zones are usually sparse immediately after bonding, they increase in size with time [16,17] indicating that water gradually replaces the other components of the hybrid layer. The lack of resin protection and presence of water leaves demineralized collagen fibrils vulnerable to time-related hydrolytic degradation.
Degradation of collagen fibrils, together with degradation of hydrophilic resin components, leads to destruction of the hybrid layer and loss of dentin bond strength over time [4,18]. The mechanisms involved in the proteolytic degradation of dentin-adhesive interfaces have been intensively studied in the recent years, and the progression in this field has been rapid. Several members of collagen-degrading enzymes matrix metalloproteinases (MMPs) and cysteine cathepsins have been identified in dentin [19,20]. Even though the role of MMPs in dentin pathologies was first suggested less than 15 years ago [21,22], and cysteine cathepsins were identified in intact and carious dentin only few years ago [23,24], the intense research activity has tremendously increased our understanding of their potential interactions in dentin physiology and diseases, including the loss of collagen matrix in the hybrid layer. A recent review discussed in detail the presence, role and function of MMPs and cysteine cathepsins in dentin [20]. Therefore, this review focuses mainly on different strategies that have been developed to control and prevent the hydrolytic enzyme-related loss of the hybrid layer collagen and bond strength.
Enzyme inhibition and hybrid layer
Because the vast majority of the experiments aimed to improve the durability of dentin bonds using an enzyme inhibition approach have been performed with CHX, we will concentrate on those studies. Other approaches use synthetic MMP inhibitors, quaternary ammonium methacrylates or benzalkonium chloride, or act indirectly by chemical chelation of calcium ion, collagen cross-linking, ethanol wet bonding, or remineralization to protect the hybrid layer from enzymatic degradation. These will be discussed separately.
Bond strength
Chlorhexidine was a logical choice as the first candidate to be tested in attempts to inhibit collagenolytic enzymes in dentin. CHX had been demonstrated to effectively inhibit MMP-2, -9 and -8 [25]. While at the time of the onset of the experiments, only MMP-2 was known to be present in dentin [26], the data demonstrating the presence of MMP-9 [27] and -8 [28] was soon published. CHX is well-known and widely used in dentistry as an antimicrobial compound. Most importantly, Pashley et al. [29] had presented convincing evidence of its efficacy in inhibiting dentin collagenolytic enzymes. The first study to show the potential of CHX in preserving hybrid layer integrity came from Hebling and others [30]. In that study, pediatric patients with carious primary molars on both sides of the mouth were selected. The experimental tooth was restored as follows: after acid etching and rinsing, the cavity was scrubbed with 2% chlorhexidine for 30 seconds, gently dried to remove excess moisture but to leave the exposed collagen matrix slightly moist to prevent matrix collapse, and then restored in a normal fashion using Single Bond 2 (3M ESPE), an etch-and-rinse adhesive and composite. The contralateral control molar was treated similarly, except that water was used instead of CHX. The teeth were collected six months later when they exfoliated, and were processed for TEM. The results showed practically perfect hybrid layers in teeth receiving CHX-treatment under the restorations in primary teeth after six months in function. Control teeth exhibited large voids in the hybrid layers. The study not only proved that hybrid layer preservation is possible with MMP-inhibition; it also demonstrated that hybrid layer can be destroyed in alarming speed in vivo, contrary to the common belief at that time [30]. More evidence came from in vitro [31] and in vivo [32] studies using the similar protocol, which used 2% CHX to decrease dentin bond strength loss in vitro by preventing the loss of the base of hybrid layer (the area typically exhibiting nanoleakage in non-inhibited controls). Surprisingly, in vivo the bond strength was preserved even better than in vitro: while in vitro the loss of bond strength was 23% in six months, in vivo it was only 1.5% after 14 months of function [32]!
The findings of these first studies have since been confirmed in numerous studies with different CHX concentrations and different adhesives (Table 1). With simplified etch-and-rinse adhesives [4,31,33–39] the loss of bond strength in control teeth (i.e. uninhibited teeth) over 1–2 years has been approximately 50%. Experimental teeth pretreated with CHX have shown 20–25% loss of bond strength over comparable times. The effective concentrations have varied between 0.002% and 4% [37], 0.2% and 2% CHX being the most common concentrations used (Table 1). Stanislawczuk et al. [39] incorporated 2% CHX into 37% phosphoric acid. After 2 yrs of storage, control teeth lost 46–53% of their original bond strength, while the CHX treated teeth only lost between 16–21% of their initial strength [39]. One study failed to show statistically significant difference between the CHX-treated and control group. In that study, 0.05% CHX was incorporated directly into Adper Scotchbond 1 XT, and bond strengths tested three, six and 12 months later showed no difference between the groups and even higher loss of bond strength in 12-month testing with CHX [40]. It is likely that little CHX could leach from the polymerized resin. The effect of CHX on bond durability with 3-step E&R adhesives (Adper Scotchbond MultiPurpose (SBMP, 3M ESPE) and All-Bond 2, Bisco) has been tested only in two studies [4,41]. While in both studies, the use of CHX resulted with slightly better long-term bond strengths, with the best outcomes of 42 and 44% lower bond strength loss with CHX applied under SBMP and All-Bond 2, respectively [4], no statistically significant differences could be found in these studies. The reason may be the relatively low bond strength loss in the controls, demonstrating again the better long-term function of 3-step etch-and-rinse adhesives compared to their simplified 2-step versions.
Table 1.
Experiments aiming to improve the durability of dentin bond strength by elimination of collagen degradation. Material names as presented in the article. Permanent teeth with microtensile bond strength testing method were used, unless otherwise mentioned.
| Bond strength (BS) | BS reduction (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Author, Year | Storage solution | Adhesive | Experiment groups | Duration (months) | Immediate | Aged | Total | Per month |
| Carrilho et al. 2007 [31] | AS | Single Bond (2-step E&R) | Control | 6 | 38.2 (5.0) | 20.9 (3.9) | 45.3 | 7.5 |
| CHX 2% | 37.8 (4.1) | 28.9 (4.4) | 23.4 | 3.9 | ||||
| AS + inhibitorsa | Control | 38.2 (5.0) | 23.7 (5.2) | 38.0 | 6.3 | |||
| CHX 2% | 37.8 (4.1) | 30.1 (6.5) | 20.3 | 3.4 | ||||
|
| ||||||||
| Carrilho et al. 2007 [32] | In vivo | Single Bond (2-step E&R) | Control | 14 | 29.3 (9.2) | 19.0 (5.2) | 35.2 | 2.5 |
| CHX | 32.7 (7.6) | 32.2 (7.2) | 1.5 | 0.1 | ||||
|
| ||||||||
| Breschi et al. 2009 [35] | AS | Single Bond (2-step E&R) | Control | 6 | 43.9 (9.5) | 27.2 (8.4) | 38.0 | 6.3 |
| 12 | 20.1 (5.4) | 54.2 | 4.5 | |||||
| CHX 0.2% | 6 | 41.9 (9.6) | 35.0 (9.7) | 16.5 | 2.7 | |||
| 12 | 33.2 (8.3) | 20.8 | 1.7 | |||||
| CHX 2% | 6 | 39.1 (11.9) | 34.8 (8.6) | 11.0 | 1.8 | |||
| 12 | 29.5 (12.7) | 24.6 | 2.0 | |||||
| XP-Bond (2- step E&R) | Control | 6 | 39.6 (9.4) | 14.2 (5.0) | 64.1 | 10.7 | ||
| 12 | 14.2 (5.0) | 64.1 | 5.3 | |||||
| CHX 0.2% | 6 | 38.3 (8.9) | 33.3 (8.5) | 13.1 | 2.2 | |||
| 12 | 26.5 (10.9) | 30.8 | 2.6 | |||||
| CHX 2% | 6 | 37.6 (5.6) | 32.2 (7.9) | 14.4 | 2.4 | |||
| 12 | 28.5 (7.5) | 24.2 | 2.0 | |||||
|
| ||||||||
| Campos et al. 2009b [36] | Water | Single Bond (2-step E&R) | Control | 6 | 24.2 (1.7) | 13.7 (1.8) | 43.6 | 7.3 |
| CHX 0.2% | 23.4 (2.1) | 17.9 (2.8) | 23.8 | 4.0 | ||||
| CHX 2% | 23.7 (2.8) | 17.4 (1.7) | 26.4 | 4.4 | ||||
| Clearfil 3S (1- step SE) | Control | 21.6 (2.8) | 12.8 (2.5) | 40.9 | 6.8 | |||
| CHX 0.2% | 20.2 (3.1) | 12.7 (2.6) | 37.1 | 6.2 | ||||
| CHX 2% | 20.5 (2.1) | 16.0 (1.4) | 22.1 | 3.7 | ||||
|
| ||||||||
| Loquercio et al. 2009 [37] | Water | Single Bond (2-step E&R) | Control | 6 | 34.1 (4.6) | 24.2 (5.4) | 29.3 | 4.8 |
| CHX 0,002% | 35.2 (6.2) | 31.1 (5.3) | 11.7 | 1.9 | ||||
| CHX 0,02% | 30.2 (4.3) | 27.3 (5.1) | 9.6 | 1.6 | ||||
| CHX 0,2% | 34.2 (4.1) | 36.2 (4.0) | −5.8b | −1.0 | ||||
| CHX 2% | 32.4 (6.1) | 28.3 (3.5) | 12.7 | 2.1 | ||||
| CHX 4% | 26.3 (4.2) | 24.3 (4.2) | 7.6 | 1.3 | ||||
| Prime & Bond 2.1 (2-step E&R) | Control | 32.0 (3.2) | 21.3 (2.4) | 33.4 | 5.6 | |||
| CHX 0,002% | 28.2 (3.4) | 25.1 (2.4) | 11.0 | 1.8 | ||||
| CHX 0,02% | 29.9 (5.3) | 30.1 (4.2) | −0.7 | −0.1 | ||||
| CHX 0,2% | 30.9 (4.7) | 27.4 (4.6) | 11.3 | 1.9 | ||||
| CHX 2% | 34.2 (5.1) | 31.3 (4.1) | 8.5 | 1.4 | ||||
| CHX 4% | 26.7 (4.7) | 21.1 (3.5) | 21.0 | 3.5 | ||||
|
| ||||||||
| Zhou et al. 2009 [42] | 0.9% NaCl | Clearfil SE Bond (2-step SE) | Control | 12 | 64.9 (9.2) | 52.8 (20.1) | 18.7 | 1.6 |
| CHX 0.05% | 62.8 (9.4) | 44.7 (13.5) | 28.8 | 2.4 | ||||
| Control | 68.6 (11.9) | 57.7 (15.9) | 16.0 | 1.3 | ||||
| CHX 0.1% | 67.9 (15.5) | 68.4 (12.3) | −0.8 | −0.1 | ||||
| Control | 67.4 (14.4) | 52.7 (16.9) | 21.8 | 1.8 | ||||
| CHX 0.5% | 68.6 (12.7) | 64.5 (13.1) | 5.9 | 0.5 | ||||
| Control | 63.1 (12.8) | 53.2 (13.1) | 15.6 | 1.3 | ||||
| CHX 1.0% | 66.5 (14.4) | 64.6 (11.9) | 2.9 | 0.2 | ||||
|
| ||||||||
| Breschi et al. 2010 [38] | AS | SB1 XT (2- step E&R) | Control | 24 | 40.8 (8.7) | 13.4 (4.9) | 67.1 | 2.8 |
| CHX 0.2% | 39.2 (9.3) | 32.6 (8.3) | 16.8 | 0.7 | ||||
| CHX 2% | 41.2 (9.6) | 28.5 (7.2) | 30.8 | 1.3 | ||||
|
| ||||||||
| Breschi et al. 2010 [81] | AS | SB1 XT (2- step E&R) | Control | 12 | 41.4 (5.9) | 22.6 (5.4) | 45.4 | 3.8 |
| Galardin | 44.1 (7.3) | 32 (6.6) | 27.4 | 2.3 | ||||
|
| ||||||||
| Ricci et al. 2010c [48] | In vivo | Prime & Bond NT (2-step E&R) | Control | 5 | 31.0 (11.7) | 21.5 (11.6) | 30.6 | 6.1 |
| CHX | 5 | 29.7 (10.6) | 27.5 (11.5) | 7.4 | 1.5 | |||
| Control | 12 | 31.0 (11.7) | 17.4 (6.8) | 43.9 | 3.7 | |||
| CHX | 12 | 29.7 (10.6) | 21.9 (9.5) | 26.3 | 2.2 | |||
| Control | 20 | 31.0 (11.7) | 13.5 (5.1) | 56.5 | 2.8 | |||
| CHX | 20 | 29.7 (10.6) | 18.7 (6.4) | 37.0 | 1.9 | |||
|
| ||||||||
| Kim et al. 2010 [162] | Single Bond (2-step E&R) | Control | 12 | 39.8 (6.0) | 23.5 (4.1) | 41.0 | 3.4 | |
| BMR | 41.6 (7.2) | 38.2 (6.0) | 8.2 | 0.7 | ||||
| One Step (2- step E&R) | Control | 12 | 37.0 (6.2) | 20.8 (6.9) | 43.8 | 3.6 | ||
| BMR | 39.2 (4.7) | 37.7 (4.9) | 3.8 | 0.3 | ||||
|
| ||||||||
| Sadek et al. 2010 [145] | AS | SBMP (3-step E&R) | Control | 6 | 41.2 (3.3) | 38.3 (4.0) | 7.0 | 1.2 |
| EWB 1d | 45.6 (5.9) | 43.1 (3.2) | 5.5 | 0.9 | ||||
| EWB 2 | 40.0 (3.1) | 38.6 (3.2) | 3.5 | 0.6 | ||||
| EWB 3 | 35.5 (4.3) | 33.7 (7.1) | 5.1 | 0.8 | ||||
| EWB 4 | 34.6 (5.7) | 25.9 (4.1) | 25.1 | 4.2 | ||||
| EWB 5 | 24.7 (4.9) | 18.2 (4.2) | 26.3 | 4.4 | ||||
|
| ||||||||
| Sadek et al. 2010 [128] | AS | SBMP (3-step E&R) | Control | 12 | 40.6 (2.5) | 27.5 (3.3) | 32.3 | 2.7 |
| Exp.adhesivee (3-step E&R) | EWB | 43.7 (7.4) | 39.8 (2.7) | 8.9 | 0.7 | |||
|
| ||||||||
| Bedran-Russo et al. 2010 [116] | Water | Single Bond (2-step E&R) | Control | 12 | 51.7 (17.7) | 26.6 (13.4) | 48.5 | 4.0 |
| Carbodiimide | 52.4 (11.4) | 39.4 (17.2) | 24.7 | 2.1 | ||||
| One Step Plus (2-step E&R) | Control | 12 | 41.6 (13.2) | 20.8 (12.0) | 49.9 | 4.2 | ||
| Carbodiimide | 46.9 (13.2) | 43.2 (14.2) | 7.9 | 0.7 | ||||
|
| ||||||||
| Sadek et al. 2010 [41] | AS | Single Bond 2 (2-step E&R) | Control | 9 | 42.3 (7.4) | 34.4 (4.9) | 18.7 | 2.1 |
| CHX 2% | 42.6 (5.2) | 38.4 (4.7) | 9.9 | 1.1 | ||||
| Control | 18 | 42.3 (7.4) | 31.5 (4.3) | 25.5 | 1.4 | |||
| CHX 2% | 42.6 (5.2) | 28.8 (8.3) | 32.4 | 1.8 | ||||
| SBMP (3-step E&R) | Control | 9 | 44.2 (3.5) | 37.4 (5.6) | 15.4 | 1.7 | ||
| CHX 2% | 41.3 (8.1) | 37.4 (5.6) | 9.4 | 1.0 | ||||
| Control | 18 | 44.2 (3.5) | 32.6 (7.1) | 26.2 | 1.5 | |||
| CHX 2% | 41.3 (8.1) | 30.5 (8.0) | 26.2 | 1.5 | ||||
| Exp. (EWB) | Control | 9 | 45.8 (7.2) | 44.4 (6.9) | 3.1 | 0.3 | ||
| CHX 2% | 46.8 (5.1) | 44.6 (5.6) | 4.7 | 0.5 | ||||
| Control | 18 | 45.8 (7.2) | 44.2 (7.8) | 3.5 | 0.2 | |||
| CHX 2% | 46.8 (5.1) | 43.6 (5.5) | 6.8 | 0.4 | ||||
| Mobarak 2011f,g [43] | AS | Clearfil SE Bond: intact dentin | Control | 24 | 24.3 (5.1) | 9.5 (3.4) | 61.1 | 2.5 |
| CHX 2% | 23.8 (5.9) | 8.7 (3.2) | 63.3 | 2.6 | ||||
| CHX 5% | 25.9 (6.4) | 11.0 (3.3) | 57.7 | 2.4 | ||||
| AS | Clearfil SE Bond: caries- affected dentin | Control | 21.7 (6.0) | 10.0 (3.5) | 54.1 | 2.3 | ||
| CHX 2% | 20.8 (6.2) | 10.0 (3.4) | 52.1 | 2.2 | ||||
| CHX 5% | 20.6 (5.1) | 14.7 (4.5) | 28.8 | 1.2 | ||||
|
| ||||||||
| De Munck et al. 2010 [40] | Water | Scotchbond 1 XT (2-step E&R) | Control | 3 | 41.0 (12.2) | 32.7 (14.2) | 20.2 | 6.7 |
| CHX 0.05% | 3 | 28.5 (13.9) | 23.7 (23.8) | 16.8 | 5.6 | |||
| Control | 6 | 41.0 (12.2) | 23.4 (25.5) | 42.9 | 7.2 | |||
| CHX 0.05% | 6 | 28.5 (13.9) | 16.7 (17.2) | 41.4 | 6.9 | |||
| Control | 12 | 41 (12.2) | 20.7 (18.0) | 49.5 | 4.1 | |||
| CHX 0.05% | 12 | 28.5 (13.9) | 6.0 (6.6.) | 78.9 | 6.6 | |||
| SB-3CT | 3 | 50.3 (16.8) | 17.5 (11.8) | 65.2 | 21.7 | |||
| 6 | 11.8 (17.3) | 76.5 | 12.8 | |||||
| 12 | 3.2 (4.2) | 93.4 | 7.8 | |||||
| Clearfil Protect Bond (2-step SE) | Control | 3 | 55.3 (21.3) | 55.4 (14.2) | −0.2 | −0.1 | ||
| CHX 0.05% | 3 | 60.5 (16.6) | 49.3 (10.2) | 18.5 | 6.2 | |||
| Control | 6 | 55.3 (21.3) | 43.7 (14.3) | 21.0 | 3.5 | |||
| CHX 0.05% | 6 | 60.5 (16.6) | 40.5 (17.5) | 33.1 | 5.5 | |||
| Control | 12 | 55.3 (21.3) | 36.8 (17.1) | 33.5 | 2.8 | |||
| CHX 0.05% | 12 | 60.5 (16.6) | 31.3 (16.9) | 48.3 | 4.0 | |||
| SB-3CT | 3 | 57.2 (19.0) | 50.4 (22.8) | 11.9 | 4.0 | |||
| 6 | 39.8 (19.6) | 30.4 | 5.1 | |||||
| 12 | 24.3 (8.2) | 57.5 | 4.8 | |||||
| G-Bond (1- step SE) | Control | 3 | 14.2 (8.5) | 12.8 (9.6) | 9.9 | 3.3 | ||
| CHX 0.05% | 3 | 13.9 (12.8) | 23.9 (26.3) | −71.9 | −24.0 | |||
| Control | 6 | 14.2 (8.5) | 6.8 (6.4) | 52.1 | 8.7 | |||
| CHX 0.05% | 6 | 13.9 (12.8) | 9.0 (10.4) | 35.3 | 5.9 | |||
| Control | 12 | 14.2 (8.5) | 4.8 (4.8) | 66.2 | 5.5 | |||
| CHX 0.05% | 12 | 13.9 (12.8) | 2.9 (2.7) | 79.1 | 6.6 | |||
| SB-3CT | 3 | 20.4 (7.5) | 19.2 (12.2) | 5.9 | 2.0 | |||
| 6 | 7.7 (5.6) | 62.3 | 10.4 | |||||
| 12 | 8.0 (7.1) | 60.8 | 5.1 | |||||
|
| ||||||||
| Cova et al. 2011 [112] | AS | XP Bond (2- step E&R) | Control | 12 | 37.3 (10.3) | 17.7 (9.0) | 52.6 | 4.4 |
| Riboflavin 0.1% | 44.4 (10.4) | 30.9 (12.2) | 30.4 | 2.5 | ||||
|
| ||||||||
| Erhardt et al. 2011 [172] | Water | Single Bond (2-step E&R) | Control | 12 | 48.5 (6.7) | 39.7 (5.7) | 18.1 | 1.5 |
| 48 | 48.5 (6.7) | 37.4 (10.3) | 22.9 | 0.5 | ||||
| Ascorbic acid 10% | 12 | 44.7 (8.7) | 48.3 (7.3) | −8.1 | −0.7 | |||
| 48 | 44.7 (8.7) | 39.8 (11.0) | 11.0 | 0.2 | ||||
| Clearfil SE Bond (2-step SE) | Control | 12 | 42.0 (6.1) | 39.5 (7.1) | 6.0 | 0.5 | ||
| 48 | 42.0 (6.1) | 28.1 (4.3) | 33.1 | 0.7 | ||||
