Abstract
HIV facilitates an increase in human papillomavirus (HPV) associated conditions. HIV-positive men living in a substance use context in Los Angeles were recruited using Respondent Driven Sampling, completed a questionnaire and had biological samples including an anal HPV swab taken. 316 evaluable men were enrolled in the study. The prevalence of all HPV, high-risk (HR) infection, and multiple type infection was highest for men who have sex with men (MSM) (93.9%, 64.6%, 29.7% respectively). When all HPV and HR-HPV prevalence in all men was stratified by age, the youngest group had 100% and 68.2% prevalence respectively with similarly high rates maintained up to 49 years. The individual’s use of alcohol, marijuana, cocaine, methamphetamine or heroin was not significantly associated with anal HPV isolation. In this marginalized population, high anal HPV and HR-HPV prevalence rates over a wide age range may increase the individual’s risk for anal dysplasia and anal cancer.
Keywords: human papillomavirus (HPV), substance use, anal, HIV, men who have sex with men (MSM)
Introduction
There is a large proportion of the adult population that is both human papillomavirus (HPV) exposed, infected, and at risk of HPV associated complications, and for whom the available prophylactic HPV vaccines has no proven efficacy. Men who have sex with men (MSM) have emerged as a societal group that is disproportionately affected by HPV disease. In particular, manifestations of HPV infection such as anogenital warts, anal dysplasia and anal cancer are seen in high and increasing numbers in this population. (1–3) This group also has the highest prevalence and incidence of HIV infection in the United States with particularly high rates reported in young minority men.(4)
Following infection, HPV is predominantly controlled by cell meditated immunity (CMI), and when this control is impaired by either HIV or iatrogenic immunosuppression, the prevalence of HPV associated conditions increases.(5–6) In the context of HIV infection, both HPV isolation and detection of multiple HPV types increases with a reduction in CD4 T lymphocyte count below 200 cells/mm3.(7) The effects of HPV burden, immunosuppression, and the possible effect of HIV per se are reflected in the current incidence rates of anal squamous cell cancer, which in the general population approximates 2:100 000 while in HIV-positive MSM is approximately 174:100 000.(8)
The prevalence of HPV among HIV-positive men has been well documented, but risk and type of infection among those who live in a substance use context is not known.(9) While substance use is considered to be associated with an impairment in immune function, there is little compelling evidence for an effect on progression of HPV associated dysplasia in the uterine cervix.(10) While the cervix is in many ways analogous to the anal canal, in HIV-positive women anal prevalence rates far exceed those in the cervix and a different mechanism of immune surveillance may be operating. In the specific context of methamphetamine use, there is evidence of impaired antigen processing that may lead to compromise of mucosal CMI and facilitate HPV infection and persistence.(11)
This study investigated the prevalence and risk factors for anal HPV in HIV-positive men and will contribute to the understanding of the effect of substance use in this population.
Materials and Methods
Participants were males who participated in one of the two waves of data collection (2005–2007) at the Los Angeles site for National Institute on Drug Abuse’s Sexual Acquisition and Transmission of HIV - Cooperative Agreement Program (SATH-CAP). The primary goal of the SATH-CAP study was to examine the role of drugs in the sexual diffusion of HIV from high-risk individuals (MSM and/or drug users) to the general population. The sample for each wave was recruited using Respondent Driven Sampling (RDS).(12) Briefly, for each of the two waves of data collection, an initial set of ‘seeds’ who were MSM or drug users were passively recruited via advertisements posted on walls and online. Each ‘seed’ was seen at the study site where he completed a questionnaire, provided biological samples, and was compensated for his time. Participants were provided with coupons and received compensation for others recruited to the study who were either an MSM and/or drug user or was their sexual partner. Participants had to be 18 years of age or older and: (1) MSM or men who have sex with men and women (MSMW) who reported engagement in any anal intercourse (AI) in the past six months and/or (2) a male or female who used powder cocaine, crack cocaine, heroin or methamphetamine in the past six months; or recruited by one of the latter as a sexual partner or drug using partner. In the parent study 285 women and 246 drug using men who have sex with women (MSW) were recruited.
