Abstract
Objective
To evaluate the relationship between chronic glucocorticoid (GC) exposure and bone mineral density (BMD) in children with rheumatic diseases and inflammatory bowel disease.
Study Design
Lumbar spine BMD was measured by DXA in 86 GC-treated children (66% female, age 8-20 years, mixed ethnicity) screened for a multi-center intervention trial. Predictors of spine BMD Z-score and vitamin D [25(OH)D] were examined by multivariable linear regression.
Results
Mean prior year and lifetime cumulative GC exposure was 77.8 mg/kg and 224.6 mg/kg respectively. BMD Z-scores ranged from −3.7 to 2.2 SD (−1.1 ± 1.2, mean ± SD). Lower BMD Z-scores were associated with increased prior year average daily GC dose (p=0.03), decreased height Z-score (p=0.003), and decreased 25(OH)D concentrations (p=0.03), but explained only a small proportion of BMD variability (adjusted R2 =0.29). The 25(OH) D levels were < 20 ng/mL in 45% of subjects, and low 25(OH)D was associated with non-Caucasian ethnicity (p<0.001), increased age (p=0.004), increased PTH (p=0.03), and residing in the Boston area (p<0.001).
Conclusions
Although GC exposure is significantly associated with BMD Z-score, the association is too variable to serve as a consistent predictor of reduced BMD in children. Vitamin D insufficiency is common and may contribute to skeletal deficits in this population.
Keywords: bone density, vitamin D, rheumatic disease, inflammatory bowel disease
Introduction
Glucocorticoid (GC) excess resulting from endogenous or exogenous sources is a recognized risk factor for bone loss and fragility fractures. Supraphysiological GC concentrations can adversely affect bone through multiple pathways, including reduced osteoblast function, enhanced bone resorption, and inhibition of sex steroid production. Many of the direct effects of GC on bone are mediated through changes in the RANK/osteoprotegerin pathways1. The majority of adults exposed to systemic GC will experience measurable bone loss2, and 30-50% will fracture3. Since fractures occur more commonly than predicted by bone density measurements alone, GC exposure may adversely affect bone quality or micro-architecture independent of bone mass4-6.
Evidence linking GC exposure to osteoporosis in adults has led to the development of practice guidelines for the treatment and prevention of osteoporosis7-8. Adult patients requiring GC therapy are advised to take supplemental vitamin D and calcium, and bisphosphonates are recommended if chronic treatment beyond six months is anticipated. Far less is known about the effects of chronic GC exposure on the growing skeleton. Numerous publications describe the association between GC therapy and poor linear growth and low BMD9-16, and a large study in the UK has demonstrated a significant association between GC exposure and low impact fractures in children6. It is frequently difficult to distinguish the contribution of GC from other factors such as undernutrition, inflammation and reduced mobility in patients with reduced bone mass, and few reports comment on the effect of the GC exposure on BMD. However, there has been sufficient evidence linking GC therapy to reduced bone health in younger patients to generate interest in prevention and treatment of bone fragility. Increasingly, practitioners are prescribing bisphosphonates for children receiving GC therapy, despite a paucity of safety and efficacy data, and the absence of standardized guidelines for children.
The aim of this study was to examine the relationship between chronic GC therapy and bone mass in children and adolescents with rheumatologic disorders or inflammatory bowel disease. A secondary aim was to examine the contribution of host, disease and other treatment-related factors to bone mass in this population. Data were derived from the screening visit of an intervention study for reduced bone mass in GC-treated pediatric patients.
Methods
Study Design and Subjects
All subjects were enrolled during the screening phase of a treatment trial for reduced BMD at a Glaser Pediatric Research Network Site between 2002 and 2004. Potential subjects were identified by clinicians in the rheumatology and gastroenterology clinics. Informed consent/assent was obtained according to local institutional review board requirements. Subjects with Ulcerative Colitis (UC), Crohn’s Disease (CD), Systemic-onset Juvenile Rheumatoid Arthritis (sJRA), Juvenile Dermatomyositis (JDM), Systemic Lupus Erythematosus (SLE), Mixed Connective Tissue Disease (MCTD) or vasculitis were eligible for screening if they were between 8 and 22 years, had received at least 6 months of GC therapy, were not taking medications known to alter bone density (calcium and vitamin D supplementation were permitted), and did not meet any of the following exclusion criteria: significant upper gastrointestinal disease; hyperthyroidism; hyperparathyroidism; malignancy; rickets; osteomalacia; planned or current pregnancy; breastfeeding; non-ambulation; renal dysfunction; hepatic insufficiency; uncorrected hypocalcemia; or weight greater >136 kg. Subjects with a spine BMD Z-score ≤−1.5 and 25(OH)D concentration ≥20 ng/mL were eligible for the treatment phase.