| Ascorbic acid 10% | 12 | 38.2 (10.6) | 23.3 (8.0) | 39.0 | 3.3 | |||
| 48 | 38.2 (10.6) | 12.4 (6.0) | 67.4 | 1.4 | ||||
|
| ||||||||
| Leitune et al. 2011c,g [173] | Water | SBMP (3-step E&R) | Control | 6 | 22.4 (3.7) | 19.9 (2.1) | 10.9 | 1.8 |
| CHX 2% | 22.3 (3.7) | 24.5 (2.2) | −9.8 | −1.6 | ||||
|
| ||||||||
| Stanislawczuk et al. 2011 [39] | Water | Single Bond (2-step E&R) | Control | 24 | 32.3 (4.1) | 17.2 (5.9) | 46.7 | 1.9 |
| CHX 2% | 32.2 (5.2) | 26.1 (5.4) | 18.9 | 0.8 | ||||
| CHX 2% in etchant | 31.3 (6.1) | 26.2 (5.3) | 16.3 | 0.7 | ||||
| Prime & Bond NT (2-step E&R) | Control | 29.0 (4.5) | 13.5 (6.3) | 53.4 | 2.2 | |||
| CHX 2% | 32.8 (4.2) | 26.5 (4.6) | 19.2 | 0.8 | ||||
| CHX 2% in etchant | 34.8 (6.7) | 27.2 (5.1) | 21.8 | 0.9 | ||||
|
| ||||||||
| Pashley et al. 2011 [4] | AS | SBMP (3-step E&R) | Control | 18 | 37.8 (4.9) | 27.4 (5.4) | 27.5 | 1.5 |
| EWB | 43.5 (4.5) | 43.6 (3.4) | −0.2 | 0.0 | ||||
| CHX 2% | 35.8 (5.8) | 30.1 (5.0) | 15.9 | 0.9 | ||||
| CHX 2%/EWB | 46.1 (4.5) | 44.4 (3.0) | 3.7 | 0.2 | ||||
| All-Bond 2 (3-step E&R) | Control | 34.4 (6.1) | 22.3 (4.6) | 35.2 | 2.0 | |||
| EWB | 41.3 (7.3) | 30.1 (5.2) | 27.1 | 1.5 | ||||
| CHX 2% | 32.5 (4.9) | 26.1 (5.6) | 19.7 | 1.1 | ||||
| CHX 2%/EWB | 42.9 (4.9) | 38.7 (6.5) | 9.8 | 0.5 | ||||
| Single Bond (2-step E&R) | Control | 35.7 (5.7) | 17.1 (4.3) | 52.1 | 2.9 | |||
| EWB | 42.0 (5.6) | 30.6 (4.7) | 27.1 | 1.5 | ||||
| CHX 2% | 34.4 (6.0) | 26.2 (5.3) | 23.8 | 1.3 | ||||
| CHX 2%/EWB | 40.4 (6.1) | 36.3 (5.9) | 10.1 | 0.6 | ||||
|
| ||||||||
| Almahdy et al. 2012 [85] | Water | Optibond FL (3-step E&R) | Control | 3 | 34.7 (18.7) | 36.8 (17.0) | −6.1 | −2.0 |
| Galardin | 42.0 (18.7) | 30.9 (15.7) | 26.4 | 8.8 | ||||
| Batimastat | 48.0 (20.3) | 34.2 (17) | 28.8 | 9.6 | ||||
| Prime&Bond NT (2-step E&R)d | Control | 48.2 (20.4) | 42.7 (17.5) | 11.4 | 3.8 | |||
| Galardin | 41.9 (15.8) | 31.4 (16.0) | 25.1 | 8.4 | ||||
| Batimastat | 43.2 (18.4) | 46.4 (14.5) | −7.4 | −2.5 | ||||
| G-Bond (1- step SE) | Control | 25.3 (15.7) | 12.2 (10.0) | 51.8 | 17.3 | |||
| Galardin | 41.7 (17.7) | 17.0 (13.2) | 59.2 | 19.7 | ||||
| Batimastat | 34.8 (19.2) | 18.4 (16.1) | 47.1 | 15.7 | ||||
|
| ||||||||
| Yiu et al. 2012 [174] | AS | Exp. 2-step E&R adhesives with increasing hydrophilicity | Exp.adhesive I | 12 | 20.2 (2.6) | 12.7 (4.6) | 37.1 | 3.1 |
| Exp.adhesive I + CHX 2.0% | 19.0 (2.0) | 16.3 (0.9) | 14.2 | 1.2 | ||||
| Exp.adhesive II | 33.1 (2.9) | 25.1 (2.5) | 24.2 | 2.0 | ||||
| Exp.adhesive II + CHX 2.0% | 30.2 (3.4) | 26.3 (3.3) | 12.9 | 1.1 | ||||
| Exp.adhesive III | 37.3 (1.7) | 13.1 (3.4) | 64.9 | 5.4 | ||||
| Exp.adhesive III + CHX 2.0% | 34.8 (2.7) | 24.8 (1.7) | 28.7 | 2.4 | ||||
|
| ||||||||
| Manfro et al. 2012c [175] | AS | Single Bond (2-step E&R) | Control | 12 | 50.8 (12.8) | 20.4 (3.7) | 59.8 | 4.9 |
| CHX 0.5% | 49.3 (2.6) | 32.3 (7.9) | 34.5 | 2.9 | ||||
| CHX 2.0% | 44.0 (8.7) | 34.6 (5.1) | 21.4 | 1.8 | ||||
|
| ||||||||
| Fawsy et al. 2012 [113] | Water | Single Bond 2 (2-step E&R): separate cure for riboflavin and adhesive | Control | 4 | 37.6 (7.3) | 24.2 (6.9) | 35.6 | 8.9 |
| 0.1% riboflavin/BL | 39.8 (8.8) | 31.7 (7.7) | 20.4 | 5.1 | ||||
| 0.1% riboflavin/UVA | 46.8 (12.4) | 41.9 (9.5) | 10.5 | 2.6 | ||||
| 1% riboflavin/BL | 42.4 (10.9) | 33.6 (8.7) | 20.8 | 5.2 | ||||
| 1% riboflavin/UVA | 50.2 (9.2) | 40.2 (10.8) | 19.9 | 5.0 | ||||
| Single Bond 2: one-step BL cure | 0.1% riboflavin | 36.4 (9,6) | 30.6 (8.5) | 15.9 | 4.0 | |||
| 1% riboflavin | 22.8 (7.3) | 11.7 (6.3) | 48.7 | 12.2 | ||||
AS: artificial saliva; E&R: etch-andf-rinse; SE: self-etch; CHX: chlorhexidine; Exp.: experimental adhesive; BMR: biomimetic remineralization; EWB: ethanol-wet bonding; UVA: Ultaviolet A irradiation; BL: blue light irradiation
: storage solution containing commercial enzyme inhibitors;
: simulated pulpal pressure, mastication and thermal cycling;
: primary teeth;
: different ethanol treatments, with increasing simplicity with higher group number;
: not described in the article - alternative “enamel only” etching option suggested by the manufacturer;
: simulated pulpal pressure;
: microshear
With SE adhesives, fewer studies have been performed, and the results have been conflicting. Campos and others [36] used 1-step SE adhesive (Clearfil 3S Bond, Kuraray) with 45% better outcome using 2% CHX, while 0.2% was much less effective. Zhou et al. [42] demonstrated markedly better 12-month bond strengths with 0.1% to 1% CHX with 2-step SE (Clearfil SE Bond). However, 0.05% CHX in adhesive using Clearfil Protect Bond (2-step SE) and G-Bond (1-step SE, GC) could not prevent bond strength loss [40] (for details, see below). Mobarak [43] using Clearfil SE Bond and 2 and 5% CHX concentrations in both carious and intact dentin could not find statistically significant difference between the groups, even though in caries-affected dentin the decrease in 24-month bond strength was 45% lower than with controls. The comparison with other studies is a bit difficult because the microshear testing method was used [43], while all the other studies used microtensile method. Sectioning of large specimens into microtensile beams before storage reduces cross-sectional area of specimens, allowing faster water diffusion through the hybrid layer [44]. This mimicks the in vivo conditions where dentinal fluid flow enters hybrid layer from the tubules due to pulpal hydrostatic pressure, a phenomen called micropermeability [4,45]. Indeed, composite restorations aged in vivo have demonstrated comparable reduction in microtensile bond strengths to the specimens aged as beams in in vitro experiments [17,32,46–48], and in vitro introduced pulpal pressure is almost as effective as beam storage [49].
CHX has been also added to SE primer with slightly conflicting results. De Munck and co-authors [40] demonstrated that 0.05% CHX in Clearfil Protect Bond (Kuraray) primer could not prevent the loss of bond strength in 12-month storage. However, at the same time Zhou et al. [42] published a study comparing the effect of various CHX concentrations in Clearfil SE Bond primer and demonstrated that while 0.05% CHX was indeed ineffective in preserving long-term bond strength, 0.1, 0.5 and 1.0% CHX effectively inhibited the loss of bond strength after 12 month artificial aging (Table 1). The same group also demonstrated that 0.05% CHX was not able to inhibit the increase of collagenolytic activity of dentin powder treated with the same primer, but higher concentrations (0.5%, 1.0% and 2.0%) dose-dependently inhibited the primer-induced increase in enzyme activity when the primer was applied as recommended by the manufacturer (20 s) [50]. Interestingly, even though the inhibitory action with these concentrations was far from complete (from 15.6 to 56.7%, respectively) [50], the 0.1%, 0.5% and 1.0% CHX concentrations used in a same fashion did not affect the immediate bond strength [42,51], and completely inhibited the bond strength loss for 12 months in vitro [42]. The authors speculate that the dose-dependency is related to the mode of MMP-inhibiting action of CHX. CHX has been shown to lose its MMP inhibition in the presence of calcium chloride [25], indicating that CHX-related MMP inhibition would be related to its chelating property. Since 0.05% CHX alone completely inhibited the collagenolytic activity of untreated (undemineralized) dentin powder but 5% to 20% higher concentrations in SE primer were able to only partially inhibit activity induced with acidic SE primer, the authors concluded that calcium ions released by the primer are responsible for the loss of enzyme inhibition by CHX [50]. This is supported by the finding that treating dentin powder with Clearfil SE Bond primer for 2 minutes instead of 20 s not only increased the collagenolytic activity, but also caused the loss of inhibition by 0.5% and 1.0% CHX, with only 2.0% CHX showing statistically significant inhibition [50]. However, CHX has also been shown to inhibit cysteine cathepsins by a direct interaction of the enzyme active site [52]. To the best of our knowledge, respective molecular docking analysis with CHX and MMPs have not been performed, but the possibility remains that chelating action is not the only reason for CHX-induced MMP inhibition. Even if this is accepted, the CHX inhibion would still be dose-dependent [42,52]: stronger acid treatment of dentin (regardless of whether the etchant is phosphoric acid or acidic SE primer) exposes more MMPs and cysteine cathepsins, and higher concentration of inhibitor would thus be needed for effective inhibition.
For the successful application of CHX or other inhibitors in adhesives to preserve dentin bonding, not only the optimum inhibitor concentration but also the optimal mechanical properties of the adhesives need to be examined. The effect of CHX on mechanical and other properties of experimental adhesives has been studied in some articles. Incorporation of high levels of CHX into copolymers increases water sorption due to the osmotic effect [53] and prevents proper polymerization [54] with resultant reduction in mechanical properties [55]. Pallan and co-workers [56] investigated the effects of two MMP-inhibiting drugs, CHX and epigallocatechin gallate (EGCG, a green tea flavonoid belonging to catechins), incorporated in different ratios into bisGMA-based experimental adhesive diluted with TEGDMA, to analyze their effects on the degree of copolymer conversion, water sorption and solubility and mechanical properties. Incorporation of either CHX or EGCG at the used, reasonably low (0.2%, 1.0% and 2.0%) concentration into methacrylate comonomers had no effect on water sorption, confirming previous findings that demonstrated that the water sorption of different resins was proportional and attributed to their hydrophilicity instead of the presence of CHX [57]. Both 2% CHX and EGCG caused some decrease in conversion rate. However, with CHX, flexural strength and modulus of elasticity even increased, while respective effect was observed with EGCG only with lower concentrations. As expected, drug release form the polymerized adhesive was concentration-dependent and markedly higher during the first 24 h of the experiment, but retained slow steady-state level for the duration of the experiment, supporting previous findings [53,57]. Since the idea behind the incorporation of inhibitors into adhesive is their continuous release to prevent collagen degradation for a prolonged time, these results are promising, especially since the authors conclude that the CHX concentrations would still be sufficient to inhibit MMPs in levels present dentin and saliva [56]. The clinical efficacy of CHX has been shown especially in in vivo studies, where preservation of hybrid layer integrity [30,32] and bond strength [32] have been demonstrated.
Chlorhexidine has recently been shown to also inhibit cysteine cathepsins [52] (Figure 2), which may also contribute to its effect on preserving hybrid layer collagen. Since cysteine cathepsins have their best functional activity in acidic pH, it is possible that in acidic conditions (during acid etching, or before neutralization of acidic monomers in SE primers), cysteine cathepsins are activated, and they further activate neighboring latent MMPs by proteolytic degradation of their prodomains. At least some cysteine cathepsins are active in or close to neutral pH, so they may also independently be responsible of the degradation of the hybrid layer or for producing nanoleakage areas in the collagen matrix.
Figure 2.
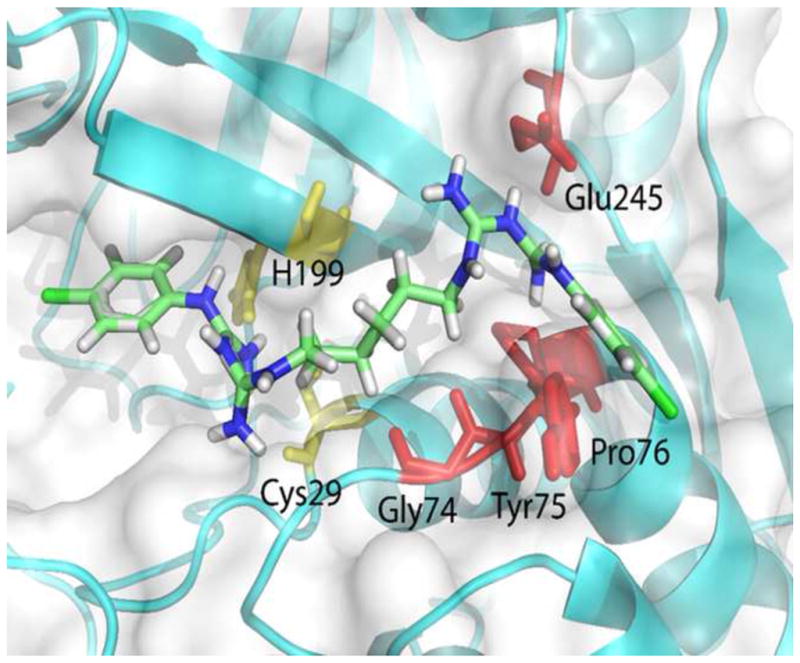
Schematic of the complex of CHX with cysteine cathepsins B, showing CHX (stick model) completely engulfed in the cathepsin B active site cavity from S2 to S2′: Cys29 and His199 are the active site residues (light yellow). The amino acid residues Tyr75, Pro76, and Glu245 (red) are at the subsite S2; Gly74 (red) and Cys29 (light yellow) residues are at the subsite S1, and the His199 (light yellow) residue is at the subsite S1′. The enzyme secondary structure elements are shown as cyan blue schematics (arrows for extended strands and cylinders for helices); the carbon atoms of CHX are indicated by light green sticks. (Reproduced from Scaffa et al. 2012 [52], with permission)
Fractographic analyses
Usually, failure modes are examined by stereomicroscopy and are classified as cohesive in dentin or composite, adhesive, or mixed failures, even though there is no clear consensus in the literature regarding their classification [58]. While this information may be useful, it has also been recognized that evaluation only with low power microscopy may cause erroneous interpretations of the materials at the fractured surface and of the distinction of failure modes [58]. Affirmative decision on the failure mode for the adhesive interface or mixed failures can only be properly made with scanning electron microscopy (SEM) at high magnification [58,59]. The laborious sample preparation and large number of samples seriously limit the use of TEM, while the less demanding sample preparation and practicality of different magnification with SEM make it more convenient, but still effective. However, often only “representative” samples are examined with SEM. This makes it difficult to make definitive conclusions on the exact site of failures especially within the hybrid layer and at the interface of demineralized and mineralized dentin.
In a study where the effect of CHX on bond strength was examined during six-month storage in artificial saliva in vitro, Carrilho et al. [31] examined both sides of all the fractured samples bonded using the 2-step etch-and-rinse adhesive (Single Bond). In controls, the failures at the bottom of the HL (Figure 3A) increased dramatically after 6-months of storage (Figure 4A). Application of CHX prior to adhesive application significantly reduced the failures at the bottom of the HL when compared to the control group, and completely eliminated the cohesive failures in dentin in 6-month aged samples (Figure 4A). The cohesive failures in the adhesive layer (Figure 3B) and composite in CHX-treated aged samples increased significantly, while in controls, clear reductions were seen especially in cohesive composite failures (Figure 4A). These findings indicate that with E&R adhesives, especially in the totally or partially denuded collagen at the bottom or below the hybrid layer, where the fibrils lack appropriate protection by adhesive, the weakest link in the bonded complex was the area where hydrolytic collagen degradation is fastest [31] (Figure 3C). Inhibiting collagenolytic enzymes using CHX eliminates or slows down collagen degradation, and shifts the major site of failure elsewhere. This conclusion has later been supported by other studies [32,60,61], even though it must be mentioned that only one of these studies [32] examined all the fractured surfaces with SEM (Figure 4B). It is logical to assume that “naked” acid-etched collagen fibrils were degraded faster than resin-encapsulated collagen fibrils that would have less access to water and where resins may sterically hinder the access of MMPs to collagen fibrils.