Participants completed Audio Computer Assisted Self Interviews that collected detailed information on the following: (1) demographic characteristics; (2) drug use (powder cocaine, crack cocaine, methamphetamine or heroin); (3) sexual risk behaviors – number of male and female sexual partners in the prior 30 days and the past six months, number of specific behaviors engaged in over the past six months while having sex with male and with female partners; (4) specific sexual and drug use behaviors with each of up to six sexual partners (in Wave 1 the last three sexual partners and a main or female partner but in Wave 2 the language was changed to allow for any three partners and a main or female partner), and characteristics and behaviors of each of those sex partners. In addition, all individuals underwent an oral HIV rapid test with confirmatory blood test by Western Blot for those testing positive as well as a quantitative HIV viral load at one time point only. Participants also provided urine specimens for detection of metabolites of recent powder cocaine, crack cocaine, methamphetamine or heroin use. Participants had specimens taken for sexually transmitted infections including a urine specimen (Neisseria gonorrhea and Chlamydia trachomatis), rectal swab (Neisseria gonorrhea) and venous blood draw for syphilis rapid plasma reagin (RPR). If the RPR was positive a confirmatory Treponema pallidum particle agglutination test was done and correlated with syphilis treatment history from the Los Angeles County Health Department records.
This HPV sub study involved a clinician or self taken internal anal swab as previously described.(13) The swab was tested for HPV deoxyribonucleic acid (DNA) types using the polymerase chain reaction (PCR) as described below.
HPV PCR testing
AmpliTaq Gold (Applied Biosystems, Foster City, CA) PCR was performed on purified HPV DNA using MY09/MY11 consensus HPV L1 primers as well as primers for amplification of the human beta-globin as an indicator of specimen adequacy as described previously.(9, 14) After 30 amplification cycles, specimens were probed with a biotin-labeled HPV L1 consensus probe mixture. A separate membrane was probed with a biotin-labeled probe to the human beta-globin gene. Specimens were also typed by hybridizing to 29 different HPV types, 6, 11, 16, 18, 26, 31, 32, 33, 35, 39, 40, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 66, 68, 69, 70, 73, 82 variant, 83, 84, as well as the following 10 types together in a probe mixture: HPV 2, 13, 34, 42, 57, 62, 64, 67, 72, and 82. Specimens negative for beta-globin gene amplification were excluded from analysis.(15)
HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68 and 73 were considered to be high-risk. Three separate analyses of HPV samples were conducted, each using a different HPV classification scheme: HPV vs. no HPV, HR-HPV vs. no HR-HPV and having multiple HR-HPV infections vs. no multiple HR-HPV infections.
Chi-square tests were used to test independence between HPV status and demographic characteristics, self reported and urine verified substance use, HIV viral load (VL) and HIV antiretroviral (ARV) resistance. Partnership level outcomes such as partner race, partner type, partner drug use and unprotected receptive AI (RAI) were assessed with 1–3 sexual partners within the past 6 months. Generalized linear random intercept models with age, race, history of homelessness, history of incarceration, reported methamphetamine use, and gender of sexual partners as covariates were used to predict partnership level outcomes. Fitted values (including fixed effects and random effects) from the generalized linear random intercept models were then used along with demographic and substance abuse variables as covariates in multivariate logistic regressions predicting HPV status. Analyses were fit using SAS (version 9.1). All scientific and research procedures were overseen by the UCLA Human Subjects Protection Committee and the RAND Institutional Review Board.
Results
316 beta-globin positive HPV swabs were included in the analysis. Participant demographics are presented in Table 1 and show that the population was predominantly US born (71%), single (72%), unemployed (84%), Black or Hispanic (75%), and MSM (67%). Over half of the participants had been incarcerated and reported a monthly income below $500.
Table 1.
