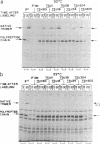
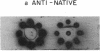
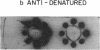
Abstract
Temperature-sensitive mutations in the gene encoding the trimeric tail spike protein of phage P22 interfere with protein maturation at 39 degrees C. We show here that temperature-sensitive mutations at many sites block the folding pathway prior to accumulation of the partially folded protrimer intermediate. Temperature-shift experiments indicate that at least some of the mutants accumulate an earlier intermediate in the folding pathway. Immunoprecipitation experiments suggest that the conformation of the isolated temperature-sensitive polypeptide chains is closer to that of the unfolded chain than to that of the mature spike formed at permissive temperature. The sites of these mutations probably represent amino acid sequences that play key roles during the folding of the tail spike polypeptide chain but are not important in the mature protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anfinsen C. B. Principles that govern the folding of protein chains. Science. 1973 Jul 20;181(4096):223–230. doi: 10.1126/science.181.4096.223. [DOI] [PubMed] [Google Scholar]
- Baldwin R. L. Intermediates in protein folding reactions and the mechanism of protein folding. Annu Rev Biochem. 1975;44:453–475. doi: 10.1146/annurev.bi.44.070175.002321. [DOI] [PubMed] [Google Scholar]
- Berget P. B., Poteete A. R. Structure and functions of the bacteriophage P22 tail protein. J Virol. 1980 Apr;34(1):234–243. doi: 10.1128/jvi.34.1.234-243.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein D., Waddell C. H., King J. Mechanism of head assembly and DNA encapsulation in Salmonella phage p22. I. Genes, proteins, structures and DNA maturation. J Mol Biol. 1973 Nov 15;80(4):669–695. doi: 10.1016/0022-2836(73)90204-0. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. Conformational restrictions on the pathway of folding and unfolding of the pancreatic trypsin inhibitor. J Mol Biol. 1977 Jun 25;113(2):275–293. doi: 10.1016/0022-2836(77)90142-5. [DOI] [PubMed] [Google Scholar]
- Creighton T. E., Dyckes D. F. Refolding of S-methylmethionyl basic pancreatic trypsin inhibitor. J Mol Biol. 1981 Mar 5;146(3):375–387. doi: 10.1016/0022-2836(81)90394-6. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. Energetics of folding and unfolding of pancreatic trypsin inhibitor. J Mol Biol. 1977 Jun 25;113(2):295–312. doi: 10.1016/0022-2836(77)90143-7. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. Experimental studies of protein folding and unfolding. Prog Biophys Mol Biol. 1978;33(3):231–297. doi: 10.1016/0079-6107(79)90030-0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Goldenberg D. P., Berget P. B., King J. Maturation of the tail spike endorhamnosidase of Salmonella phage P22. J Biol Chem. 1982 Jul 10;257(13):7864–7871. [PubMed] [Google Scholar]
- Goldenberg D. P., King J. Temperature-sensitive mutants blocked in the folding or subunit of the bacteriophage P22 tail spike protein. II. Active mutant proteins matured at 30 degrees C. J Mol Biol. 1981 Feb 5;145(4):633–651. doi: 10.1016/0022-2836(81)90307-7. [DOI] [PubMed] [Google Scholar]
- Goldenberg D., King J. Trimeric intermediate in the in vivo folding and subunit assembly of the tail spike endorhamnosidase of bacteriophage P22. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3403–3407. doi: 10.1073/pnas.79.11.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grütter M. G., Hawkes R. B., Matthews B. W. Molecular basis of thermostability in the lysozyme from bacteriophage T4. Nature. 1979 Feb 22;277(5698):667–669. doi: 10.1038/277667a0. [DOI] [PubMed] [Google Scholar]
- Israel J. V., Anderson T. F., Levine M. in vitro MORPHOGENESIS OF PHAGE P22 FROM HEADS AND BASE-PLATE PARTS. Proc Natl Acad Sci U S A. 1967 Feb;57(2):284–291. doi: 10.1073/pnas.57.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashita S., Kanegasaki S. Enzymic and molecular properties of base-plate parts of bacteriophage P22. Eur J Biochem. 1976 May 17;65(1):87–94. doi: 10.1111/j.1432-1033.1976.tb10392.x. [DOI] [PubMed] [Google Scholar]
- Kim P. S., Baldwin R. L. Specific intermediates in the folding reactions of small proteins and the mechanism of protein folding. Annu Rev Biochem. 1982;51:459–489. doi: 10.1146/annurev.bi.51.070182.002331. [DOI] [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- King J., Lenk E. V., Botstein D. Mechanism of head assembly and DNA encapsulation in Salmonella phage P22. II. Morphogenetic pathway. J Mol Biol. 1973 Nov 15;80(4):697–731. doi: 10.1016/0022-2836(73)90205-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowe P. A., Aebi U., Gross C., Burgess R. R. In vitro thermal inactivation of a temperature-sensitive sigma subunit mutant (rpoD800) of Escherichia coli RNA polymerase proceeds by aggregation. J Biol Chem. 1981 Feb 25;256(4):2010–2015. [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Privalov P. L. Stability of proteins: small globular proteins. Adv Protein Chem. 1979;33:167–241. doi: 10.1016/s0065-3233(08)60460-x. [DOI] [PubMed] [Google Scholar]
- Sauer R. T., Krovatin W., Poteete A. R., Berget P. B. Phage P22 tail protein: gene and amino acid sequence. Biochemistry. 1982 Nov 9;21(23):5811–5815. doi: 10.1021/bi00266a014. [DOI] [PubMed] [Google Scholar]
- Smith D. H., Berget P. B., King J. Temperature-sensitive mutants blocked in the folding or subunit assembly of the bacteriophage P22 tail-spike protein. I. Fine-structure mapping. Genetics. 1980 Oct;96(2):331–352. doi: 10.1093/genetics/96.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. H., King J. Temperature-sensitive mutants blocked in the folding or subunit assembly of the bacteriophage P22 tail spike protein. III. Intensive polypeptide chains synthesized at 39 degrees C. J Mol Biol. 1981 Feb 5;145(4):653–676. doi: 10.1016/0022-2836(81)90308-9. [DOI] [PubMed] [Google Scholar]
- Sternberg M. J., Thornton J. M. Prediction of protein structure from amino acid sequence. Nature. 1978 Jan 5;271(5640):15–20. doi: 10.1038/271015a0. [DOI] [PubMed] [Google Scholar]
- Yura T., Ishihama A. Genetics of bacterial RNA polymerases. Annu Rev Genet. 1979;13:59–97. doi: 10.1146/annurev.ge.13.120179.000423. [DOI] [PubMed] [Google Scholar]