Figure 3.
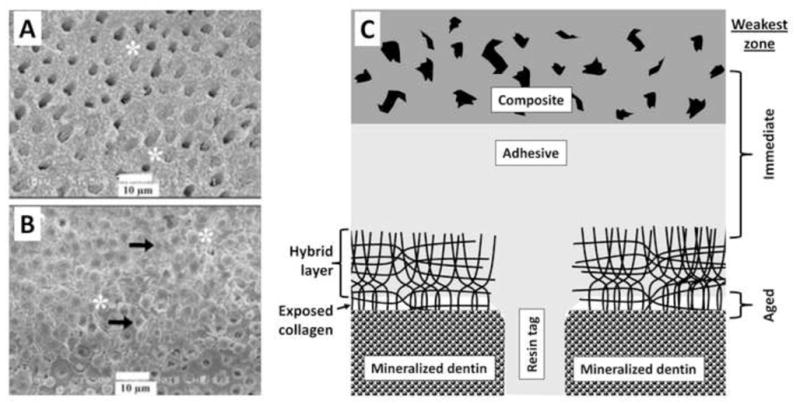
A: SEM image of the fracture occurring at the bottom of the hybrid layer. Dentinal tubules are mostly exposed, with few dentinal tubules containing remaining resin tags. Partially degraded collagen at the bottom of the hybrid layer gives can be seen (asterisk). B: SEM image of the fracture cohesive failure localized in the middle of the hybrid layer. Dentinal tubules are completely filled by resin tags (black arrow), and intertubular dentin is covered by adhesive (asterisk). C: Schematic presentation of resin-bonded acid-etched dentin covered with resin composite. The acid-etched tubules no longer contain peritubular dentin, making the tubules twice their normal diameter. Resin tags extend down from the adhesive layer. The tags are hybridized with the surrounding demineralized dentin as they pass through the hybrid layer. There is no such hybridization of the resin tags as it passes into mineralized dentin. As poorly infiltrated hybrid layers age, the collagen fibrils degrade and disappear. In such hybrid layers, water replaces the collagen. The spaces in the composite are due to hydrolysis of nanofillers of silica from the resin composite. These, too become filled with water. (Figures A and B reproduced from Carrilho et al. 2007 [31], with permission.)
Figure 4.
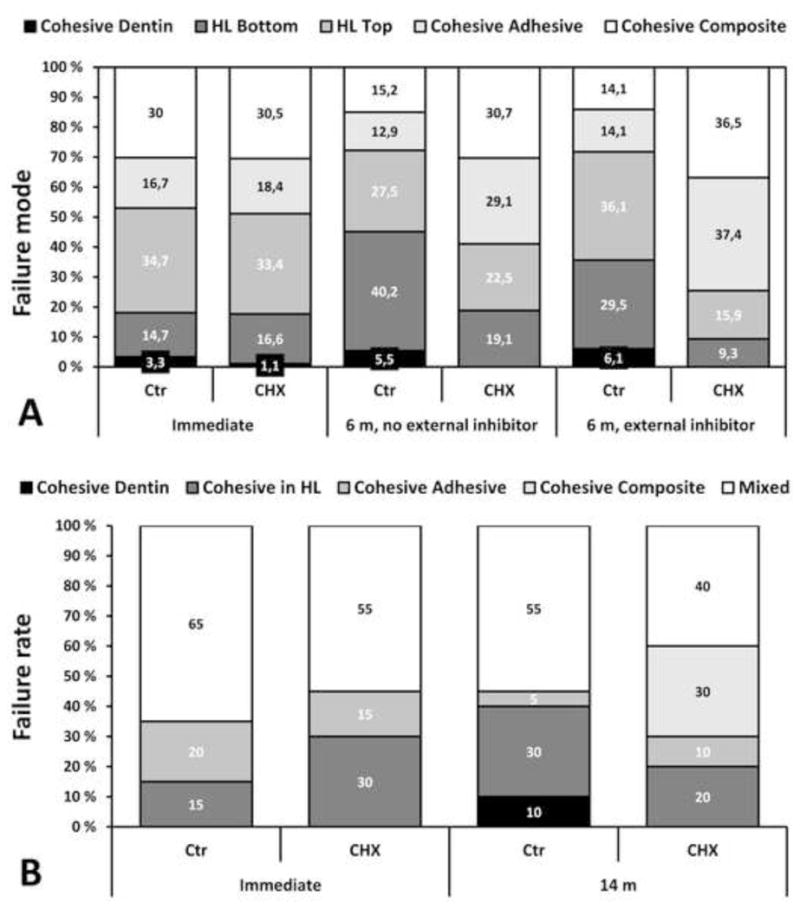
The effect of 2% CHX pretreatment on the distribution of failure modes (in percentage) as observed with SEM in vitro (A) and in vivo (B). External inhibitor indicates the absence or presence of a protease inhibitor cocktail used in incubation medium (artificial saliva, AS).
A) In immediate testing, no differences between the fracture modes were detected. After 6-month incubation, statistically significant increase in failures located at the bottom of the hybrid layer were seen in control group, but not in CHX group. External inhibitors in AS significantly reduced the failures at the bottom of the hybrid layer in controls, indicating partial elimination of endogenic enzyme function; respective effect in CHX-treated samples was non-significant. (Data from Carrilho et al. 2007 [31])
B) Failure modes in restorations tested immediately or after 14 months (14 m) in service in vivo. After 14 months, cohesive failures in the hybrid layer and dentin increased in controls, while in CHX-pretreated group failures in the hybrid layer decreased and no cohesive failures in dentin were observed. (Data from Carrilho et al. 2007 [32]).
Other MMP inhibitors
EDTA
MMPs are known to require calcium to maintain their tertiary structure and zinc ions for their catalytic hydrolase activity [62]. Chelation of calcium and zinc from acid-etched dentin, most often with ethylene diamine tetraphosphonic acid (EDTA), inactivates dentinal MMPs. Indeed, 2 to 10 mM EDTA is often used as a nonspecific inhibitor of MMPs in many laboratory studies. EDTA-demineralized dentin beams have been shown to retain their mechanical properties for a prolonged period of time with no apparent degradation of collagen matrix [63]. This finding was recently challenged by a study demonstrating significant reduction in mechanical properties of EDTA-demineralized dentin beams accompanied with degradation of collagen already in one-week incubation [64] and the release of C-terminal telopeptides related to MMP- and cysteine cathepsin functional activity (ICTP and CTX, respectively) [65]. The difference between these two studies is that in the latter, the beams were incubated in simulated body fluid containing Ca- and Zn-ions necessary for the MMP function [64] instead of zinc-free phosphate-buffered saline. The importance of Ca- and Zn-ions in the aging medium for the collagenytic function of endogenous MMPs has only recently been demonstrated [66]. Recently, Thompson et al. [67] demonstrated a significant and time-related reduction in MMP activity in EDTA-demineralized dentin beams. EDTA as an etchant has also been suggested to create a HL that would be more resistant to degradation [68–70] and produce higher immediate bond strengths with experimental ethanol wet bonding technique [71]. However, the HL collagen resistance has in these studies been tested with exposure to NaOCl [68,69], and long-term aging studies, allowing dentin endogenous collagenolytic enzyme function, have not yet been done. Also, even though the procedure may be feasible in endodontic treatment [67], where the main aim for EDTA is the removal of smear layer prior to obturation, the time needed for efficient etching for restorative adhesive purposes limits its clinical use in adhesive dentistry [4].
Synthetic MMP inhibitors
In the oral cavity, huge interest has been demonstrated towards the MMP inhibitory effect of tetracycline and its derivatives and chemically modified analogs (chemically modified tetracyclines, CMTs) and bisphosphonates because of their potential use as adjunct therapy in the treatment of periodontitis [72]. Tetracyclines are antibiotics with cationic chelating properties, and they inhibit MMP extracellularly, including gingival crevicular fluid and saliva [72–75]. Low-dose doxycycline medication for MMP inhibition is currently used as an adjuvant therapy for adult periodontitis [72,74]. CMTs are modified tetracyclines that lack the antibacterial activity, but have more or less retained their MMP-inhibition [72]. CMT-3 (aka Metastat, COL-3) is one of the most potent CMTs against collagenases (as effective as doxycycline, which is considered the most potent collagenase-MMP inhibitor in this group), but also effective against gelatinases [76,77], and is particularly effective in inhibiting MMPs in dentinal caries lesions [78]. Bisphosphonates (BPs) are pyrophosphate analogs with a high affinity for hydroxyapatite crystals and they are used to treat conditions involving increased bone resorption, e.g. Paget’s disease and osteoporosis. BPs impair calcification by inhibiting the dissolution and formation of calcium phosphate crystals; however, their anti-resorptive effect on bone is mediated mainly via cellular mechanisms [79]. BPs are also MMP down-regulating and inhibiting agents with chelating activity. Because phosphonates contain very metabolically stable R-C-P bonds instead of the R-O-P ester bonds of phosphates, single doses last a very long time. Hydroxamate-based inhibitors, such as Batimastat, Galardin and Marimastat, also act by chelating active-site zinc [80]. Zoledronic acid (zoledronate) is also effective in inhibiting carious dentin MMPs [78].
It is quite reasonable to speculate that specific MMP-inhibitors that are effective even in extremely low concentrations could be even more effective than CHX in increasing the durability of hybrid layers. Galardin (also known as GM6001 or Ilomastat) has been shown to demonstrate this effect [81]. Galardin has a collagen-like backbone that binds to the active site of MMPs and a hydroxamate structure which chelates the MMP catalytic domain zinc ion [82]. Galardin has potent inhibitory activity against MMP-1, -2, -3, -8 and -9, thus including all the MMPs currently known to present in dentin matrix, in nanomolar concentrations [83,84]. When Breschi et al. [81] treated acid-etched dentin with Galardin, after 12 months of storage in the microtensile bond strength of the untreated controls fell 45%, while that of the Galardin-treated group only fell 26.5%. Galardin was also shown to be effective against dentin gelatinases in zymographic analyses [81]. Since Galardin is a specific synthetic MMP-inhibitor (contrary to CHX, that also inhibits cysteine cathepsins [52]), the work supports the concept that the poor stability of hybrid layers is at least partly related to the activity of endogenous dentinal MMPs. Perhaps the 26.5% fall in microtensile bond strength in the Galardin-treated specimens was due to the hydrolytic action of cysteine cathepsins which are not inhibited by Galardin.
The very recent study using two hydroxyamate-based inhibitors, Batimastat (aka BB94) and Galardin, incorporated into three commercially available adhesives demonstrated partially conflicting results [85]. Inhibitor-modified adhesives significantly inhibited both recombinant and dentin MMPs. With inhibitors, the immediate microtensile bond strength was enhanced for Optibond FL (3-step E&R, Kerr) and G-Bond (1-step SE), but not for Prime&Bond NT (2-step E&R, Dentsply DeTrey) when used with composites. After three month storage in distilled water, the bond strength was retained only with Batimastat in Prime&Bond NT. With E&R adhesives, the reduced bond strength was accompanied with significant increase in cohesive failures in dentin or composite, except with Batimastat in Optibond FL. In control samples, only G-Bond demonstrated significant reduction in bond strength. With Prime&Bond NT and G-Bond (but not with Optibond FL), both Batimastat and Galardin also reduced the micropermeability of the hybrid layer in the immediate samples (the aged samples were not tested) [85]. Because of these contradictory results, it is difficult to draw any definitive conclusions of this study. The authors speculate that the absence of a decrease in micropermeability with Optibond FL is related to its better sealing ability when compared with other adhesives [85], but the explanation for the significant decrease in micropermeability in the immediate samples remains unknown. The authors also speculate that reduced bond strengths in aged samples may be related to the increased cohesive failures [85], which also is surprising considering relatively short aging (3 months) of the samples in distilled water. There are two major differences in the above study compared to the Breschi et al. study [81]. The mode of use of the inhibitor and the concentration used in these studies differed. Breschi and co-workers applied a water solution of Galardin directly on the acid-etched dentin [81], while Almahdy and others incorporated the inhibitors directly to the adhesives [85]. More importantly, while Breschi et al. [81] used 0.2 mM Galardin (the highest possible concentration to achieve a saturated water solution), Almahdy et al. [85] used 40 times lower 5 μM concentration. Even though hydroxyamate-based MMP inhibitors have been shown to be effective in nanomolar concentration, it must been noted that most inhibition studies have been performed using soluble enzymes. Since dentin MMPs in the hybrid layer remain bound to collagen, the concentration of inhibitors (including bisphosphonates) required to complete inhibition may be much higher in dentin than with unbound soluble MMPs [81]. Therefore, use of higher concentration of hydroxyamate-based inhibitors may be necessary to facilitate effective MMP inhibition.
SB-3CT belongs to the new generation thiol-based MMP inhibitors, and is a uniquely selective gelatinase (MMP-2 and -9) inhibitor [80]. The effect of SB-3CT on hybrid layer durability has been tested in two studies [40,86]. In both studies, the MMP inhibitors chlorhexidine and SB-3CT were mixed into the adhesive primers to final concentrations of 0.05% and 10 μM, respectively, and stored for variable times in water. Neither one of the inhibitors was capable of completely eliminate the loss of bond strength either with 3-step E&R (Optibond FL), 2-step E&R (Singe Bond 1 XT), or 2-step SE (Clearfil SE Bond, Clearfil Protect Bond) or 1-step SE (G-Bond) [40,86]. However, using Optibond FL and Clearfil SE Bond, the long-term (12 months) bond strengths did not show statistically significant differences when compared to the immediate values [86]. Even though the findings appear disappointing with respect to the attempted improvement in bond strength durability, the results may be interpreted with caution. The use of gelatinase-specific MMP-inhibitor SB-3CT can be justified by the assumption that MMP-2 most likely is the most abundant MMP in human dentin [19]. However, even though MMP-2 is capable of degrading collagen at a slow rate, it is still most potent against gelatinases [19] and its ability to alone degrade highly cross-linked acid-insoluble dentin collagen remains to be demonstrated. It may be that SB-3CT inhibits MMP-2, which blocks its telopeptidase activity, so that the C-terminal telopeptides in collagen sterically prevents access of collagen by collagenase like MMP-8. However, SB-3CT is not known to inhibit MMP-8 or cysteine cathepsins, collagenolytic and/or telopeptidase enzymes also present in dentin and presumably participating into matrix degradation [19,23,24,28,87]. CHX, on the other hand, inhibits soluble MMP-2, -8 and -9 in low concentrations [25], and has recently been shown to also inhibit soluble cysteine cathepsins [52]. Unfortunately, the CHX concentration used in these studies (0.05%) may be too low to exert sufficient inhibitory action on matrix-bound cathepsins. Zhou and others [42] demonstrated with one of the adhesives used in these studies (Clearfil SE Bond) that 0.1% or higher CHX concentrations, but not 0.05%, completely preserved the bond strengths up to 12 months in vitro. As discussed above, the concentrations needed for the long-term MMP inhibition in dentin matrix may be higher than those that are effective against soluble recombinant enzymes [81], which may perhaps be the case also in these studies with SB-3CT. The recent reports of chlorhexidine binding by dentin collagen [88,89] suggests that collagen may compete with MMPs for CHX binding, requiring the use of relatively high CHX concentrations.
Quaternary ammonium group
Both E&R [90] and SE [91] adhesives have been shown to activate dentin MMP activity, and they may at least partially be responsible for the gelatinolytic activity observed in the hybrid layer [20,92,93]. Adhesive monomers that would possess enzyme-inhibiting properties offer an appealing alternative to prevent hydrolytic degradation of hybrid layer collagen.
Polymerizable quaternary ammonium methacrylates (QAMs), especially 12-methacryloyloxydodecylpyridinium bromide (MDPB) have been incorporated into SE primers because they possess antimicrobial properties and can copolymerize with adhesive monomers [94,95]. Similar to CHX, these compounds are cationic, water-soluble, but unlike CHX they may not leach out of bonded interfaces. These similarities lead to testing whether QAMs might also inhibit the endogenous dentin MMPs [96]. Four out of six QAMs inhibited soluble MMP-9 as or more effectively as Galardin, which was used as control, and all of them almost completely inhibited the release of hydroxyproline-containing collagen peptides and loss of dentin dry mass over time. MDPB (a component of Clearfil Protect Bond and Clearfil Protect SE) proved to be among the most effective in spite that it was used in 5% concentration (the concentration used in commercial adhesives) in contrast to 30% with the other QAMS [96]. Some in vitro and clinical experiments have also indicated that QAMs (namely MDPB in Clearfil Protect Bond) may inhibit collagenolytic enzymes in the hybrid layer. Donmez and coworkers [17] demonstrated comparative immediate and 12-month bond strengths both in vitro and in vivo. Other studies have shown some reduction in bond strength that did not reach statistically significant differences [15,97], and in some studies the bond strengths have even increased [98,99]. However, other studies have reported reductions in bond strength comparable to other adhesives [40,100] (Table 2), so it may be too early to make any definitive conclusions of the clinical efficacy of MDPB in the preservation of hybrid layer.
Table 2.