Cross classifications of demographics and HPV, HR-HPV, multiple HR-HPV among HIV-positive Males
| Covariate | ALL MALES 1 (n=316) | HPV 1 (n=292) | χ2 P-Value | HR-HPV1 (n=203) | χ2 P-Value | Multiple HR-HPV 1 (n=90) | χ2 P-Value | |||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| N | N | % | N | % | N | % | ||||
| Age | 0.04 | 0.06 | 0.11 | |||||||
| < 30 | 22 | 22 | 100.0 | 15 | 68.2 | 6 | 27.3 | |||
| 30 – 39 | 85 | 81 | 95.3 | 57 | 67.1 | 28 | 32.9 | |||
| 40 – 49 | 160 | 148 | 92.5 | 108 | 67.5 | 49 | 30.6 | |||
| > 50 | 49 | 41 | 83.7 | 23 | 46.9 | 7 | 14.3 | |||
| Race | 0.04 | 0.3 | 0.28 | |||||||
| White, non-Hispanic | 62 | 61 | 98.4 | 45 | 72.6 | 20 | 32.3 | |||
| Black, non-Hispanic | 118 | 103 | 87.3 | 69 | 58.5 | 26 | 22.0 | |||
| Hispanic | 118 | 111 | 94.1 | 77 | 65.3 | 38 | 32.2 | |||
| Others | 18 | 17 | 94.4 | 12 | 66.7 | 6 | 33.3 | |||
| Country of birth | 0.41 | 0.6 | 0.43 | |||||||
| US | 223 | 207 | 92.8 | 147 | 65.9 | 68 | 30.5 | |||
| Mexico | 51 | 45 | 88.2 | 30 | 58.8 | 13 | 25.5 | |||
| Other | 42 | 40 | 95.2 | 26 | 61.9 | 9 | 21.4 | |||
| Marital Status | 0.6 | 0.2 | 0.27 | |||||||
| Single | 229 | 210 | 91.7 | 149 | 65.1 | 65 | 28.4 | |||
| Married/cohabitating | 33 | 30 | 90.9 | 24 | 72.7 | 13 | 39.4 | |||
| Formerly married | 48 | 46 | 95.8 | 26 | 54.2 | 11 | 22.9 | |||
| Gender of Sex Parnters (6 months) | 0.006 | 0.93 | 0.59 | |||||||
| No Partners | 41 | 41 | 100.0 | 27 | 65.9 | 11 | 26.8 | |||
| MSM | 212 | 199 | 93.9 | 137 | 64.6 | 63 | 29.7 | |||
| MSW | 10 | 8 | 80.0 | 6 | 60.0 | 1 | 10.0 | |||
| MSMW | 40 | 33 | 82.5 | 24 | 60.0 | 11 | 27.5 | |||
| Education | 0.88 | 0.02 | 0.67 | |||||||
| Less than High School | 80 | 73 | 91.3 | 52 | 65.0 | 21 | 26.3 | |||
| High School | 90 | 83 | 92.2 | 48 | 53.3 | 24 | 26.7 | |||
| More than High School | 145 | 135 | 93.1 | 103 | 71.0 | 45 | 31.0 | |||
| Employment | 0.25 | 0.9 | 0.69 | |||||||
| Employed part/full-time | 52 | 46 | 88.5 | 33 | 63.5 | 16 | 30.8 | |||
| Unemployed | 264 | 246 | 93.2 | 170 | 64.4 | 74 | 28.0 | |||
| Income past month | 0.15 | 0.43 | 0.06 | |||||||
| $0–$500 | 172 | 155 | 90.1 | 106 | 61.6 | 47 | 27.3 | |||
| $501–$1000 | 88 | 85 | 96.6 | 61 | 69.3 | 33 | 37.5 | |||
| More than $ 1000 | 52 | 49 | 94.2 | 35 | 67.3 | 10 | 19.2 | |||
| Homeless (past year) | 0.75 | 0.65 | 0.64 | |||||||
| Yes | 148 | 136 | 91.9 | 97 | 65.5 | 44 | 29.7 | |||
| No | 168 | 156 | 92.9 | 106 | 63.1 | 46 | 27.4 | |||
| Incarcerated (ever) | 0.34 | 0.11 | 0.72 | |||||||
| Yes | 170 | 160 | 94.1 | 116 | 68.2 | 50 | 29.4 | |||
| No | 138 | 126 | 91.3 | 82 | 59.4 | 38 | 27.5 | |||
Total N for a given variable does not sum to column total due to missing values
Anal HPV prevalence was high in all groups with 92.4% of all men having detectable infection. The percent prevalence of all HPV and HR-HPV infection respectively was MSM (93.9, 64.6%), MSMW (82.5, 60%), and MSW (80, 60%). The presence of multiple HPV infections in MSM and MSMW was similar at 29.7% and 27.5% respectively, and 10.0% in MSW. The ANOVA analysis for all HPV prevalence by sexuality was 0.006. (See Table 2)
Table 2.