Densitometry
Posteroanterior (PA) lumbar spine (L1-L4) BMD (g/cm2) was determined by DXA (Hologic 4500 scanners at all 4 sites) using the fast array scan mode. Standard phantoms were circulated between sites for cross-calibration. All scans were analyzed at UCSF using Hologic Delphi Manual Low-Density spine software; average L1-L4 was converted using standard calibration factors17 prior to generation of Z-scores. Z-scores were calculated using the chronologic age, for comparison to age- and gender-specific reference curves (Hologic Pediatric Research Reference Database [adapted from Faulkner reference data18]). Ethnicity was controlled for in the analysis. Longitudinal quality control scans were obtained on each day that subjects were scanned, with a minimum of 3 times per week, utilizing cross-calibration and longitudinal site-specific Hologic spine phantoms. An air scan was performed once a week to evaluate table top uniformity. The in vitro precision was 0.5%.
Clinical Evaluation
A physical examination was performed by the site investigator. Ethnicity, medical, and family histories were obtained by interview. GC exposure was determined by medical record review. Oral and parenteral GC formulations were converted to prednisone equivalents using standard conversion factors19. Current GC exposure was calculated as mg/kg/d and prior year and lifetime exposures were calculated as cumulative dose (mg/kg) and average daily dose (mg/kg/d). Weight and height were measured in triplicate utilizing a digital scale to the nearest 0.1 kg, and a wall-mounted stadiometer to the nearest 0.1 cm, respectively. Skeletal delay was assessed by comparing chronologic age with bone age from non-dominant hand radiographs. The subset of 26 subjects who were eligible for the treatment phase of the trial underwent dietary assessment for calcium and vitamin D utilizing a validated food frequency questionnaire20.
Laboratory Measures
Ionized calcium determinations were performed in local CLIA-certified labs and other assays were completed at a centralized, commercial reference laboratory. Intact PTH concentration was determined by immunochemiluminometry and 25(OH)D concentration by competitive protein binding radioassay (Esoterix, Calabasas Hills, CA).
Statistical Analysis
Associations between clinical variables and spine BMD Z-score and 25(OH)D concentration were evaluated by ANOVA for categorical variables and Spearman rank correlation coefficient for continuous variables due to skewness. Pearson correlation coefficients were calculated when bivariate normality assumptions were satisfied. Multivariable linear regression models were constructed to identify predictors of BMD Z-score and serum 25(OH)D concentration. Explanatory variables were considered for inclusion in the multivariable model if the p-value was ≤0.1 in the univariate analysis, or if the variables were important confounders. Backward stepwise methods were utilized to determine the final models. For all analyses, p=0.05 (2-tailed) tests were considered significant. Statistical analyses were performed utilizing STATA 8.
Results
Clinical characteristics
The 86 subjects (29 male, 57 female) ranged in age from 8-20 years (13.8 ± 3.4 yrs, mean ± SD) and were ethnically diverse (Caucasian 37%, Hispanic 26%, Asian 15%, African American 10%, mixed 12%). Underlying inflammatory disorders included SLE (n = 25), JDM (n= 24), vasculitis (n=11), sJRA (n = 10), CD (n=9), UC (n=5) and MCTD (n = 2). Age at diagnosis was 9.4 ± 4.0 yrs (range 0.2-17.6) and disease duration was 4.5 ± 2.9 yrs (range 0.6-14.0). For all analyses, subjects with UC and CD were combined as Inflammatory Bowel Disease (IBD), and subjects with SLE and MCTD as SLE/MCTD. In general, subjects were short, slightly overweight, and had delayed skeletal maturation (Table 1).
Table 1.