Percentage change of the bond strength between immediate and aged values in the experiments in which Clearfil Protect Bond has been evaluated.
| Author, year | Duration (months) | Adhesive | % change |
|---|---|---|---|
| Nakajima et al. 2003 [97] | 6 | Clearfil SE Bond | −47.1* |
| Clearfil Protect Bond | −7.5 | ||
|
| |||
| Donmez et al. 2005 (in vivo) [17] | 12 | Clearfil SE Bond | −35,2* |
| Clearfil Protect Bond | 3.9 | ||
|
| |||
| Donmez et al. 2005 (in vitro) [17] | 12 | Clearfil SE Bond | −36.9* |
| Clearfil Protect Bond | 0.7 | ||
|
| |||
| Ansari et al. 2008 (microshear) [98] | 12 | Clearfil SE Bond | −25.0 |
| Clearfil 3S Bond | −9.7 | ||
| Clearfil Protect Bond | 35.1* | ||
|
| |||
| Erhardt et al. 2008 [176] | 6 | Scotchbond 1 | −9.9 |
| AdheSE | −3.5 | ||
| Clearfil Protect Bond | 0.8 | ||
|
| |||
| Reis et al. 2008 [177] | 12 | Prime&Bond NT | −41.2* |
| Single Bond | −25.2* | ||
| Clearfil SE Bond | −45.5* | ||
| One-up Bond | −29.1 | ||
| Clearfil Protect Bond | −21.7* | ||
|
| |||
| Shinohara et al. 2009 [99] | 3 | SB MultiPurpose | −14.0* |
| Clearfil SE Bond | 0.2 | ||
| Clearfil Protect Bond | 13.2* | ||
|
| |||
| De Munck et al. 2010 [40] | 12 | Single Bond | −49.5* |
| G-Bond | −66.2* | ||
| Clearfil Protect Bond | −33.5* | ||
|
| |||
| Van Landuyt et al. 2010 [15] | 12 | Optibond FL | −13.1 |
| Clearfil SE Bond | −42.8* | ||
| G-Bond | −53.1* | ||
| iBond | −58.7* | ||
| Clearfil 3S Bond | −62.0* | ||
| Clearfil Protect Bond | −29.1 | ||
|
| |||
| De Munck et al. 2011 [100] | 12 | OptiBond FL | −47.2* |
| ScotchBond 1 XT | −70.0* | ||
| Clearfil SE Bond | −47.9* | ||
| G-Bond | −72.4* | ||
| Protect Bond | −51.6* | ||
indicates statistically signficant difference to the respective immediate bond strength as reported in the publication
Benzalkonium chloride (BAC) is a mixture of alkylbenzyldimethylammonium chlorides of various alkyl chains. It is a cationic surface-acting agent with a quaternary ammonium group used as antimicrobial agent and surfactant [101]. BAC-containing etchants can be used with E&R adhesives without affecting immediate bond strength to enamel or dentin [102]. Tezvergil-Mutluay et al. [101] demonstrated that demineralized dentin could take up high (up to 10 times more) concentrations of BAC than mineralized powder, with about half remaining after water rinsing. 0.5% BAC concentrations completely inhibited soluble MMP-2, -8 or -9, and produced up to 66% and 81% reduction in demineralized dentin collagen degradation when measured as mass loss or as hydroxyproline release into medium, respectively [101]. The results indicate that BAC is effective at inhibiting dentin matrix enzymatic degradation. It is possible that acid-etching with BAC-containing acid may leave some (perhaps about half of the original concentration) of this MMP-inhibiting quaternary ammonium compound into demineralized matrix, possibly contributing to the longer durability of the hybrid layer. Further experiments are needed to test whether BAC could be incorporated into adhesive primers and whether the enzyme inhibition would be effective to further improve the long-term bonding in dentin.
Other approaches to eliminate collagen degradation
Even though the concept of chemical bonding with self-etch adhesives to dentin hydroxyapatite is, in a strict sense, not aiming to inhibit dentin enzymatic function, it has been suggested to preserve the long-term collagen integrity in the hybrid layer (for details, see the recent review by Van Meerbeek et al. 2011 [103]), and is therefore also briefly considered here.
The effects of acid-etching mineralized dentin with phosphoric acid versus acidic monomers
Phosphoric acid (PA) has a molecular weight of 100 g/mole. Thirty-seven percent PA contains 370 g/L of phosphoric acid which, divided by the molecular weight of PA, gives a concentration of 3.7 moles/L. The pH of 37% PA is −0.37; that is the pH is minus 0.37, making it relatively acidic. When this etchant is applied to dentin, the protons of the acid combine with trivalent phosphate (PO4−3) in apatite to form HPO4−2 which strains the crystalline lattice of apatite so much that it disintegrates within seconds. All the extrafibrillar apatite crystallites that occupy the space around mineralized collagen fibrils are dissolved to a depth of 8–10 μm in 15 sec. It is likely that there is some buffering of the PA by apatite but not much because the PA is present in great excess. Immediately after dissolving the extrafibrillar apatite crystallites, the PA begins to diffuse into the mineralized collagen fibrils. Toroian et al. [104] carefully studied the molecular size exclusion characteristics of demineralized bone collagen. They concluded that molecules smaller than 6 kDa can diffuse into or out of the water within demineralized bone matrix. As collagen fibrils are not more than 100 nm in diameter, PA can diffuse from both sides in seconds to completely solubilize all of the intrafibrillar apaptite minerals. While these crystals are dissolving, the PA is also activating all preforms of MMPs and cysteine cathepsins to their active forms.
In marked contrast, when self-etching primers or single-step self-etching adhesives are applied to dentin, most of them have pHs between 2.1–2.8. This is acidic enough to etch its way through most smear layers and into the underlying mineralized dentin to a depth of 0.5–1.5 μm. These acidic monomers also attack extrafibrillar apatite crystallites, they protonate the trivalent phosphate ions in apatite crystallites causing it to rapidly dissolve. About half of the ionized calcium ions that are liberated from apatite react with the acidic monomers to create their relatively insoluble calcium salts. Two processes stop acid-etching. First, within 10–20 sec of application, the pH of the acidic monomers increases from its original pH 2.1–2.5 to reach a pH of 5.6–6.8 [105]. Second, the formation of insoluble calcium salts precipitates the acids from solution. The result of this is that not all the apatite crystallites are removed from the extrafibrillar compartment and almost all of the intrafibrillar apatite crystallites remain intact. Wherever there are nanometer-sized apatite crystallites remaining in collagen, all of the associated noncollagenous proteins, including MMPs/cysteine cathepsins will remain mineralized and inactive. Most of the activation of MMPs produced by self-etching adhesives [42,51,91] was probably extrafibrillar. That is, the endogenous proteases on the outside of collagen fibrils became uncovered and activated, while those on the inside of the fibril (i.e. the vast majority of the total theoretical MMPs) probably remain fossilized and inactive. Much more research is required to prove or disprove these speculations
Preservation of collagen hydroxyapatite at the interface to protect collagen
Functional monomers in mild self-etch adhesives, such as 10-methacryloyloxydecyl dihydrogen phosphate (10-MDP), 4-methacryloxyethyl trimellitic acid (4-MET) and 2-(methacryloyloxyethyl)phenyl hydrogenphosphate (phenyl-P) mimick the effect seen with polyalkenoic acids in glass ionomer cements and leads to an electrostatic chemical bond to calcium ions of the hydroxyapatite crystals. ‘Mild’ self-etch adhesives and glass ionomers interact only superficially with dentin, forming a thin (even submicron) hybrid layer, and the bond is considered to result both from micro-mechanical and chemical bonding mechanisms. This is different form the effect observed with “strong” or “aggressive” SE adhesives, which create an etching effect fairly similar to phosphoric acid in etch-and-rinse systems, leading to unstable bonds with the dissociation of the electrostatic bond and subsequent loss of bond strength [103]. A recent study also demonstrated inherent deficiencies in hybrid layers created by an “aggressive” 1-step SE that could be identified only after the resin-dentin interfaces were aged for six weeks in the alkaline glycine buffer [106]. The use of “mild” SE adhesives is believed to minimize nanoleakage, leave a substantial amount of hydroxyapatite around the collagen fibrils to mask the collagen cleavage site and keep the enzymes “fossilized” in the mineralized portion. Thus, the collagen could not be degraded. The functional monomers mentioned above differ in the stability of the bond formed with Ca ion, 10-MDP being the most stable [107]. The importance of bond stability has been confirmed in thermocycling studies, in which the bond strength to dentin of the 10-MDP-based Clearfil SE Bond has been shown to remain high, while those of 4-MET-containing Unifil Bond and phenyl-P-containg Clearfil Liner Bond II gradually decreased over time [103,108]. However, most of the studies showing “chemical bonding” of mild self-etch adhesives were done under extremely artificial conditions. They were usually done on synthetic apatite or enamel. No comonomers like HEMA, TEGDMA or BisGMA were mixed with the self-etching adhesives and the adhesives were not required to etch into the substrate at high concentrations for only 20 sec before they were light-cured.
Even though the TEM analyses in long-term studies in vitro and in vivo indicate good preservation of the hybrid layer with the “mild” SE adhesives [15,109], there is evidence that even with 10-MDP – that is currently considered to form the most stable chemical bond with hydroxyapatite – the bond strengths do decrease with time, both in vitro and in vivo [15,17,36,42,47]. Apparently the weakest zone in aged samples with SE adhesives is located immediately below the hybrid layer observed with TEM [15,47], as the cohesive fractures of dentin (when observed with SEM) increased significantly in an in vivo study [47]. It is possible that the loss of collagen integrity still occurs at the base of the hybrid layer due to the voids and nanoleakage practically undetectable with current techniques [110]. This is supported by the study by Kim et al. [106], in which alkaline glycine buffer incubation caused the basal hybrid layer to disappear, creating a 1 to 2 μm gap between the intact top of the hybrid layer and the mineralized dentin base that was readily detectable with SEM and confocal microscopy in spite of seemingly perfect hybrid layers in their respective TEM images. As the acidic monomers require water for their ionization and etching, the monomers at the bottom of the hybrid layer might be less polymerized, causing a loss of bond strength due to the hydrolytic degradation of the hydrophilic monomers.
If it is accepted that the enzymatic degradation of collagen is not completely eliminated in the deepest parts of the hybrid layer, even with the “mild” SE adhesive functional monomers that partially preserve and chemically bond with the collagen minerals, the need for enzyme inhibition is still apparent with these adhesives. This assumption is actually supported by some studies. Adding CHX into self-etching primer of Clearfil SE Bond significantly improved the 12-month bond strength, as long as the CHX concentration was at least 0.1% [42]. Also, the use of the self-etching primer containing MDPB (Clearfil Protect Bond) with MMP-inhibitory activity (see details above) has been shown to preserve the dentin bond strength both in vivo [17] and in vitro [15,17,97,17,99].
Collagen cross-linking
The realization that two different types of endogenous proteases in dentin matrix may be involved in the hybrid layer collagen destruction complicates the enzyme inhibition approach to preserving hybrid layer integrity. If we used inhibitors of these two classes of inhibitors, we might have to use two different inhibitors. Even though CHX inhibits both MMPs [38] and cysteine cathepsins [52], the potential disadvantage is that CHX may leach out of hybrid layers within 18 to 24 months [41,48].
To find a permanent solution to the presence of two different types of endogenous proteases in the dentin matrix, inactivation of all endogenous proteases of dentin matrix using a cross-linking agent is an interesting option. One approach is to apply 0.1% riboflavin to acid-etched dentin that is then irradiated with UVA light for 2 min prior to resin-bonding. Such treatment has been successfully used in ophthalmology to treat keratoconus [111]. These treatments did improve the durability of resin-dentin bonds [112]. Recently, Fawzy and co-authors [113] showed that treating dentin with 0.1% riboflavin before the application of the 2-step E&R adhesive adhesive (Adper Singlebond 2, 3M ESPE) and curing separately with blue light (2-step photocuring) significantly improved the durability of dentin bond strength, although not as much as using UVA [113]. With 1% riboflavin, the immediate bond strengths were higher, but the relative decrease was also higher than with lower riboflavin concentration (Table 1). When blue light was used to cure the adhesive placed on riboflavin-treated dentin without separate irradiation of riboflavin (1-step photocuring), 0.1% riboflavin treatment still showed markedly better long-term bond strength, even though statistical significance was not reached. However, with 1-step photocuring 1.0% riboflavin demonstrated catastrophically lower immediate and long-term bond strengths than all the other groups, including untreated controls [113] (Table 1). The findings indicate that using riboflavin to cross-link collagen might be advantageous also with simplified application technique using blue light irradiation, but further work is needed to find optimum concentration and to shorten the time needed for cross-linking (5 minutes). Glutaraldehyde and grape-seed extract [114] have also been also tried with promising results, but were found to take too long (minimum exposure time 10 minutes) to be clinically useful. A recent study indicates, though, that an increase in immediate dentin bond strength may be achiavble with grape-seed extract-based preconditioners even in reduced, clinically applicable treatment times [115], but the durability of long-term bond strength remains to be examined. Glutaraldehyde works well but is considered to be too toxic. Bedran-Russo et al. [116] had better success with carbodiimide, a cross-linking agent with very low cytotoxicity. When we applied 0.3 M carbodiimide to acid-etched dentin, we found that it completely blocked the total endogenous protease activity of dentin in as little as 1 min of topical treatment [117]. Because dentin does not turnover, if all of the exposed proteases bound to matrix collagen are inactivated by a nontoxic cross-linker like carbodiimide, such treatment should lead to a dentin matrix that is permanently stable.
Carbodiimide contains a functional group with the formula RN=C=NR. The cabodiimide reacts with ionized carboxyl groups in proteins to form an O-acylisourea intermediate. This intermediate reacts with a nonproteinated amino group and an adjacent protein chain to form a stable covalent amide bond between the two proteins, with the only by-product being urea. It is considered one of the least cytotoxic cross-linkers. These cross-links are very stable. They may inactivate the active sites of dentin proteases by reducing the molecular mobility of the active site or by changing negatively charged ionized carboxyl groups into positively charged amides. Additionally, carbodiimides can cross-link both helical and especially telopeptide domains in collagen. They may prevent telopeptidase activity that would normally remove bulky telopeptides from the specific peptide bond of collagenases.
The use of carbodiimide to inactivate all endogenous proteases in dentin looks like a very promising procedure. It is currently designed to be used just after acid-etching, in etch-and-rinse systems. It may be possible to reduce the current treatment time of 60 s to less than 30 s. Preliminary evaluation of the cytotoxicity of carbodiimide on pulpal cells found no cytotoxicity. In that study (Scheffel et al. 2012, unpublished observations) dentin disks 0.4 mm thick were prepared from mid-coronal dentin. After acid-etching both sides of the disks to remove smear layers and maximize permeability, odontoblast-like cells (MDPC-23 cells) were attached to the pulpal side of the disks. Then the disks were placed in split chambers that permitted the cells to be bathed in tissue culture media, while the “occlusal side” of the disks were treated with 0.05, 0.1, 0.3 or 0.5 M EDC (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride) made up in pH 6 phosphate buffer, that was applied to the acid-etched dentin for 60 s. Excess unreacted EDC was rinsed away with a copious stream of water for 10 s. The split chambers were than incubated for 3 days to see if the EDC had any negative effects on the cells on the other side of the disk. The MTT assay shown no cytotoxicity of any of the EDC concentrations (Figure 5) compared to water treatment. The positive control was 37% H2O2 which caused cell death for most of the cells.
Figure 5.
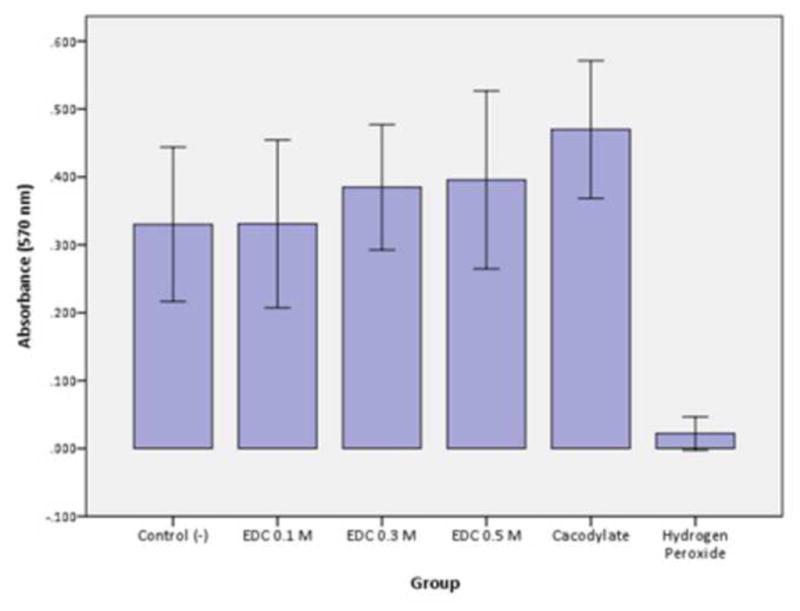
Cellular metabolism (SDH enzyme production detected by MTT assay) of odontoblast-like cells on the pulpal side of dentin disks following different concentrations of EDC solutions applied on the occlusal side of 0.4 mm-thick dentin discs. Columns are mean absorbance (570 nm) and error bars are standard-deviations, n=12. Columns connected by the horizontal line do not differ statistically.
The cross-linking that we propose is very different from the natural, time-dependent cross-linking that occurs in dentin collagen. The natural cross-linking involves oxidative deamination of free ε-amino groups in lysine and hydroxylysine and lysine hydroxylation of residues on adjacent peptides to form covalently linked pyridinium containing compounds [118–121]. These natural cross-links are not altered by acid-etching dentin with 37% phosphoric acid [122].
Ethanol wet bonding
To ensure proper hybridization of wet collagen matrix, increasing concentrations of hydrophilic and ionic monomers have been added to new adhesives [123]. These polymers are vulnerable to water sorption and/or hydrolysis due to the presence of ester linkages [124], which significantly weakens the mechanical properties of adhesive with time [123,125,126]. Therefore, hydrolytic degradation of adhesives is the other “weak link” in bond strength durability. The relative importance of the destruction of the collagen or resin components can be only indirectly estimated. For example, in a recent study Van Landuyt et al. [15] compared the immediate and 6-month bond strengths of six commercially available adhesives both in enamel and in dentin in vitro. Enamel bonds fell in average 13.7% (minimum 1.6% and maximum 27.3%). Because enamel bonding does not rely on collagen, the loss of bond strength was presumably due to the water sorption and/or hydrolytic degradation of adhesive. With dentin, the respective loss of bond strength was 43.1% (minimum 13.1% and maximum 58.7%) (Figure 6). In TEM analysis of the fractured surfaces, the most common failure site with SE adhesives was in dentin just beneath the hybrid layer, which the authors speculate to be associated with insufficient encapsulation of residual smear [15]. However, since smear layer is located at the surface of dentin, it can be argued that the smear components should remain at the top half of the hybrid layer. Instead, the fracture site may represent the partially denuded collagen zone with less-than-ideal protection by polymerized SE monomers and subsequent enzymatic degradation of exposed collagen. Alternatively, it might be due to the slow solubilization of the calcium salts of the acidic monomers used in self-etching adhesives. Although they are relatively insoluble, they probably solubilize over many months to leave a porous zone containing demineralized collagen fibrils containing activated collagenolytic enzymes. This speculation is supported by other studies with TEM analysis of the hybrid layers created with E&R adhesives after artificial aging. It is a frequent finding that the collagen matrix at the top of the hybrid layer is better preserved than in the middle or lower thirds [30,32,127–129]. The difference may be caused by the phase separation of the hydrophobic and hydrophilic monomers, where the hydrophobic monomers (such as BisGMA) are more or less restricted to the upper parts of the hybrid layer, and the bottom is HEMA- and water rich [4,130,131].
Figure 6.
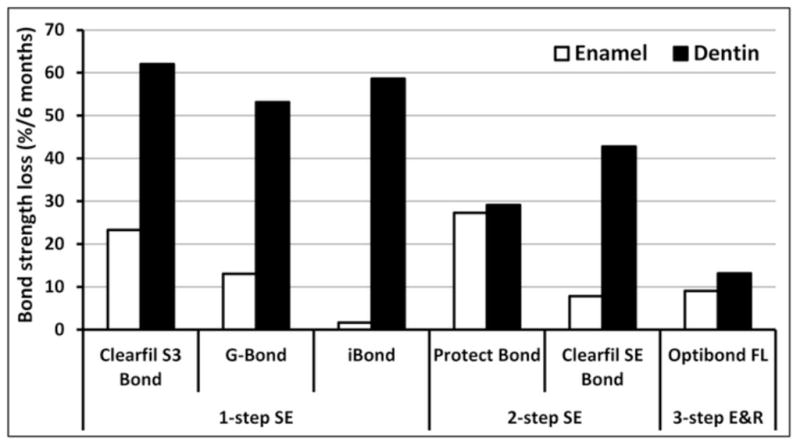
Comparison of the loss of bond strength between enamel and dentin in vitro after 6 month water storage within the same study [15]. Bond strengths in enamel after the storage did not show statistically significant difference with any adhesive when compared to the intial bond strengths. In dentin, significant decrease (p<0.05) was observed with all adhesives except Protect Bond and Optibond FL.