Cross classification of risk factors by HPV, HR-HPV, multiple HR-HPV among HIV-positive SATH-CAP Males
| Covariate | n | Any HP %(n) | High-Risk HPV %(n) | Multiple High-Risk HPV %(n) |
|---|---|---|---|---|
|
| ||||
| All Males (n = 316)1 | 316 | 92.4(292) | 64.2(203) | 28.5(90) |
| Gender of Sexual Partners (6 months) | ||||
| 0 partners in the past 6 months | 41 | 100(41) | 65.9(27) | 26.8(27) |
| MSM | 212 | 93.9(199) | 64.6(137) | 29.7(137) |
| MSW | 10 | 80 (8) | 60 (6) | 10 (6) |
| MSMW | 40 | 82.5(33) | 60(24) | 27.5(24) |
| Χ2 comparing % across gender of sexual partners | 303 | 0.006 | 0.93 | 0.59 |
|
| ||||
| Age Group | ||||
| < 30 | 22 | 100(22) | 68.2 (15) | 27.3 (6) |
| 30–39 | 85 | 95.3(81) | 67.1(57) | 32.9(28) |
| 40–49 | 160 | 92.5(148) | 67.5(108) | 30.6(49) |
| ≥ 50 | 49 | 83.7(41) | 46.9(23) | 14.3 (7) |
| Χ2 comparing % across age groups | 316 | 0.04 | 0.06 | 0.11 |
|
| ||||
| Viral Load | ||||
| Detectable | 148 | 95.3(141) | 66.9(99) | 32.4(48) |
| Undectable | 164 | 90.2(148) | 62.2(102) | 25.0(41) |
| Χ2 comparing % across viral load | 312 | 0.09 | 0.39 | 0.15 |
Covariate totals may not sum to all males due to missing values
When all HPV and HR-HPV prevalence in all HIV-positive men was stratified by age the youngest group had 100% and 68.2% prevalence respectively. These high rates were maintained in the 30–39 year and 40–49 years age groups with all HPV and HR-HPV prevalence of 95.3/67.1% and 92.5/67.5% respectively. Only in the older age range of greater or equal to 50 years did all HPV prevalence fall to 83.7% and HR-HPV to 46.9%. The ANOVA for isolation of any HPV by age was 0.04. (See Table 2)
The presence of a detectable HIV VL was associated with the presence of any anal HPV although this did not reach statistical significance (p=0.09). (See Table 2) No CD4 lymphocyte counts were collected as part of this study.
The self reported use of alcohol, marijuana, cocaine or heroin was not significant for the isolation of anal HPV. However, although individuals who had ever injected methamphetamine, injected methamphetamine in the last 30 days, or provided a urine sample positive for metabolite of methamphetamine had no significantly increase in anal HPV detection, still individuals who verified methamphetamine use in the last 30 days did have a significantly increased risk of anal HPV infection (p=0.03). (See Table 3) The presence of either internal or external anal symptoms or the presence of bleeding was not associated with anal HPV detection.
Table 3.
Drug Cross classification of drug use and HPV, HR-HPV, multiple HR-HPV among HIV-positive SATH-CAP Males
| Covariate | MALES (n=316) | HPV (n=292) | χ2 P-Value | HR-HPV (n=203) | χ2 P-Value | Multiple HR-HPV (n=90) | χ2 P-Value | |||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| N | N | % | N | % | N | % | ||||
| Ever injected drugs | 76 | 71 | 93.4 | 0.70 | 52 | 68.4 | 0.38 | 20 | 26.3 | 0.63 |
| Ever injected meth | 51 | 50 | 98.0 | 0.1 | 38 | 74.5 | 0.09 | 15 | 29.4 | 0.87 |
| Injected drugs in past 30 days | 38 | 37 | 97.4 | 0.22 | 28 | 73.7 | 0.2 | 14 | 36.8 | 0.23 |
| Used amph/meth in past 30 days | 104 | 101 | 97.1 | 0.03 | 73 | 70.2 | 0.15 | 30 | 28.8 | 0.95 |
| Injected meth in past 30 days | 11 | 11 | 100.0 | 0.32 | 7 | 63.6 | 1.02 | 3 | 27.3 | 0.96 |
| Recent Marijuana use (Lab test) | 96 | 91 | 94.8 | 0.29 | 63 | 65.6 | 0.73 | 23 | 24.0 | 0.24 |
| Recent Amph/Meth use (Lab test) | 42 | 41 | 97.6 | 0.17 | 31 | 73.8 | 0.16 | 10 | 23.8 | 0.47 |
| Recent Cocaine use (Lab test) | 50 | 47 | 94.0 | 0.64 | 30 | 60.0 | 0.5 | 10 | 20.0 | 0.15 |
| Recent Opiate/Heorin use (Lab test) | 11 | 11 | 100.0 | 0.33 | 8 | 72.7 | 0.55 | 4 | 36.4 | 0.56 |
Amph = amphetamine, meth = methampletamine
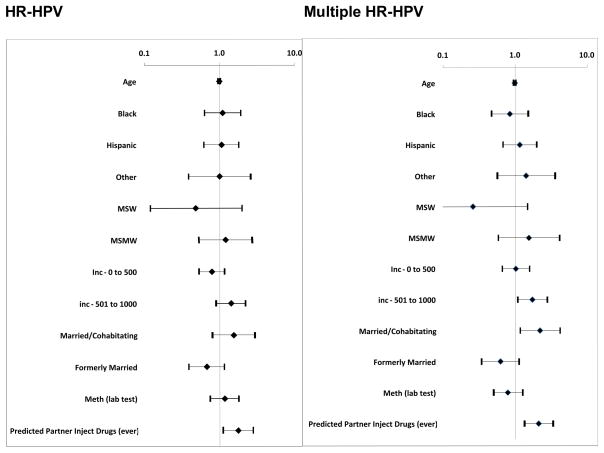
The multivariate analyses controlled for age, race, income, country of birth, marital status, and reported current methamphetamine use and found that having a sex partner who injected drugs was associated with having HR-HPV (adjusted odds ratio (AOR) 1.54, 95%CI 1.02, 2.33) and having infection with multiple strains of HR-HPV (AOR 1.64, 95%CI 1.09, 2.48). Being currently married was also associated with having multiple HR-HPV (AOR 2.05, 95% CI 1.08, 3.89). (See Figure 1)
Figure 1.
Multivariate Logistic Regressions
Discussion
As RAI is a risk factor for anal HPV it is expected that individuals who affirm this behavior frequently have quantifiable anal HPV. Findings show a sequential increase in the frequency of HPV isolation from MSW, MSMW to MSM that likely reflects concomitant increasing frequency of RAI. Still the HPV prevalence observed in the MSW is much higher than previously identified, which may be explained by either underreporting of RAI or the presence of other risk factors not measured in this study such as cigarette smoking. Anal HPV prevalence rates for HIV-positive MSM are similar to those previously reported.(9)
Similar to the trend seen for cervical infection in women, there is increased HPV isolation observed for younger compared to older age groups of men in this study. However, by contrast, the prevalence of cervical HPV in younger Western women approximates 20% whereas anal HPV prevalence in this sample was 100% in men below 30 years and only dropped to 83.7% for men aged over 50 years.(16) Anal HR-HPV prevalence approximated 65% in all age strata below 50 years and should these rates persist over time, as is suggested by the EXPLORE HPV sub-study, these individuals may be at high-risk of HPV associated anal dysplasia and cancer.(1)
We found that having a sexual partner who injected drugs was associated with having HR-HPV and infection with multiple strains of HR-HPV after controlling for other factors including the methamphetamine use of the individual. Given that the only other variable associated with HPV infection with multiple types was being married or co-habiting, it is likely that sexual exposure is frequent and occurs over a long period of time with some partners suggesting the possibility of repeated infection within the partnership. Moreover, it suggests the behavior of the partner seems to create more risk for the individual than their own behavior, similar to our other research that has shown that partners behavior is associated with risk of cervical HR-HPV in women.(17)
Among MSM, methamphetamine use corresponds with increased risks for HIV infection (18), an effect that may be related to methamphetamine-facilitated episodes of anorectal sexual intercourse that are more frequent, longer and rougher than when not under the influence (27). In contrast to expectation, there were no multivariate differences in the prevalence of any type of HPV observed with reported or biological markers of methamphetamine or other substance use. The univariate finding that report of recent methamphetamine use correlated with higher prevalence of anal HPV isolation suggests that there may be a weak association, but it loses significance when controlling for other covariates. There was no data collected on rectal administration that could feasibly be associated with local mucosal immune impairment that could facilitate HPV transmission or expression of established infection.