Proteoglycans (PGs) form interfibrillar bridges that absorb water with their carbohydrate side chains, glycosaminoglycans (GAGs) in dentin collagenous matrix [110]. In demineralized dentin, PG/GAG interfibrillar hydrogel may allow the penetration of smaller hydrophilic non-cross-linking monomers (such as HEMA), but restrict the entrance of larger cross-linking monomers (such as BisGMA) into deeper parts of the hybrid layers [4,20]. Several MMPs may bind to PGs or GAGs, which may affect their substrate binding, activation and activity; GAGs also have an important role in cysteine cathepsin function (for details, see [20]). This may not only allow enzymatic collagen degradation of collagen, but also facilitate hydrolytic degradation of the bonding monomers, both leading to increased nanoleakage. Brackett and co-authors [129] demonstrated the water-related loss of nano-fillers within a water-rich zone in the adhesive layer in 12 months in vivo irrespective of degradation of the hybrid layer collagen or its preservation with CHX. Since most studies with CHX and other enzyme inhibitors show some loss of bond strength (Table 1), that part of bond strength loss may be attributed to the water sorption or degradation of the adhesive monomers or polymerized hydrophilic adhesives. In addition, degradation of collagen leaves the subsequent voids filled with water and increases nanoleakage in the hybrid layer [30,37,38,81,127,128]. This may increase microfluid fluxes at the interface, accelerating adhesive component hydrolytic degradation. Since all these aspects may be related to PGs and GAGs, extracting them prior to adhesive application sounds like an attractive alternative. However, since removal of PGs and GAGs from dentin has resulted in conflicting results in bond strength [132–134], and because of 24h treatment required for their enzymatic removal, this is not clinically relevant approach.
The problem of water hydrolysis of ester-bonds in adhesive polymers and peptide bonds in collagen might be eliminated if water could be excluded from the bonded interface. This has been the aim in ethanol wet bonding [4,135,136], where ethanol is used to chemically dehydrate acid-etched demineralized dentin matrices to reduce dentin hydrophilicity and facilitate the infiltration of more hydrophobic monomers to dentin [4]. Organic solvents, including ethanol and acetone, cause a collapse of GAG gels in connective tissues by removing water [137].
Wang and his colleagues have described the distribution of HEMA and BisGMA across hybrid layers using confocal μRaman spectroscopy [138–140]. They reported that BisGMA was only seen in the top half of hybrid layers, while HEMA was uniformly distributed across the entire hybrid layer. However, when they employed ethanol wet bonding, BisGMA penetrated much deeper into hybrid layers [131]. Ethanol wet bonding coaxes hydrophobic monomers into a demineralized collagen matrix with limited matrix shrinkage. The proof-of-concept was provided in a study in which ethanol-solvated BisGMA bonding resulted with wider interfibrillar spaces, more extensive collagen fibril shrinkage and narrower hybrid layers (due to the absence of water) accompanied by high immediate bond strength, acid-/base-resistant hybrid layers, resin tags, and nanoleakage distribution comparable to water-wet bonding with commercial adhesive [141]. Infiltration of hydrophobic monomers decreases water sorption/solubility and resin plasticization, but it has been suggested that the elimination of residual water also reduces or eliminates enzyme-catalyzed hydrolytic collagen degradation [41,142], which would also contribute to improved durability of the resin bonds [4,143]. That the enzyme inhibition is due to removal of water instead of directly inhibiting or denaturating the enzymes is supported by the study demonstrating that different alcohols have different abilities to inhibit recombinant MMP-9 [144]. While ethanol (used in ethanol wet bonding) and methanol were relatively poor inhibitors of MMP-9 (12% and 14%, respectively), 1- and 2-propanol, 1-and 2-butanol and tert-butanol were very effective, with 52% to 91% inhibition [144]. The authors speculate that alcohols that inhibit MMPs do so by hydrophobic interactions between the catalytic zinc and the oxygen atom of the hydroxyl group of the alcohol [144]. To the best of our knowledge there are no studies published where alcohols other than ethanol would have been used to test the durability of dentin bonding. We have, however, preliminary results where pretreatment of acid-etched dentin with 0.5% CHX diluted into 64% mixture of alcohols (ethanol, 2-propanol and tetr-butanol) completely inhibited the reduction in dentin bond strength reduction with 2-step E&R adhesive for 6 and 12 months, while in controls the reduction was 37 and 26%, respectively (Tjäderhane et al. unpublished observation). The findings are in line with those using 2% CHX with 3-step E&R adhesive (Scotchbond MultiPurpose) in ethanol-wet dentin, in which bonding with and without CHX resulted with unchanged bond strengths even after 18 months of aging, thus leaving the role of CHX in the preservation of bond strength open when used in ethanol-wet dentin [4]. With other adhesives (All-Bond 2 and SB1), combining 2% CHX with ethanol wet bonding produced additional 50% decrease in bond strength reduction when compared to CHX-treatment alone [4]. However, while “traditional” ethanol wet bonding requires reasonably long treatment times [145], in our unpublished study acid-etched dentin was only treated with alcohol-CHX mixture for 30 seconds before applying the adhesive, the time being clinically acceptable (Tjäderhane et al. unpublished observation). Another unpublished observations by Manso et al. suggested that ethanol bonding under clinically acceptable time was effective in preventing bonding degradation, regardless of the adjunctive use of CHX or not in the bonding protocol. Additionally, the same study indicated that bonding technique may play important role in the bonding effectiveness over time as control groups, in which neither ethanol, CHX or combination of both were used, the bond strength values did not decrease after 15 months using either All Bond 3 (Bisco) or Excite (Ivoclar Vivadent) adhesive systems. Together, these findings indicate that with alcohols capable of direct MMP inhibition it may be possible to create an ethanol wet bonding technique – perhaps in combination with other MMP inhibitors, such as CHX - that would be clinically usable yet offer improved bond strength durability.
However, the concept of completely removing water from the collagen matrix – or at least complete replacement of the space previously occupied by mineral crystals, with adhesive monomers - has been questioned based on the space available between collagen molecules and size of monomers. As discussed above, Bertassoni et al. [110] believe that the lateral distance between collagen molecules within a fibril is too small (1.26 to 1.33 nm) for even the smallest monomer triethyleneglycoldimethacrylate (TEGDMA) (appr. 2 nm/molecule) to fit in. However, if osteocalcin (molecular weight of 5700 Da, 18 nm diameter can equilibrate with collagen water [104] then dental adhesive monomers should easily permeate into the available space, especially if water has not been removed. Others might argue that the catalytic zinc of MMPs is located at the bottom of a deep cleft with the entrance of this groove being only 0.5 nm wide [146], so how could adhesive resin monomers inhibit MMPs by flowing into ethanol-saturated dentin matrix and polymerizing in the catalytic sites of MMPs, as earlier speculated [4].
However, Carvalho et al. [147] using zymography, reported that 5% HEMA inhibited MMP-2 in polyacrylamide gels. More recently, HEMA was shown to inhibit soluble rhMMP-9 as well as matrix-bound MMPs [144]. Those results suggest that HEMA must be able to reach the catalytic site of the enzyme. Water is also believed to be quite tightly bound to collagen molecule [110]. This concern is certainly valid from the collagen nanostructural point of view, but whether it creates a problem regarding collagen preservation and dentin bond durability is another question. The lateral shrinkage (about 20% [141]) of collagen fibrils in ethanol wet bonding may be caused by the removal of intrafibrillar water. If this space is not refilled with adhesive monomers, degradation of collagen may still be prevented provided that infusion of water is also prohibited. Alternatively, increases in the width of interfibrillar spaces has been suggested to be caused by the ethanol-induced shrinkage of interfibrillar proteoglycans [128], leading to a higher resin/collagen ratio within the hybrid layer. The increase of the width of interfibrillar spaces and reduced hydrophilicity of collagen may, in turn, allow better encapsulation of collagen fibrils with hydrophobic monomers, thus resulting in water-tight envelopment of collagen fibrils. This assumption is supported by the recent study by Sadek et al. [128], in which relatively thin (appr. 3 μm compared to 5 μm in controls) hybrid layer created with ethanol wet bonding demonstrated almost complete absence of nanoleakage, as well as excellent durability of bond strength, even after 12-month storage in artificial saliva.
In their book, Nakabayashi and Pashley [148] suggested that if resins could truly infiltrate collagen, the result would be an “interphase model”, in which resin and collagen would share the elongation stress in ethanol wet-bonded dentin due to equalizing the mechanical properties of collagen and resin [4]. In general, resins with one or two hydrophilic compounds produced the highest bond strengths and excellent resistance to cyclic mechanical stressing [149]. The authors hypothesize that hydrophilic comonomers, through their tendency to absorb water after polymerization may have plasticized the resin–dentin interface enough to tolerate cyclic mechanical stressing without fracturing [149]. If this assumption is accepted, it not only explains the good bond strength and excellent ability to tolerate mechanical forces, it may also offer a plausible explanation for the complete absence of enzymatic collagen degradation in resin-dentin bonds created by ethanol wet bonding after 12-month storage in artificial saliva [128]. Even though the adhesive used in that study was basically hydrophobic, the residual ethanol in collagen would allow BisGMA to dissolve in that ethanol in a manner similar to the hydrophilic comonomers in the study by Sauro and others [149]. That would leave collagen- and PG-bound hydrolytic enzymes devoid of water necessary for the activity and thus prevent time-related enzymatic collagen degradation. In other words: if ethanol wet bonding is capable of removing most of intrafibrillar water, hydrophilic components of adhesive resins may absorb the rest and actually improve both the mechanical properties and inhibit the enzymatic degradation. That way, hydrophilic monomers may be beneficial, provided that the amount of water is not high enough to allow hydrolytic degradation of polymerized resin.
In summary, even if ethanol wet bonding may still be considered more as a “bonding philosophy” [150], it offers both important information of the role of solvent or intrinsic water in the hybrid layer degradation process and interesting potential for the clinically useable bonding techniques to be developed. As pointed out also in recent reviews [4,110], these ideas must be regarded as speculations until new nanotechnologies allow testing of these concepts. Ethanol wet bonding is in spite of recent progress especially in application time, still demanding and at the current state may not be applicable to everyday clinical work. Further studies are also needed to create technically less demanding, reproducible and clinically relevant adhesive bonding methods.
Remineralizing the hybrid layer collagen
The physical packing of the collagen fibril is believed to act as a gatekeeper, preventing molecules larger than a 40 kDa protein from entering demineralized dentin fibrils [104]. In turn, during demineralization either by acids or acidic monomers, minerals are removed and replaced by rinse-water (E&R adhesives) or water used in primer-adhesives (E&R and SE adhesives) as a solvent. The loss of protection by the mineral phase of dentin renders collagen vulnerable to enzymatic degradation in several ways. Acid-etching activates inactive endogenous proteases bound to collagen, the enzymes themselves are freed from their “fossilized” state, and chemical and/or mechanical damage may result with the exposure of the critical collagen cleavage site, facilitating collagen molecule cleavage. The mechanical damage may be especially important in the hybrid layer. Tay and co-workers [151] proposed that collagen fibrils in poorly infiltrated hybrid layers, being unsupported by resin, undergo various degrees of irreversible mechanical disruption under occlusal loads. This part of collagen fibril network has a much lower modulus of elasticity than resin-infiltrated fibrils allowing if to strain more than resin-enveloped fibrils causing microdisruptions [151]. Since strains caused by physical loading may induce local molecular damage that makes collagen more susceptible to MMP cleavage [152]. Physical loading of restorations may speed up and escalate enzymatic collagen degradation. For these reasons, remineralization of the hybrid layer collagen to restore mechanical properties to its mineralized state is an attractive approach to improving the durability of resin-dentin bonds.
Remineralization by fluoride-containing adhesives
Fluoride-containing adhesives have been suggested to eliminate the time-dependent decrease in dentin bond strength [17,97,153] or even improve it in a short-term indirect exposure experiment [99]. However, the ability of fluoride in adhesive resin to remineralize the collagen matrix [154] has been questioned [17,150], at least with acid-etched dentin or with more aggressive SE adhesives [150]. Remineralization of collagen requires seed apatite crystallites that determine the orientation of remineralized crystalline lattices. This limitation severely restricts the remineralization of hybrid layers that contain little or no residual apatite [150]. Interestingly, several studies have been performed where the preservation of long-term bond strength has been attributed to fluoride in the adhesive primer of Clearfil Protect Bond [15,17,97,99], an adhesive containing 12-methacryloyloxydodecylpyridinium bromide (MDPB) monomer with antibacterial properties [94,95]. As described above, MDPB belongs to the group of quaternary ammonium methacrylates (QAMs) that were recently shown to have MMP-inhibiting properties comparable to CHX [96]. So, at least part of the preservation of dentin bond strength durability with this fluoride-containing adhesive resin may be attributable to the inhibition of collagen degradation by MDPB. If endogenous dentin proteases are not inhibited, demineralized collagen may degrade and solubilize before it can remineralize [143]. The use of quaternary ammonium methacrylates as functional monomers to inhibit enzymatic collagen degradation offers interesting possibilities in the development of new adhesives with better dentin bond durability.
Chlorhexidine can be simultaneously incorporated into the resin matrix together with fluoride, and the release of either agent may be optimized for therapeutic purposes [155]. Combination of fluoride with an enzyme-inhibiting agent might have synergistic effects in retaining matrix integrity in hybrid layer. In dentin erosion, where fluoride has long been known to slow lesion progression, significantly better protective effect was observed in situ with FeSO4, which also was shown to effectively inhibit dentinal MMPs [156]. This anti-MMP effect of heavy metals has also been shown for excess zinc [157,158].
Remineralization by adhesives containing bioactive particles
Hydrophilic biodegradable polymers and bioactive silicates have been proposed as useable materials for remineralizing scaffolds (reviewed by Hoppe et al. 2011 [159]). Osorio and co-authors [160] investigated the effect of calcium/sodium phosphosilicate (Bioglass 45S5, BAG) and tricalcium phosphate (TCP)-modified silicate cement incorporated as bioactive microfillers in adhesive resin containing hydrophilic and –phobic monomers. When phosphoric acid- or EDTA-demineralized dentin beams were treated with BAG-containing adhesive, significantly lower degrees of collagen degradation were seen compared with neat-resin or even non-demineralized dentin samples after four week incubation in artificial saliva. At the same time, remineralization detected as a calcium phosphate (Ca/P) surface layer and mineral precipitates with SEM and energy-dispersive x-ray analysis (EDX) was observed. With TCP-modified silicate cement inhibition of MMP-related collagen degradation was less pronounced than with BAG, and only thin Ca/P layer was observed in EDTA-demineralized dentin, while only scattered microglobular crystals were observed with phosphoric acid-demineralized dentin [160]. The authors concluded that ionic dissolution products when exposed to biological fluids are responsible for the biological activity of the tested inorganic materials. Formation of CaP-MMP complexes may be responsible for the reduced collagen degradation [160]. However, the question remains whether the remineralization of the hybrid layer that is based on the degradation of the adhesive components could lead to the reduction of the mechanical properties of adhesive. Experiments analyzing the long-term bonding durability together with the analysis of the structural changes in the hybrid layer are essential to evaluate the potential advantages of the use of bioactive components in the adhesives aiming to inhibit the hybrid layer collagen degradation by endogenous enzymes.
Biomimetic remineralization – imitating nature
Due to all of the structural and chemical limitations associated with dentin bonding, and the clinicians “need for speed”, most resin-dentin bonds are less than perfect. The retention of rinse water in etch-and-rinse adhesives or of the intrinsic water (about 25%) that must be added to SE adhesives to allow the acidic monomers to dissociate, permit water-filled voids to exist in adhesive and hybrid layers, even after resin infiltration. “Water trees” tend to grow over time [16] increasing nanoleakage. All of this water promotes extraction of unreacted monomers and hydrolysis of resins over time.
In resin-dentin bonding, the mineral phase of dentin that is responsible for its high stiffness (ca. 20 GPa) is removed by acid-etching and partially to almost fully replaced with cross-linked resins with a much lower stiffness (ca. 3.4 GPa). Stress concentrations develop in resin-bonded assembles where there is a mismatch in the modulus of elasticity of its constituents, such as at the junction between the bottom of the hybrid layer (E = 3–4 GPa) and the underlying mineralized dentin (E = 20 GPa).
While protease inhibitors or cross-linking inactivation of proteases can block matrix degradation, they do nothing to improve the mechanical properties of the hybrid layer above that of adhesive resins. A new approach to resin-dentin bonding is to perfect the bonds after they have been made, using remineralizing reagents to backfill the water-filled voids with nanometer-sized apatite crystallites.
In biomimetic remineralization of resin-dentin bonds, one covers a polymerized resin-dentin bond with a “therapeutic” flowable composite that contains a source of amorphous calcium phosphate (ACP). Biomimetic polyanionic analogs of noncollagenous phosphoproteins are included so that they can bind to collagen and serve as templates to induce nucleation and growth of apatite in completely demineralized regions of collagen. Polyanions like polyacrylic acid or polyaspartic acid are included to cluster around nanoprecursors of ACP to prevent it from growing into crystals that are too large to fit into the gap zone of collagen. The key to remineralization of dentin is to create “fluidic” nanoprecursors [161] that can pass through polymerized dental adhesives. This process is thought to use water-filled voids (i.e. water trees) that extend from the surface of the adhesive layer, through the thickness of the layer into contiguous water filled spaces in the hybrid layer. When the remineralization process is finished, the water-trees become saturated with nanoprecursors and ACP and mineralize. That is, the water-trees become mineral trees. This process is self-limiting. When it is over, the channels that transported the mineral salts become closed. Indeed, the process of mineralization involves replacing water with mineral. Mineralization is a form of dehydration [162].
There are two forms of remineralization: 1) In demineralized enamel, the residual mineral crystals can serve as templates for appositional regrowth of apatite crystallites by epitaxial growth [163]. In dentin that has only been partially demineralized as in caries-affected dentin, seed apatite crystallites remain and can serve as templates for additional epitaxial growth. However, remineralization of dentin will not occur in locations where seed apatite crystals are absent. An example would be in dentin that has been acid-etched with 32–37% phosphoric acid in preparation for resin bonding. In these situations, all of the apatite crystallites have been dissolved and extracted from both extrafibrillar and intrafibrillar collagen fibril compartments. There are no seed crystals remaining and some of the noncollagenous, developmental phosphoproteins necessary to attract calcium to collagen may have been extracted. In carious dentin or even in caries-affected dentin, these phosphoproteins may be masked by bacterially derived proteins or polysaccharides. Remineralization of such dentin becomes more complex. 2) One of the mechanisms for apatite nucleation in the absence of seed crystals is to include polyanions such as polyacrylic acid or polyaspartic acid. These polyanions bind to collagen and serve as templates for stereo specific calcium binding and promotes apatite nucleation.