Resistance to current ARV drugs is associated with loss of control of HIV replication and disease progression leading to increased clinical events and a reduced prognosis when compared to individuals with undetectable plasma HIV VL.(19) The rates of ARV resistance in this study population are extremely high for all ARV drug classes. This likely reflects poor ARV adherence previously reported in methamphetamine/substance using individuals and may represent advanced HIV infection with immune compromise in this population.(20) A failure in local immunological surveillance with unchecked HPV replication may be responsible for the high rates of prevalent infection seen in participants without a sexual exposure in the last 6 months (See Table 1).
Although benefiting from a non-clinic based sample, this study has clear limitations that relate to generalization of findings based on the population that was predominantly urban poor, minority and marginalized. The very small sample of MSW (n=10) greatly limits the ability to understand correlations in that group. Overall, the RDS method may have produced homogeneous samples that lack the variability needed to detect associations between demographics, drug use and anal HPV.
Summary
Substance using men are at increased risk of a number of medical conditions. In this population of HIV-positive men we see very high rates of anal HPV, HR-HPV and multiple types of HR-HPV in all age ranges up to 50 years. This may place substance using men at increased risk of HPV associated diagnoses such as anal dysplasia and anal cancer that may be explored in future longitudinal studies addressing these parameters.
Acknowledgments
Sources of support: The authors gratefully acknowledge the support of NIDA grant U01DA017394 for this work.
Footnotes
Declarations:
This study was approved by the University of California human subjects protection committee and the RAND institutional review board.
No author has any conflict of interest.
Dr. Palefsky has previously received research funding from Merck and Co.
References
- 1.Chin-Hong PV, Vittinghoff E, Cranston RD, Buchbinder S, Cohen D, Colfax G, et al. Age-Specific prevalence of anal human papillomavirus infection in HIV-negative sexually active men who have sex with men: the EXPLORE study. J Infect Dis. 2004 Dec 15;190(12):2070–6. doi: 10.1086/425906. [DOI] [PubMed] [Google Scholar]
- 2.Chin-hong PVHM, Benet D, Buchbinder S, Colfax G, Cranston RD, Da Costa M, Darragh T, Judson F, Koblin B, Mayer K, Vittinghoff E, Palefsky J. IAS 2005. Rio de Janeiro: 2005. Human papillomavirus is associated with HIV acquisition: the EXPLORE study. [Google Scholar]
- 3.D’Souza G, Wiley DJ, Li X, Chmiel JS, Margolick JB, Cranston RD, et al. Incidence and epidemiology of anal cancer in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2008 Aug 1;48(4):491–9. doi: 10.1097/QAI.0b013e31817aebfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celentano DD, Sifakis F, Hylton J, Torian LV, Guillin V, Koblin BA. Race/ethnic differences in HIV prevalence and risks among adolescent and young adult men who have sex with men. J Urban Health. 2005 Dec;82(4):610–21. doi: 10.1093/jurban/jti124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palefsky JM, Holly EA, Ralston ML, Arthur SP, Jay N, Berry JM, et al. Anal squamous intraepithelial lesions in HIV-positive and HIV-negative homosexual and bisexual men: prevalence and risk factors. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17(4):320–6. doi: 10.1097/00042560-199804010-00005. [DOI] [PubMed] [Google Scholar]
- 6.Scholefield JH, Castle MT, Watson NF. Malignant transformation of high-grade anal intraepithelial neoplasia. Br J Surg. 2005 Sep;92(9):1133–6. doi: 10.1002/bjs.4994. [DOI] [PubMed] [Google Scholar]