Hybrid layers created by etch-and-rinse adhesives have been shown to be remineralizable using a biomimetic mineralization approach [164,165]. Apatite crystallites were detected in both extrafibrillar and intrafibrillar compartments after remineralization in phosphoric acid etched resin-bonded dentin. Self-etching adhesives also have water-filled defects that can be remineralized [166], but the apatite deposition is largely limited to the intrafibrillar spaces, because the extrafibrillar spaces were better filled with adhesives (Figure 7). Even the water-filled spaces (e.g. water trees) in many adhesive layers become filled with apatite nanocrystals [162,166–168], although they have no hierarchical order because they contain no collagen.
Figure 7.
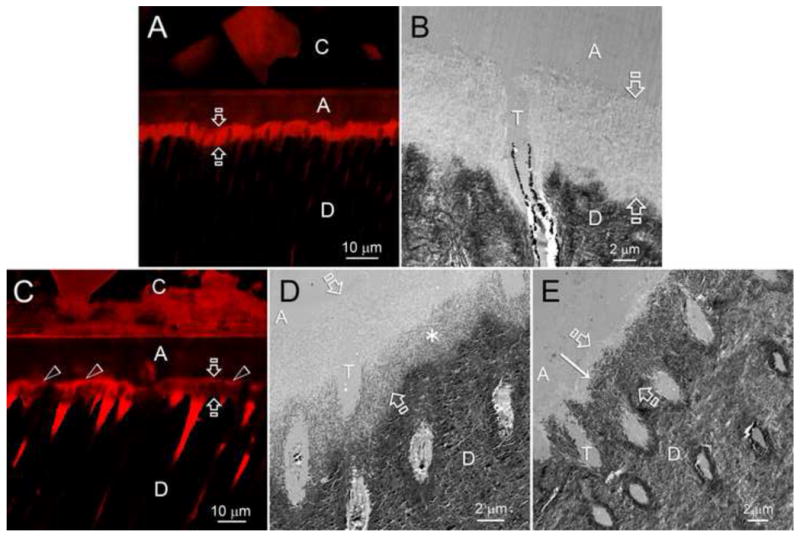
A–E show adjacent confocal laser scanning microscopy (CLSM) and transmission electron microscopy images obtained from dentin bonds made with the self-etching dental adhesive, Adper Prompt-L-Pop. After bonding, the bonded tooth was sectioned vertically into 1 mm thick slabs and incubated in control media (Figs. A and B) or in biomimetic remineralization medium for 6 months. The specimen in Fig. A was immersed in 0.1% rhodamine B overnight. The red fluorescent tracer diffused into water-rich, resin-poor spaces within the hybrid layer (the area between the opposite arrows). In the absence of remineralizing reagents, no mineralization of the water-filled spaces occurred. The adjacent TEM shows no remineralization of the hybrid layer (area between opposing arrows). Fig. C is a CLSM image of the same resin-dentin bond that was allowed to remineralize for 6 months with biomimetic reagents. Note that the hybrid layer (area between opposing arrows) is less fluorescent and more grey indicating that much of the water was replaced by apatite mineral. This is better shown in the adjacent TEM images showing mineralization (asterisk region) of the bottom of the hybrid layer in 2 months and most of the hybrid layer in 6 months (from Kim et al. 2010 [166], with permission).
Unlike epitaxial apatite growth over seed crystals that can occur in weeks, the remineralization of completely demineralized dentin is much slower, usually taking 3–4 months. This is why we recommend the use of a therapeutic flowable composite that can be placed over typical polymerized resin-dentin bonds, to allow slow back-filling of any water-rich, resin-poor regions that might have developed during bonding. When all water-filled spaces have been filled, the flowable composite mineralizes.
In a proof-of-concept biomineralization strategy, the authors used polyvinylphosphoric acid as both a templating molecule and to inhibit the endogenous MMPs of dentin, to prevent collagen degradation during mineralization [169].
The goal of mineralizing resin-dentin bonds is to back-fill water-rich, resin-poor hybrid layers. However, as etch-and-rinse hybrid layers are only 8–10 μm thick, how can one demonstrate that local depositions of mineral within hybrid layers can actually improve the stiffness of hybrid layers. This can be accomplished using a nanoindenter coupled to a dynamic mechanical analyzer [170] to imaged control unmineralized hybrid layers vs. experimental mineralized hybrid layers (Figure 8, Figure 9). The results revealed that statistically significant stiffening of mineralized hybrid layers occurred.
Figure 8.
A: The effect of biomineralization on the dynamic mechanical behavior of hybrid layers (between opposing white arrowheads). Specimens were examined in the hydrated condition after 2 months of biomineralization. R: hydrophilic adhesive resin; T: resin-filled dentinal tubule; M: underlying mineralized dentin. The color scale shows the complex modulus of elasticity from 0–30 GPa. Much of the hybrid layer contains blue and green colors corresponding to complex moduli of 10 to 15 GPa. B: The right image is a TEM micrograph from the same specimen showing that much of the lower half of the hybrid layer (area between opposing white arrowheads) is filled with mineralized collagen (dark appearance). The top of the hybrid layer is electron transparent and not mineralized because the collagen fibrils were enveloped by adhesive resin.
Figure 9.
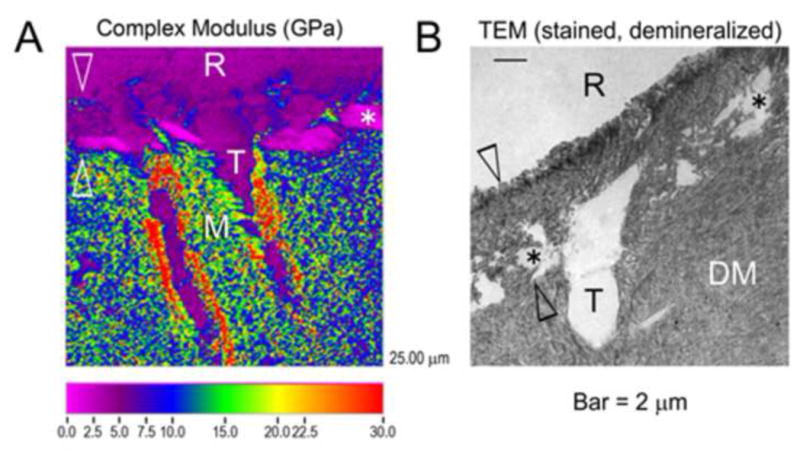
A: Control resin-bonded dentin that was subjected to 2 months of immersion in a simulated body fluid without biomimetic mineralizing reagents. Note that the overlying adhesive resin (R) has a complex modulus of 2.5 GPa, as does most of the hybrid layer (area between opposing white arrowheads) indicating that the hybrid layers remained unmineralized and relatively soft (complex modulus of 2.5 GPa). B: TEM micrographs from the same resin-bonded dentin specimen that show areas where the collagen within the hybrid layer has been solubilized by endogenous dentin protease activity (asterisks).
When Kim et al. [162] compared the durability of typical resin-dentin bonds with that of resin-dentin bonds that had been remineralized, the control bond strength fell 43% over 12 months, while the remineralized resin-dentin bonds showed 3.8% decrease in bond strength over 12 months. Although such treatments take extra time and need to be optimized, they offer the promise of being very durable over time because mineralization inactivates the endogenous proteases of dentin [150] by embedding them with apatite crystallites.
Conclusions
In the NIDCR 2009–2013 strategic plan on tooth-colored restorations, the authors reported that the average replacement time for such restorations is only 5.7 years. Replacing defective esthetic restorations costs five billion dollars per year in the U.S. alone [171].
Resin-dentin bonding is a unique form of tissue engineering in which a demineralized collagen matrix is used as scaffold for resin infiltration to create a hybrid layer that is relatively hydrophobic, very organic, soft, acid-resistant and tough. This is done in about 2 min. These physicochemical properties are completely different from mineralized dentin or enamel that are hydrophilic, crystalline, acid-labile, relatively brittle, and very hard. In most forms of tissue engineering, the scaffold is designed to be resorbed by host cells and replaced by natural tissue within weeks to months. In resin-bonded hybrid layers, the collagen scaffold should last for decades! It should be as stable as possible even though it is seeded with endogenous proteases that can destroy it. While there are some unresolved problems regarding the durability of resin-dentin bonds, it is truly remarkable to see how far adhesive bonding has come in the past 50–60 years. Direct repair of fractured incisors is routinely accomplished by general practitioners in less than one hour. Fifty years ago, those teeth would receive full crowns. We predict that stable resin-dentin bonds will be routinely available in 10–15 years and that tooth-colored restorations will last as long as amalgam restorations.
Acknowledgments
This work was supported, in part, by grants DE 015306 from the NIDCR to DHP, P.I.; by the Academy of Finland to LT, P.I. and AT-M, P.I; and FIRB RBAP1095CR and PRIN 2009SAN9K5 TO L.B. from Italy.
References
- 1.Shono Y, Terashita M, Shimada J, Kozono Y, Carvalho RM, Russell CM, et al. Durability of resin-dentin bonds. Journal of Adhesive Dentistry. 1999;1:211–8. [PubMed] [Google Scholar]
- 2.Loguercio AD, Moura SK, Pellizzaro A, Dal-Bianco K, Patzlaff RT, Grande RHM, et al. Durability of enamel bonding using two-step self-etch systems on ground and unground enamel. Operative Dentistry. 2008;33:79–88. doi: 10.2341/07-42. [DOI] [PubMed] [Google Scholar]
- 3.Tjäderhane L, Carrilho MR, Breschi L, Tay FR, Pashley DH. Dentin basic structure and composition – an overview. Endodontic Topics. 2012;20:3–29. [Google Scholar]
- 4.Pashley DH, Tay FR, Breschi L, Tjäderhane L, Carvalho RM, Carrilho M, et al. State of the art etch-and-rinse adhesives. Dental Materials. 2011;27:1–16. doi: 10.1016/j.dental.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sano H, Takatsu T, Ciucchi B, Horner JA, Matthews WG, Pashley DH. Nanoleakage: leakage within the hybrid layer. Operative Dentistry. 1995;20:18–25. [PubMed] [Google Scholar]
- 6.Sano H, Yoshiyama M, Ebisu S, Burrow MF, Takatsu T, Ciucchi B, et al. Comparative SEM and TEM observations of nanoleakage within the hybrid layer. Operative Dentistry. 1995;20:160–7. [PubMed] [Google Scholar]
- 7.Tay FR, King NM, Chan KM, Pashley DH. How can nanoleakage occur in self-etching adhesive systems that demineralize and infiltrate simultaneously? Journal of Adhesive Dentistry. 2002;4:255–69. [PubMed] [Google Scholar]
- 8.Tay FR, Pashley DH. Water treeing–a potential mechanism for degradation of dentin adhesives. American Journal of Dentistry. 2003;16:6–12. [PubMed] [Google Scholar]
- 9.Agee KL, Pashley EL, Itthagarun A, Sano H, Tay FR, Pashley DH. Submicron hiati in acid-etched dentin are artifacts of desiccation. Dental Materials. 2003;19:60–8. doi: 10.1016/s0109-5641(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 10.Breschi L, Prati C, Gobbi P, Pashley D, Mazzotti G, Teti G, et al. Immunohistochemical analysis of collagen fibrils within the hybrid layer: a FEISEM study. Operative Dentistry. 2004;29:538–46. [PubMed] [Google Scholar]
- 11.Hashimoto M, De Munck J, Ito S, Sano H, Kaga M, Oguchi H, et al. In vitro effect of nanoleakage expression on resin-dentin bond strengths analyzed by microtensile bond test, SEM/EDX and TEM. Biomaterials. 2004;25:5565–74. doi: 10.1016/j.biomaterials.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto M, Ito S, Tay FR, Svizero NR, Sano H, Kaga M, et al. Fluid movement across the resin–dentin interface during and after bonding. Journal of Dental Research. 2004;11:843–8. doi: 10.1177/154405910408301104. [DOI] [PubMed] [Google Scholar]
- 13.Carvalho RM, Chersoni S, Frankenberger R, Pashley DH, Prati C, Tay FR. A challenge to the conventional wisdom that simultaneous etching and resin infiltration always occurs in self-etch adhesives. Biomaterials. 2005;26:1035–42. doi: 10.1016/j.biomaterials.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto M. A review - micromorphological evidence of degradation in resin-dentin bonds and potential preventional solutions. Journal of Biomedical Materials Research Part B, Applied Biomaterials. 2010;92:268–80. doi: 10.1002/jbm.b.31535. [DOI] [PubMed] [Google Scholar]
- 15.Van Landuyt KL, De Munck J, Mine A, Cardoso MV, Peumans M, Van Meerbeek B. Filler debonding & subhybrid-layer failures in self-etch adhesives. Journal of Dental Research. 2010;89:1045–50. doi: 10.1177/0022034510375285. [DOI] [PubMed] [Google Scholar]
- 16.Tay FR, Hashimoto M, Pashley DH, Peters MC, Lai SCN, Yiu CKY, et al. Aging affects two modes of nanoleakage expression in bonded dentin. Journal of Dental Research. 2003;82:537–41. doi: 10.1177/154405910308200710. [DOI] [PubMed] [Google Scholar]
- 17.Donmez N, Belli S, Pashley DH, Tay FR. Ultrastructural correlates of in vivo/in vitro bond degradation in self-etch adhesives. Journal of Dental Research. 2005;84:355–9. doi: 10.1177/154405910508400412. [DOI] [PubMed] [Google Scholar]
- 18.Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, De Stefano Dorigo E. Dental adhesion review: aging and stability of the bonded interface. Dental Materials. 2008;24:90–101. doi: 10.1016/j.dental.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Mazzoni A, Breschi L, Carrilho M, Nascimento FD, Orsini G, Ruggeri A, et al. A review on nature, role and functions of dentin non-collagenous proteins. Part II: enzymes, serum proteins and growth factors. Endodontic Topics. 2012;21:19–40. [Google Scholar]
- 20.Tjäderhane L, Nascimento FD, Breschi L, Mazzoni A, Tersariol IL, Geraldeli S, et al. Optimizing dentin bond durability: Control of collagen degradation by matrix metalloproteinases and cysteine cathepsins. Dental Materials. 2012 doi: 10.1016/j.dental.2012.08.004. (epub ahead of print: http://dx.doi.org/10.1016/j.dental.2012.08.004) [DOI] [PMC free article] [PubMed]
- 21.Tjäderhane L, Larjava H, Sorsa T, Uitto VJ, Larmas M, Salo T. The activation and function of host matrix metalloproteinases in dentin matrix breakdown in caries lesions. Journal of Dental Research. 1998;77:1622–9. doi: 10.1177/00220345980770081001. [DOI] [PubMed] [Google Scholar]
- 22.Tjäderhane L, Salo T, Larjava H, Larmas M, Overall CM. A novel organ culture method to study the function of the human odontoblasts in vitro: gelatinase expression by odontoblasts is differentially regulated by TGF-beta1. Journal of Dental Research. 1998;77:1488–98. doi: 10.1177/00220345980770070301. [DOI] [PubMed] [Google Scholar]
- 23.Tersariol IL, Geraldeli S, Minciotti CL, Nascimento FD, Pääkkönen V, Martins MT, et al. Cysteine cathepsins in human dentin–pulp complex. Journal of Endodontics. 2010;36:475–81. doi: 10.1016/j.joen.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 24.Nascimento FD, Minciotti CL, Geraldeli S, Carrilho MR, Pashley DH, Tay FR, et al. Cysteine cathepsins in human carious dentin. Journal of Dental Research. 2011;90:506–11. doi: 10.1177/0022034510391906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gendron R, Grenier D, Sorsa T, Mayrand D. Inhibition of the activities of matrix metalloproteinases 2, 8, and 9 by chlorhexidine. Clinical and Diagnostic Laboratory Immunology. 1999;6:437–9. doi: 10.1128/cdli.6.3.437-439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin-De Las Heras S, Valenzuela A, Overall CM. The matrix metalloproteinase gelatinase A in human dentine. Archives of Oral Biology. 2000;45:757–65. doi: 10.1016/s0003-9969(00)00052-2. [DOI] [PubMed] [Google Scholar]
- 27.Mazzoni A, Mannello F, Tay FR, Tonti GA, Papa S, Mazzotti G, et al. Zymographic analysis and characterization of MMP-2 and -9 forms in human sound dentin. Journal of Dental Research. 2007;86:436–40. doi: 10.1177/154405910708600509. [DOI] [PubMed] [Google Scholar]
- 28.Sulkala M, Tervahartiala T, Sorsa T, Larmas M, Salo T, Tjäderhane L. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Archives of Oral Biology. 2007;52:121–7. doi: 10.1016/j.archoralbio.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, et al. Collagen degradation by host-derived enzymes during aging. Journal of Dental Research. 2004;83:216–21. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 30.Hebling J, Pashley DH, Tjäderhane L, Tay FR. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. Journal of Dental Research. 2005;84:741–6. doi: 10.1177/154405910508400811. [DOI] [PubMed] [Google Scholar]
- 31.Carrilho MR, Carvalho RM, de Goes MF, di Hipólito V, Geraldeli S, Tay FR, et al. Chlorhexidine preserves dentin bond in vitro. Journal of Dental Research. 2007;86:90–4. doi: 10.1177/154405910708600115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carrilho MR, Geraldeli S, Tay FR, de Goes MF, Carvalho RM, Tjäderhane L, et al. In vivo preservation of hybrid layer by chlorhexidine. Journal of Dental Research. 2007;86:529–3. doi: 10.1177/154405910708600608. [DOI] [PubMed] [Google Scholar]
- 33.Brackett WW, Tay FR, Brackett MG, Dib A, Sword RJ, Pashley DH. The effect of chlorhexidine on dentin hybrid layers in vivo. Operative Dentistry. 2007;32:107–11. doi: 10.2341/06-55. [DOI] [PubMed] [Google Scholar]
- 34.Brackett MG, Tay FR, Brackett WW, Dib A, Dipp FA, Mai S, et al. In vivo chlorhexidine stabilization of hybrid layers of an acetone-based dentin adhesive. Operative Dentistry. 2009;34:381–85. doi: 10.2341/08-103. [DOI] [PubMed] [Google Scholar]
- 35.Breschi L, Cammelli F, Visintini E, Mazzoni A, Vita F, Carrilho M, et al. Influence of chlorhexidine concentration on the durability of etch-and-rinse dentin bonds: a 12-month in vitro study. Journal of Adhesive Dentistry. 2009;11:191–8. [PMC free article] [PubMed] [Google Scholar]
- 36.Campos EA, Correr GM, Leonardi DP, Barato-Filho F, Gonzaga CC, Zielak JC. Chlorhexidine diminishes the loss of bond strength over time under simulated pulpal pressure and thermo-mechanical stressing. Journal of Dentistry. 2009;37:108–14. doi: 10.1016/j.jdent.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Loguercio AD, Stanislawczuk R, Polli LG, Costa JA, Michel MD, Reis A. Influence of chlorhexidine digluconate concentration and application time on resin-dentin bond strength durability. European Journal of Oral Sciences. 2009;117:587–96. doi: 10.1111/j.1600-0722.2009.00663.x. [DOI] [PubMed] [Google Scholar]
- 38.Breschi L, Mazzoni A, Nato F, Carrilho M, Visintini E, Tjäderhane L, et al. Chlorhexidine stabilizes the adhesive interface: a 2-year in vitro study. Dental Materials. 2010;26:320–5. doi: 10.1016/j.dental.2009.11.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stanislawczuk R, Reis A, Loguercio AD. A 2-year in vitro evaluation of a chlorhexidine-containing acid on the durability of resin-dentin interfaces. Journal of Dentistry. 2011;39:40–7. doi: 10.1016/j.jdent.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 40.De Munck J, Mine A, Van den Steen PE, Van Landuyt KL, Poitevin A, Opdenakker G, et al. Enzymatic degradation of adhesive-dentin interfaces produced by mild self-etch adhesives. European Journal of Oral Sciences. 2010;118:494–501. doi: 10.1111/j.1600-0722.2010.00758.x. [DOI] [PubMed] [Google Scholar]