- 7.Palefsky JM. Human papillomavirus infection and anogenital neoplasia in human immunodeficiency virus-positive men and women. J Natl Cancer Inst Monogr. 1998;(23):15–20. doi: 10.1093/oxfordjournals.jncimonographs.a024166. [DOI] [PubMed] [Google Scholar]
- 8.Siverberg MXL, Chao C, Leyden W, Horberg M, Klein D, Towner W, Quesenberry C, Neugebauer R, Abrams D. Immunodeficiency, HIV RNA levels, and risk of non-AIDS-defining cancers. CROI; February 16–19, 2010; San Francisco. 2010. [Google Scholar]
- 9.Palefsky JM, Holly EA, Ralston ML, Jay N. Prevalence and risk factors for human papillomavirus infection of the anal canal in human immunodeficiency virus (HIV)-positive and HIV-negative homosexual men. J Infect Dis. 1998;177(2):361–7. doi: 10.1086/514194. [DOI] [PubMed] [Google Scholar]
- 10.Zaki NG, Osman A, Moustafa H, Saad AH. Alterations of immune functions in heroin addicts. Egypt J Immunol. 2006;13(1):153–71. [PubMed] [Google Scholar]
- 11.Talloczy Z, Martinez J, Joset D, Ray Y, Gacser A, Toussi S, et al. Methamphetamine inhibits antigen processing, presentation, and phagocytosis. PLoS Pathog. 2008 Feb 8;4(2):e28. doi: 10.1371/journal.ppat.0040028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heckathorn DD. Respondent-Driven Sampling: A New Approach to the Study of Hidden Populations. Social Problems. 1997;44:174–99. [Google Scholar]
- 13.Cranston RD, Darragh TM, Holly EA, Jay N, Berry JM, Da Costa M, et al. Self-collected versus clinician-collected anal cytology specimens to diagnose anal intraepithelial neoplasia in HIV-positive men. J Acquir Immune Defic Syndr. 2004 Aug 1;36(4):915–20. doi: 10.1097/00126334-200408010-00004. [DOI] [PubMed] [Google Scholar]
- 14.Castle PE, Schiffman M, Gravitt PE, Kendall H, Fishman S, Dong H, et al. Comparisons of HPV DNA detection by MY09/11 PCR methods. J Med Virol. 2002 Nov;68(3):417–23. doi: 10.1002/jmv.10220. [DOI] [PubMed] [Google Scholar]
- 15.Morrison EA, Goldberg GL, Kadish AS, Burk RD. Polymerase chain reaction detection of human papillomavirus: quantitation may improve clinical utility. J Clin Microbiol. 1992 Oct;30(10):2539–43. doi: 10.1128/jcm.30.10.2539-2543.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith JS, Melendy A, Rana RK, Pimenta JM. Age-specific prevalence of infection with human papillomavirus in females: a global review. J Adolesc Health. 2008 Oct;43(4 Suppl):S5–25. Se1–41. doi: 10.1016/j.jadohealth.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Javanbakht M, Gorbach PM, Amani B, Walker S, Cranston RD, Datta SD, et al. Concurrency, sex partner risk, and high-risk human papillomavirus infection among African American, Asian, and Hispanic women. Sex Transm Dis. 2010 Feb;37(2):68–74. doi: 10.1097/OLQ.0b013e3181bcd3e7. [DOI] [PubMed] [Google Scholar]
- 18.Plankey MW, Ostrow DG, Stall R, Cox C, Li X, Peck JA, et al. The relationship between methamphetamine and popper use and risk of HIV seroconversion in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2007 May 1;45(1):85–92. doi: 10.1097/QAI.0b013e3180417c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mellors JW, Rinaldo CR, Jr, Gupta P, White RM, Todd JA, Kingsley LA. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996 May 24;272(5265):1167–70. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 20.Colfax GN, Vittinghoff E, Grant R, Lum P, Spotts G, Hecht FM. Frequent methamphetamine use is associated with primary non-nucleoside reverse transcriptase inhibitor resistance. AIDS. 2007 Jan 11;21(2):239–41. doi: 10.1097/QAD.0b013e3280114a29. [DOI] [PubMed] [Google Scholar]