- 41.Sadek FT, Braga RR, Muench A, Liu Y, Pashley DH, Tay FR. Ethanol wet-bonding challenges current anti-degradation strategy. Journal of Dental Research. 2010;89:1499–504. doi: 10.1177/0022034510385240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou J, Tan J, Chen L, Li D, Tan Y. The incorporation of chlorhexidine in a two-step self-etching adhesive preserves dentin bond in vitro. Journal of Dentistry. 2009;37:807–12. doi: 10.1016/j.jdent.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 43.Mobarak EH. Effect of chlorhexidine pretreatment on bond strength durability of caries-affected dentin over 2-year aging in artificial saliva and under simulated intrapulpal pressure. Operative Dentistry. 2011;36:649–60. doi: 10.2341/11-018-L. [DOI] [PubMed] [Google Scholar]
- 44.Amaral FL, Colucci V, Palma-Dibb RG, Corona SA. Assessment of in vitro methods used to promote adhesive interface degradation: a critical review. Journal of Esthetic and Restorative Dentistry. 2007;19:340–53. doi: 10.1111/j.1708-8240.2007.00134.x. [DOI] [PubMed] [Google Scholar]
- 45.Griffiths BM, Watson TF, Sherriff M. The influence of dentine bonding systems and their handling characteristics on the morphology and micropermeability of the dentine adhesive interface. Journal of Dentistry. 1999;27:63–71. doi: 10.1016/s0300-5712(97)90022-1. [DOI] [PubMed] [Google Scholar]
- 46.Hashimoto M, Ohno H, Kaga M, Endo K, Sano H, Oguchi H. In vivo degradation of resin-dentin bonds in humans over 1 to 3 years. Journal of Dental Research. 2000;79:1385–91. doi: 10.1177/00220345000790060601. [DOI] [PubMed] [Google Scholar]
- 47.Koshiro K, Inoue S, Tanaka T, Koase K, Fujita M, Hashimoto M, et al. In vivo degradation of resin-dentin bonds produced by a self-etch vs. a total-etch adhesive system. European Journal of Oral Sciences. 2004;112:368–75. doi: 10.1111/j.1600-0722.2004.00141.x. [DOI] [PubMed] [Google Scholar]
- 48.Ricci HA, Sanabe ME, de Souza Costa CA, Pashley DH, Hebling J. Chlorhexidine increases the longevity of in vivo resin-dentin bonds. European Journal of Oral Sciences. 2010;118:411–6. doi: 10.1111/j.1600-0722.2010.00754.x. [DOI] [PubMed] [Google Scholar]
- 49.Feitosa VP, Leme AA, Sauro S, Correr-Sobrinho L, Watson TF, Sinhoreti MA, et al. Hydrolytic degradation of the resin-dentine interface induced by the simulated pulpal pressure, direct and indirect water ageing. Journal of Dentistry. 2012 doi: 10.1016/j.jdent.2012.09.011. (Epub ahead of print.) [DOI] [PubMed] [Google Scholar]
- 50.Zhou J, Tan J, Yang X, Xu X, Li D, Chen L. MMP-inhibitory effect of chlorhexidine applied in a self-etching adhesive. Journal of Adhesive Dentistry. 2011;13:111–5. doi: 10.3290/j.jad.a18783. [DOI] [PubMed] [Google Scholar]
- 51.Zhou J, Tan J, Yang X, Cheng C, Wang X, Chen L. Effect of chlorhexidine application in a self-etching adhesive on the immediate resin-dentin bond strength. Journal of Adhesive Dentistry. 2010;12:27–31. doi: 10.3290/j.jad.a17543. [DOI] [PubMed] [Google Scholar]
- 52.Scaffa PM, Vidal CM, Barros N, Gesteira TF, Carmona AK, Breschi L, et al. Chlorhexidine inhibits the activity of dental cysteine cathepsins. Journal of Dental Research. 2012;91:420–5. doi: 10.1177/0022034511435329. [DOI] [PubMed] [Google Scholar]
- 53.Riggs PD, Braden M, Patel M. Chlorhexidine release from room temperature polymerizing methacrylate systems. Biomaterials. 2000;21:345–51. doi: 10.1016/s0142-9612(99)00187-8. [DOI] [PubMed] [Google Scholar]
- 54.Anusavice KJ, Zhang NZ, Shen C. Controlled release of chlorhexidine from UDMA-TEGDMA resin. Journal of Dental Research. 2006;85:950–4. doi: 10.1177/154405910608501016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jedrychowski JR, Caputo AA, Kerper S. Antibacterial and mechanical properties of restorative materials combined with chlorhexidines. Journal of Oral Rehabilitation. 1983;10:373–81. doi: 10.1111/j.1365-2842.1983.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 56.Pallan S, Furtado Araujo MV, Cilli R, Prakki A. Mechanical properties and characteristics of developmental copolymers incorporating catechin or chlorhexidine. Dental Materials. 2012;28:687–94. doi: 10.1016/j.dental.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 57.Hiraishi N, Yiu CKY, King NM, Tay FR, Pashley DH. Chlorhexidine release and water sorption characteristics of chlorhexidine-incorporated hydrophobic/hydrophilic resins. Dental Materials. 2008;24:1391–9. doi: 10.1016/j.dental.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scherrer SS, Cesar PF, Swain MV. Direct comparison of the bond strength results of the different test methods: a critical literature review. Dental Materials. 2010;26:e78–93. doi: 10.1016/j.dental.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 59.Armstrong SR, Boyer DB, Keller JC. Microtensile bond strength testing and failure analysis of two dentin adhesives. Dental Materials. 1998;14:44–50. doi: 10.1016/s0109-5641(98)00008-6. [DOI] [PubMed] [Google Scholar]
- 60.Toledano M, Osorio R, Osorio E, Aguilera FS, Yamauti M, Pashley DH, et al. Durability of resin-dentin bonds: effects of direct/indirect exposure and storage media. Dental Materials. 2007;23:885–92. doi: 10.1016/j.dental.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 61.Abdalla AI, Feilzer AJ. Four-year water degradation of a total-etch and two self-etching adhesives bonded to dentin. Journal of Dentistry. 2008;36:611–7. doi: 10.1016/j.jdent.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 62.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circulation Research. 2003;92:827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 63.Carvalho RM, Tay F, Sano H, Yoshiyama M, Pashley DH. Long-term mechanical properties of EDTA-demineralized dentin matrix. Journal of Adhesive Dentistry. 2000;2:193–9. [PubMed] [Google Scholar]
- 64.Carrilho MR, Tay FR, Donnelly AM, Agee KA, Tjäderhane L, Mazzoni A, et al. Host-derived loss of dentin matrix stiffness associated with solubilization of collagen. Journal of Biomedical Materials Research Part B, Applied Biomaterials. 2009;90:373–80. doi: 10.1002/jbm.b.31295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tezvergil-Mutluay A, Mutluay M, Seseogullari-Dirihan R, Agee KA, Key WO, Scheffel DLS, et al. Effect of phosphoric acid on the degradation of human dentin matrix. Journal of Dental Research. 2012 doi: 10.1177/0022034512466264. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tezvergil-Mutluay A, Agee KA, Hoshika T, Carrilho M, Breschi L, Tjäderhane L, et al. The requirement of zinc and calcium ions for functional MMP activity in demineralized dentin matrices. Dental Materials. 2010;26:1059–67. doi: 10.1016/j.dental.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thompson JM, Agee K, Sidow SJ, McNally K, Lindsey K, Borke J, et al. Inhibition of endogenous dentin matrix metalloproteinases by ethylenediaminetetraacetic acid. Journal of Endodontics. 2012;38:62–5. doi: 10.1016/j.joen.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Osorio R, Erhardt MC, Pimenta LA, Osorio E, Toledano M. EDTA treatment improves resin–dentin bonds’ resistance to degradation. Journal of Dental Research. 2005;84:736–40. doi: 10.1177/154405910508400810. [DOI] [PubMed] [Google Scholar]
- 69.Sauro S, Mannocci F, Toledano M, Osorio R, Pashley DH, Watson TF. EDTA or H3PO4/NaOCl dentine treatments may increase hybrid layers’ resistance to degradation: a microtensile bond strength and confocal-micropermeability study. Journal of Dentistry. 2009;37:279–88. doi: 10.1016/j.jdent.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 70.Imbery TA, Kennedy M, Janus C, Moon PC. Evaluating EDTA as a substitute for phosphoric acid-etching of enamel and dentin. General Dentistry. 2012;60:e55–61. [PubMed] [Google Scholar]
- 71.Sauro S, Toledano M, Aguilera FS, Mannocci F, Pashley DH, Tay FR, et al. Resin-dentin bonds to EDTA-treated vs. acid-etched dentin using ethanol wet-bonding. Dental Materials. 2010;26:368–79. doi: 10.1016/j.dental.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sorsa T, Tjäderhane L, Konttinen YT, Lauhio A, Salo T, Lee HM, et al. Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Annals of Medicine. 2006;38:306–21. doi: 10.1080/07853890600800103. [DOI] [PubMed] [Google Scholar]
- 73.Lauhio A, Salo T, Tjäderhane L, Lähdevirta J, Golub LM, Sorsa T. Tetracyclines in treatment of rheumatoid arthritis. Lancet. 1995;346:645–6. doi: 10.1016/s0140-6736(95)91484-6. [DOI] [PubMed] [Google Scholar]
- 74.Sorsa T, Tjäderhane L, Salo T. Matrix metalloproteinases (MMPs) in oral diseases. Oral Diseases. 2004;10:311–8. doi: 10.1111/j.1601-0825.2004.01038.x. [DOI] [PubMed] [Google Scholar]
- 75.Hannas AR, Pereira JC, Granjeiro JM, Tjäderhane L. The role of matrix metalloproteinases in oral environment. Acta Odontologica Scandinavica. 2007;65:1–13. doi: 10.1080/00016350600963640. [DOI] [PubMed] [Google Scholar]
- 76.Golub LM, Ramamurthy NS, McNamara TF, Greenwald RA, Rifkin BR. Tetracyclines inhibit connective tissue breakdown: new therapeutic implications for an old family of drugs. Critical Reviews in Oral Biology and Medicine. 1991;2:297–321. doi: 10.1177/10454411910020030201. [DOI] [PubMed] [Google Scholar]
- 77.Golub LM, Lee HM, Ryan ME, Giannobile WV, Payne J, Sorsa T. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Advances in Dental Research. 1998;12:12–26. doi: 10.1177/08959374980120010501. [DOI] [PubMed] [Google Scholar]
- 78.Sulkala M, Wahlgren J, Larmas M, Sorsa T, Teronen O, Salo T, et al. The effects of MMP inhibitors on human salivary MMP activity and caries progression in rats. Journal of Dental Research. 2001;80:1545–9. doi: 10.1177/00220345010800061301. [DOI] [PubMed] [Google Scholar]
- 79.Russell RG. Bisphosphonates: the first 40 years. Bone. 2011;49:2–19. doi: 10.1016/j.bone.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 80.Fisher JF, Mobashery S. Recent advances in MMP inhibitor design. Cancer Metastasis Reviews. 2006;25:115–36. doi: 10.1007/s10555-006-7894-9. [DOI] [PubMed] [Google Scholar]
- 81.Breschi L, Martin P, Mazzoni A, Nato F, Carrilho M, Tjäderhane L, et al. Use of a specific MMP inhibitor (galardin) for preservation of hybrid layer. Dental Materials. 2010;26:571–578. doi: 10.1016/j.dental.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Augé F, Hornebeck W, Decarme M, Laronze JY. Improved gelatinase a selectivity by novel zinc binding groups containing galardin derivatives. Bioorganic and Medicinal Chemistry Letters. 2003;13:1783–6. doi: 10.1016/s0960-894x(03)00214-2. [DOI] [PubMed] [Google Scholar]
- 83.Galardy RE, Cassabonne ME, Giese C, Gilbert JH, Lapierre F, Lopez H, et al. Low molecular weight inhibitors in corneal ulceration. Annals of the New York Academy of Sciences. 1994;732:315–23. doi: 10.1111/j.1749-6632.1994.tb24746.x. [DOI] [PubMed] [Google Scholar]
- 84.Galardy RE, Grobelny D, Foellmer HG, Fernandez LA. Inhibition of angiogenesis by the matrix metalloprotease inhibitor N-[2R-2-(hydroxamidocarbonymethyl)-4-methylpentanoyl)]-l-tryptophan methylamide. Cancer Research. 1994;54:4715–8. [PubMed] [Google Scholar]
- 85.Almahdy A, Koller G, Sauro S, Bartsch JW, Sherriff M, Watson TF, et al. Effects of MMP inhibitors incorporated within dental adhesives. Journal of Dental Research. 2012;91:605–11. doi: 10.1177/0022034512446339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.De Munck J, Van den Steen PE, Mine A, Van Landuyt KL, Poitevin A, Opdenakker G, et al. Inhibition of enzymatic degradation of adhesive-dentin interfaces. Journal of Dental Research. 2009;88:1101–6. doi: 10.1177/0022034509346952. [DOI] [PubMed] [Google Scholar]
- 87.Sulkala M, Larmas M, Sorsa T, Salo T, Tjäderhane L. The localization of matrix metalloproteinase–20 (MMP-20, Enamelysin) in mature human teeth. Journal of Dental Research. 2002;81:603–7. doi: 10.1177/154405910208100905. [DOI] [PubMed] [Google Scholar]
- 88.Carrilho RM, Carvalho RM, Sousa EN, Nicolau J, Breschi L, Mazzoni A, et al. Substantivity of chlorhexidine to human dentin. Dental Materials. 2010;26:779–85. doi: 10.1016/j.dental.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim J, Uchiyama T, Carrilho M, Agee KA, Mazzoni A, Breschi L, et al. Chlorhexidine binding to mineralized versus demineralized dentin. Dental Materials. 2010;26:771–8. doi: 10.1016/j.dental.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mazzoni A, Pashley DH, Nishitani Y, Breschi L, Mannello F, Tjäderhane L, et al. Reactivation of inactivated endogenous proteolytic activities in phosphoric acid-etched dentine by etch-and-rinse adhesives. Biomaterials. 2006;27:4470–6. doi: 10.1016/j.biomaterials.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 91.Nishitani Y, Yoshiyama M, Wadgaonkar B, Breschi L, Mannello F, Mazzoni A, et al. Activation of gelatinolytic/collagenolytic activity in dentin by self-etching adhesives. European Journal of Oral Sciences. 2006;114:160–6. doi: 10.1111/j.1600-0722.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 92.Mazzoni A, Carrilho M, Papa V, Tjäderhane L, Gobbi P, Nucci C, et al. MMP-2 assay within the hybrid layer created by a two-step etch-and-rinse adhesive: biochemical and immunohistochemical analysis. Journal of Dentistry. 2011;39:470–7. doi: 10.1016/j.jdent.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 93.Mazzoni A, Nascimento FD, Carrilho M, Tersariol I, Papa V, Tjäderhane L, et al. MMP activity in the hybrid layer detected with in situ zymography. Journal of Dental Research. 2012;91:467–72. doi: 10.1177/0022034512439210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Imazato S, Kinomoto Y, Tarumi H, Ebisu S, Tay FR. Antibacterial activity and bonding characteristics of an adhesive resin containing antibacterial monomer MDPB. Dental Materials. 2003;19:313–9. doi: 10.1016/s0109-5641(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 95.Imazato S, Tay FR, Kaneshiro AV, Takahashi Y, Ebisu S. An in vivo evaluation of bonding ability of comprehensive antibacterial adhesive system incorporating MDPB. Dental Materials. 2007;23:170–6. doi: 10.1016/j.dental.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 96.Tezvergil-Mutluay A, Agee KA, Uchiyama T, Imazato S, Mutluay MM, Cadenaro M, et al. The inhibitory effects of quaternary ammonium methacrylates on soluble and matrix-bound MMPs. Journal of Dental Research. 2011;90:535–40. doi: 10.1177/0022034510389472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nakajima M, Okuda M, Ogata M, Pereira PN, Tagami J, Pashley DH. The durability of a fluoride-releasing resin adhesive system to dentin. Operative Dentistry. 2003;28:186–92. [PubMed] [Google Scholar]
- 98.Ansari ZJ, Sadr A, Moezizadeh M, Aminian R, Ghasemi A, Shimada Y, et al. Effects of one-year storage in water on bond strength of self-etching adhesives to enamel and dentin. Dental Materials J. 2008;27:266–72. doi: 10.4012/dmj.27.266. [DOI] [PubMed] [Google Scholar]
- 99.Shinohara MS, De Goes MF, Schneider LF, Ferracane JL, Pereira PN, Di Hipólito V, et al. Fluoride-containing adhesive: durability on dentin bonding. Dental Materials. 2009;25:1383–91. doi: 10.1016/j.dental.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 100.De Munck J, Mine A, Vivan Cardoso M, De Almeida Neves A, Van Landuyt KL, Poitevin A, et al. Effect of dentin location and long-term water storage on bonding effectiveness of dentin adhesives. Dental Materials Journal. 2011;30:7–13. doi: 10.4012/dmj.2010-085. [DOI] [PubMed] [Google Scholar]
- 101.Tezvergil-Mutluay A, Mutluay MM, Gu LS, Zhang K, Agee KA, Carvalho RM, et al. The anti-MMP activity of benzalkonium chloride. Journal of Dentistry. 2011;39:57–64. doi: 10.1016/j.jdent.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kanca J., 3rd One step bond strength to enamel and dentin. American Journal of Dentistry. 1997;10:5–8. [PubMed] [Google Scholar]
- 103.Van Meerbeek B, Yoshihara K, Yoshida Y, Mine A, De Munck J, Van Landuyt KL. State of the art of self-etch adhesives. Dental Materials. 2011;27:17–28. doi: 10.1016/j.dental.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 104.Toroian D, Lim JE, Price PA. The size exclusion characteristics of type I collagen: implications for the role of noncollagenous bone constituents in mineralization. Journal of Biological Chemistry. 2007;282:22437–47. doi: 10.1074/jbc.M700591200. [DOI] [PubMed] [Google Scholar]
- 105.Iwasa M, Tsubota K, Shimamura Y, Ando S, Miyazaki M, Platt JA. pH changes upon mixing of single-step self-etching adhesives with powdered dentin. Journal of Adhesive Dentistry. 2011;13:207–212. doi: 10.3290/j.jad.a19240. [DOI] [PubMed] [Google Scholar]
- 106.Kim J, Mai S, Carrilho MR, Yiu CK, Pashley DH, Tay FR. An all-in-one adhesive does not etch beyond hybrid layers. Journal of Dental Research. 2010;89:482–7. doi: 10.1177/0022034510363665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yoshida Y, Nagakane K, Fukuda R, Nakayama Y, Okazaki M, Shintani H, et al. Comparative study on adhesive performance of functional monomers. Journal of Dental Research. 2004;83:454–8. doi: 10.1177/154405910408300604. [DOI] [PubMed] [Google Scholar]
- 108.Inoue S, Koshiro K, Yoshida Y, De Munck J, Nagakane K, Suzuki K, et al. Hydrolytic stability of self-etch adhesives bonded to dentin. Journal of Dental Research. 2005;84:1160–4. doi: 10.1177/154405910508401213. [DOI] [PubMed] [Google Scholar]
- 109.Koshiro K, Inoue S, Sano H, De Munck J, Van Meerbeek B. In vivo degradation of resin-dentin bonds produced by a self-etch and an etch-and-rinse adhesive. European Journal of Oral Sciences. 2005;113:341–8. doi: 10.1111/j.1600-0722.2005.00222.x. [DOI] [PubMed] [Google Scholar]
- 110.Bertassoni LE, Orgel JP, Antipova O, Swain MV. The dentin organic matrix - limitations of restorative dentistry hidden on the nanometer scale. Acta Biomaterialia. 2012;8:2419–33. doi: 10.1016/j.actbio.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Snibson GR. Collagen cross-linking: a new treatment paradigm in corneal disease – a review. Clinical and Experimental Ophthalmology. 2010;38:141–53. doi: 10.1111/j.1442-9071.2010.02228.x. [DOI] [PubMed] [Google Scholar]
- 112.Cova A, Breschi L, Nato F, Ruggeri A, Jr, Carrilho M, Tjäderhane L, et al. Effect of UVA-activated riboflavin on dentin bonding. Journal of Dental Research. 2011;90:1439–45. doi: 10.1177/0022034511423397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fawzy AS, Nitisusanta LI, Iqbal K, Daood U, Neo J. Riboflavin as a dentin crosslinking agent: Ultraviolet A versus blue light. Dental Materials. 2012;28:1284–91. doi: 10.1016/j.dental.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 114.Bedran-Russo AK, Pashley DH, Agee K, Drummond JL, Miescke KJ. Changes in stiffness of demineralized dentin following application of collagen crosslinkers. Journal of Biomedical Materials Research Part B, Applied Biomaterials. 2008;86:330–4. doi: 10.1002/jbm.b.31022. [DOI] [PubMed] [Google Scholar]
- 115.Fang M, Liu R, Xiao Y, Li F, Wang D, Hou R, et al. Biomodification to dentin by a natural crosslinker improved the resin-dentin bonds. Journal of Dentistry. 2012;40:458–66. doi: 10.1016/j.jdent.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 116.Bedran-Russo AKB, Vidal CMP, Santos PHD, Castellan CS. Long-term effects of carbodiimide on dentin matrix and resin-dentin bonds. Journal of Biomedical Materials Research Part B, Applied Biomaterials. 2010;94B:250–255. doi: 10.1002/jbm.b.31649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tezvergil-Mutluay A, Mutluay MM, Agee KA, Seseogullari-Dirihan R, Hoshika T, Cadenaro M, et al. Carbodiimide cross-linking inactivates soluble and matrix-bound MMPs, in vitro. Journal of Dental Research. 2012;91:192–6. doi: 10.1177/0022034511427705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rivera EM, Yamauchi M. Site comparisons of dentin collagen cross-links from extracted human teeth. Archives of Oral Biology. 1993;38:541–46. doi: 10.1016/0003-9969(93)90118-6. [DOI] [PubMed] [Google Scholar]
- 119.Knott L, Bailey AJ. Collagen cross-links in mineralizing tissues: A review of their chemistry, function, and clinical relevance. Bone. 1998;22:181–7. doi: 10.1016/s8756-3282(97)00279-2. [DOI] [PubMed] [Google Scholar]
- 120.Bornstein P. Covalent cross-links in collagen: a personal account of their discovery. Matrix Biology. 2003;22:385–91. doi: 10.1016/s0945-053x(03)00061-1. [DOI] [PubMed] [Google Scholar]
- 121.Nakornchai S, Atsawasuwan P, Kitamura E, Surarit R, Yamauchi M. Partial biochemical characterization of collagen in carious dentin of human primary dentin. Archives of Oral Biology. 2004;49:267–73. doi: 10.1016/j.archoralbio.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 122.Ritter AV, Swift E, Jr, Yamauchi M. Effects of phosphoric acid and glutaraldehyde-HEMA on dentin collagen. European Journal of Oral Sciences. 2001;109:348–53. doi: 10.1034/j.1600-0722.2001.00088.x. [DOI] [PubMed] [Google Scholar]
- 123.Van Landuyt KL, Snauwaert J, De Munck J, Peumans M, Yoshida Y, Poitevin A, Coutinho E, Suzuki K, Lambrechts P, Van Meerbeek B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials. 2007;28:3757–85. doi: 10.1016/j.biomaterials.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 124.Ferracane JL. Hygroscopic and hydrolytic effects in dental polymer networks. Dental Materials. 2006;22:211–22. doi: 10.1016/j.dental.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 125.Carrilho MR, Tay FR, Pashley DH, Tjäderhane L, Carvalho RM. Mechanical stability of resin-dentin bond components. Dental Materials. 2005;21:232–41. doi: 10.1016/j.dental.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 126.Reis AF, Carrilho MR, Ghaname E, Pereira PN, Giannini M, Nikaido T, et al. Effects of water-storage on the physical and ultramorphological features of adhesives and primer/adhesive mixtures. Dental Materials Journal. 2010;29:697–705. doi: 10.4012/dmj.2009-091. [DOI] [PubMed] [Google Scholar]
- 127.García-Godoy F, Tay FR, Pashley DH, Feilzer A, Tjäderhane L, Pashley EL. Degradation of resin-bonded human dentin after 3 years of storage. American Journal of Dentistry. 2007;20:109–113. [PubMed] [Google Scholar]
- 128.Sadek FT, Castellan CS, Braga RR, Mai S, Tjäderhane L, Pashley DH, Tay FR. One-year stability of resin-dentin bonds created with a hydrophobic ethanol-wet bonding technique. Dental Materials. 2010b;26:380–6. doi: 10.1016/j.dental.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 129.Brackett MG, Li N, Brackett WW, Sword RJ, Qi YP, Niu LN, et al. The critical barrier to progress in dentine bonding with the etch-and-rinse technique. Journal of Dentistry. 2011;39:238–48. doi: 10.1016/j.jdent.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Spencer P, Wang Y. Adhesive phase separation at the dentin interface under wet bonding conditions. Journal of Biomedical Materials Research. 2002;62:447–56. doi: 10.1002/jbm.10364. [DOI] [PubMed] [Google Scholar]
- 131.Shin TP, Yao X, Huenergardt R, Walker MP, Wang Y. Morphological and chemical characterization of bonding hydrophobic adhesive to dentin using ethanol wet bonding technique. Dental Materials. 2009;25:1050–7. doi: 10.1016/j.dental.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pereira PN, Bedran-de-Castro AK, Duarte WR, Yamauchi M. Removal of noncollagenous components affects dentin bonding. Journal of Biomedical Materials Research Part B, Applied Biomaterials. 2007;80:86–91. doi: 10.1002/jbm.b.30572. [DOI] [PubMed] [Google Scholar]
- 133.Bedran-Russo AK, Pereira PN, Duarte WR, Okuyama K, Yamauchi M. Removal of dentin matrix proteoglycans by trypsin digestion and its effect on dentin bonding. Journal of Biomedical Materials Research Part B, Applied Biomaterials. 2008;85:261–6. doi: 10.1002/jbm.b.30944. [DOI] [PubMed] [Google Scholar]
- 134.Mazzoni A, Pashley DH, Ruggeri A, Jr, Vita F, Falconi M, Di Lenarda R, et al. Adhesion to chondroitinase ABC treated dentin. Journal of Biomedical Materials Research Part B, Applied Biomaterials. 2008;86:228–36. doi: 10.1002/jbm.b.31010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nishitani Y, Yoshiyama M, Donnelly AM, Agee KA, Sword J, Tay FR, et al. Effects of resin hydrophilicity on dentin bond strength. Journal of Dental Research. 2006;85:1016–21. doi: 10.1177/154405910608501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pashley DH, Tay FR, Carvalho RM, Rueggeberg FA, Agee KA, Carrilho M, et al. From dry bonding to water-wet bonding to ethanol-wet bonding. A review of the interactions between dentin matrix and solvated resins using a macromodel of the hybrid layer. American Journal of Dentistry. 2007;20:7–21. [PubMed] [Google Scholar]
- 137.Scott JE, Thomlinson AM. The structure of interfibrillar proteoglycan bridges (‘shape modules’) in extracellular matrix of fibrous connective tissues and their stability in various chemical environments. Journal of Anatomy. 1998;192:391–405. doi: 10.1046/j.1469-7580.1998.19230391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang Y, Spencer P. Analysis of acid-treated dentin smear debris and smear layers using confocal Raman microspectroscopy. Journal of Biomedical Materials Research. 2002;60:300–8. doi: 10.1002/jbm.10108. [DOI] [PubMed] [Google Scholar]
- 139.Wang Y, Spencer P, Yao X, Brenda B. Effect of solvent content on resin hybridization in wet dentin bonding. Journal of Biomedical Materials Research Part A. 2007;82:975–83. doi: 10.1002/jbm.a.31232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang L, Nancollas GH. Pathways to biomineralization and biodemineralization of calcium phosphates: the thermodynamic and kinetic controls. Dalton Transactions. 2009;15:2665–2675. doi: 10.1039/b815887h. [DOI] [PubMed] [Google Scholar]
- 141.Tay FR, Pashley DH, Kapur RR, Carrilho MRO, Hur YB, Garrett LV, et al. Bonding BisGMA to dentin a proof of concept. Journal of Dental Research. 2007;86:1034–9. doi: 10.1177/154405910708601103. [DOI] [PubMed] [Google Scholar]
- 142.Hosaka K, Nishitani Y, Tagami J, Yoshiyama M, Brackett WW, Agee KA, et al. Durability of resin-dentin bonds to water- vs. ethanol-saturated dentin. Journal of Dental Research. 2009;88:146–51. doi: 10.1177/0022034508328910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu Y, Li N, Qi Y, Niu LN, Elshafiy S, Mao J, et al. The use of sodium trimetaphosphate as a biomimetic analog of matrix phosphoproteins for remineralization of artificial caries-like dentin. Dental Materials. 2011;27:465–77. doi: 10.1016/j.dental.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tezvergil-Mutluay A, Agee KA, Hoshika T, Uchiyama T, Tjäderhane L, Breschi L, et al. Inhibition of MMPs by alcohols. Dental Materials. 2011;27:926–33. doi: 10.1016/j.dental.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sadek FT, Mazzoni A, Breschi L, Tay FR, Braga RR. Six-month evaluation of adhesives interface created by a hydrophobic adhesive to acid-etched ethanol-wet bonded dentine with simplified dehydration protocols. Journal of Dentistry. 2010;38:276–83. doi: 10.1016/j.jdent.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 146.Nagase H, Fushimi K. Elucidating the function of non catalytic domains of collagenases and aggrecanases. Connective Tissue Research. 2008;49:169–74. doi: 10.1080/03008200802151698. [DOI] [PubMed] [Google Scholar]
- 147.Carvalho RV, Ogliari FA, de Souza AP, Silva AF, Petzhold CL, Line SR, et al. 2-hydroxyethyl methacrylate as an inhibitor of matrix metalloproteinase-2. European Journal of Oral Sciences. 2009;117:64–7. doi: 10.1111/j.1600-0722.2008.00591.x. [DOI] [PubMed] [Google Scholar]
- 148.Nakabayashi N, Pashley DH. Hybridization of Dental Hard Tissues. Chicago: Quintessence Publishing; 1998. [Google Scholar]
- 149.Sauro S, Toledano M, Aguilera FS, Mannocci F, Pashley DH, Tay FR, et al. Resin-dentin bonds to EDTA-treated vs. acid-etched dentin using ethanol wet-bonding. Part II: Effects of mechanical cycling load on microtensile bond strengths. Dental Materials. 2011;27:563–72. doi: 10.1016/j.dental.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 150.Liu Y, Tjäderhane L, Breschi L, Mazzoni A, Li N, Mao J, et al. Limitations in bonding to dentin and experimental strategies to prevent bond degradation. Journal of Dental Research. 2011;90:953–68. doi: 10.1177/0022034510391799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tay FR, Carvalho RM, Yiu CK, King NM, Zhang Y, Agee K, et al. Mechanical disruption of dentin collagen fibrils during resin-dentin bond testing. Journal of Adhesive Dentistry. 2000;2:175–92. [PubMed] [Google Scholar]
- 152.Perumal S, Antipova O, Orgel JP. Collagen fibril architecture, domain organization, and triple-helical conformation govern its proteolysis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2824–9. doi: 10.1073/pnas.0710588105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Saito A. Effect of fluoride in adhesion to dentin. Jpn Journal of Dentistry Mater. 1996;15:78–88. [Google Scholar]
- 154.Itota T, Torii Y, Nakabo S, Tashiro Y, Konishi N, Nagamine M, et al. Effect of fluoride-releasing adhesive system on decalcified dentin. Journal of Oral Rehabilitation. 2003;30:178–83. doi: 10.1046/j.1365-2842.2003.00985.x. [DOI] [PubMed] [Google Scholar]
- 155.Shen C, Zhang NZ, Anusavice KJ. Fluoride and chlorhexidine release from filled resins. Journal of Dental Research. 2010;89:1002–6. doi: 10.1177/0022034510374055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kato MT, Leite AL, Hannas AR, Oliveira RC, Pereira JC, Tjäderhane L, et al. Effect of iron on matrix metalloproteinase inhibition and on the prevention of dentine erosion. Caries Research. 2010;44:309–16. doi: 10.1159/000315932. [DOI] [PubMed] [Google Scholar]
- 157.Osorio R, Yamauti M, Osorio E, Romãn JS, Toledano M. Zinc-doped dentin adhesive for collagen protection at the hybrid layers. European Journal of Oral Sciences. 2011;119:401–410. doi: 10.1111/j.1600-0722.2011.00853.x. [DOI] [PubMed] [Google Scholar]
- 158.Osorio R, Yamauti M, Osorio E, Ruiz-Requena ME, Pashley DH, Tay FR, et al. Zinc reduces collagen degradation in demineralized human dentin explants. Journal of Dentistry. 2011;39:148–153. doi: 10.1016/j.jdent.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hoppe A, Güldal NR, Boccaccini AR. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32:2757–74. doi: 10.1016/j.biomaterials.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 160.Osorio R, Yamauti M, Sauro S, Watson TF, Toledano M. Experimental resin cements containing bioactive fillers reduce matrix metalloproteinase–mediated dentin collagen degradation. Journal of Endodontics. 2012;38:1227–32. doi: 10.1016/j.joen.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 161.Gower LB. Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization. Chemical Reviews. 2008;108:4551–627. doi: 10.1021/cr800443h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kim YK, Mai S, Mazzoni A, Liu Y, Tezvergil-Mutluay A, Takahashi K, et al. Biomimetic remineralization as a progressive dehydration mechanism of collagen matrices – Implications in the aging of resin-dentin bonds. Acta Biomaterialia. 2010;6:3729–39. doi: 10.1016/j.actbio.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Koutsoukos PG, Nancollas GH. Crystal growth of calcium phosphates-epitaxial considerations. Journal of Crystal Growth. 1981;53:10–9. [Google Scholar]
- 164.Mai S, Kim YK, Toledano M, Breschi L, Ling JQ, Pashley DH, et al. Phosphoric acid esters cannot replace polyvinylphosphonic acid as phosphoprotein analogs in biomimetic remineralization of resin-bonded dentin. Dental Materials. 2009;25:1230–9. doi: 10.1016/j.dental.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mai S, Kim YK, Kim J, Yiu CK, Ling J, Pashley DH, et al. In vitro remineralization of severely compromised bonded dentin. Journal of Dental Research. 2010;89:405–10. doi: 10.1177/0022034510363662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kim J, Vaughn RM, Gu L, Rockman RA, Arola DD, Schaffer TE, et al. Imperfect hybrid layers created by an aggressive one-step, self-etch adhesive in primary dentin are amendable to biomimetic remineralization in vitro. Journal of Biomedical Materials Research Part A. 2010;93A:1225–34. doi: 10.1002/jbm.a.32612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kim J, Arola DD, Gu L, Kim YK, Sui M, Liu Y, et al. Functional biomimetic analogs help remineralize apatite-depleted demineralized resin-infiltrated dentin via a bottom-up approach. Acta Biomaterialia. 2010;6:2740–50. doi: 10.1016/j.actbio.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kim YK, Gu LS, Bryan TE, Kim JR, Chen L, Liu Y, et al. Mineralization of reconstituted collagen using polyvinylphosphonic acid/polyacrylic acid templating matrix protein analogues in the presence of calcium, phosphate and hydroxyl ions. Biomaterials. 2010;31:6618–27. doi: 10.1016/j.biomaterials.2010.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tezvergil-Mutluay A, Agee KA, Hoshika T, Tay FR, Pashley DH. The inhibitory effect of polyvinylphosphonic acid on functional matrix metalloproteinase activities in human demineralized dentin. Acta Biomaterialia. 2010b;6:4136–42. doi: 10.1016/j.actbio.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ryou H, Niu LN, Dai L, Pucci CR, Arola DD, Pashley DH, et al. Effect of biomimetic remineralization on the dynamic nanomechanical properties of dentin hybrid layers. Journal of Dental Research. 2011;90:1122–8. doi: 10.1177/0022034511414059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jokstad A, Bayne S, Blunck U, Tyas M, Wilson N. Quality of dental restorations. FDI Commission Project 2–95. International Dental Journal. 2001;51:117–58. doi: 10.1002/j.1875-595x.2001.tb00832.x. [DOI] [PubMed] [Google Scholar]
- 172.Erhardt MC, Osorio R, Viseras C, Toledano M. Adjunctive use of an anti-oxidant agent to improve resistance of hybrid layers to degradation. Journal of Dentistry. 2011;39:80–7. doi: 10.1016/j.jdent.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 173.Leitune VC, Portella FF, Bohn PV, Collares FM, Samuel SM. Influence of chlorhexidine application on longitudinal adhesive bond strength in deciduous teeth. Brazilian Oral Research. 2011;25:388–92. doi: 10.1590/s1806-83242011000500003. [DOI] [PubMed] [Google Scholar]
- 174.Yiu CK, Hiraishi N, Tay FR, King NM. Effect of chlorhexidine incorporation into dental adhesive resin on durability of resin-dentin bond. Journal of Adhesive Dentistry. 2012;14:355–62. doi: 10.3290/j.jad.a25674. [DOI] [PubMed] [Google Scholar]
- 175.Manfro AR, Reis A, Loguercio AD, Imparato JC, Raggio DP. Effect of different concentrations of chlorhexidine on bond strength of primary dentin. Pediatric Dentistry. 2012;34:11–5. [PubMed] [Google Scholar]
- 176.Erhardt MC, Toledano M, Osorio R, Pimenta LA. Histomorphologic characterization and bond strength evaluation of caries-affected dentin/resin interfaces: effects of long-term water exposure. Dental Materials. 2008;24:786–98. doi: 10.1016/j.dental.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 177.Reis AF, Giannini M, Pereira PN. Effects of a peripheral enamel bond on the long-term effectiveness of dentin bonding agents exposed to water in vitro. Journal of Biomedical Materials Research Part B, Applied Biomaterials. 2008;85:10–7. doi: 10.1002/jbm.b.30909. [DOI] [PubMed] [Google Scholar]