Clinical and anthropomorphic characteristics of the subjects (N=86)†
| Parameter | Mean ±SD |
|---|---|
| Age (yrs) | 13.8 ± 3.4 |
| Female (%) | 66 % |
| Height Z-score | − 1.0 ± 1.4 |
| Weight Z-score | 0.02 ± 1.8 |
| BMI (mg/cm2) | 21.9 ± 4.7 |
| BMI Z-score | 0.5 ± 1.2 |
| Skeletal delay (yrs)† | − 0.9 ± 1.7 |
| PA spine BMD (g/cm2) | 0.667 ± 0.153 |
| PA spine BMD Z-score | −1.1 ± 1.2 |
N=75 for skeletal delay, defined as bone age minus chronologic age.
All subjects had a prior history of GC exposure; 70% were receiving GC therapy at the time of screening and 89% had been exposed to GC during the prior year (Table 2). The specific GC preparations were prednisone (n=84), methylprednisolone (n=31), dexamethasone (n=4), and budesonide (n=2). The 9 subjects without prior year GC exposure had been off GC therapy for 22.6 ± 6.4 mos (range 12.9-28.7). Cumulative GC dose ranged from 0-2.5 g/kg during the year prior to screening. Many patients were receiving other immunomodulatory medications including methotrexate or arava (47%); biologic agents (eg. enbrel or remicade) (14%); cellcept (11%); cyclophosphamide (7%); imuran (7%); thalidomide (5%); azulfidine (5%); asacol (3%); and 6-mercaptopurine (3%). Sixteen patients (19%) had a prior history of one (n=13) or two (n=3) fractures. Twenty percent of subjects reported osteoporosis in family members.
Table 2.
Glucocorticoid exposure (prednisone equivalent) †
| Exposure | Median (range) |
|---|---|
| Current Regimen | |
| Current dose (mg/kg/d) | 0.09 (0, 1.05) |
| Prior Year exposure | |
| Duration (days) | 364 (0, 365) |
| Cumulative dose (mg/kg) | 77.8 (0, 2497.8) |
| Average daily dose (mg/kg/d) | 0.3 (0, 15.4) |
| Lifetime exposure | |
| Duration (days) | 552 (212, 4109) |
| Cumulative dose (mg/kg) | 224.6 (35.4, 1224.1) |
| Average daily dose (mg/kg/d) | 0.3 (0.03, 1.7) |
N= 86 for current regimen and prior year exposure, N =45 for lifetime exposure.
Correlates of bone mass
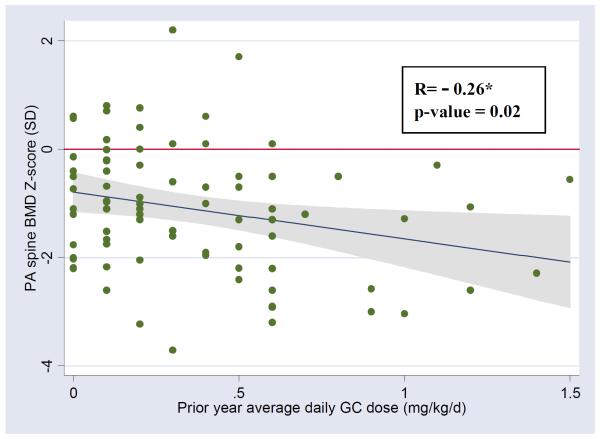
PA spine BMD ranged from 0.421 g/cm2 - 1.020 g/cm2 (0.667 ± 0.153). Spinal BMD Z-scores ranged from −3.7 to 2.2 SD (−1.1 ± 1.2). Clinical characteristics were evaluated as potential independent correlates of BMD Z-score (Table 3). In univariate analysis examining predictors of reduced BMD Z-score, factors with p≤0.1 included reduced height Z-score (p=0.0002), increased skeletal delay (p=0.006), greater current GC dose (p=0.07), greater prior year cumulative (p=0.01) or average daily (p=0.008) GC dose, history of exposure to cellcept (p=0.09) or MTX/Arava (p=0.09), and underlying diagnosis (p=0.10). Mean spinal BMD Z-score was −1.5 ± 1.1 for SLE/MCTD, −1.3 ± 1.4 for IBD, −0.9 ± 1.0 for JDM, −0.8 ± 1.3 for vasculitis, and −0.5 ± 1.0 for sJRA (Table 4). As shown in Figure 1, despite the overall significant association between GC exposure during the prior year and BMD Z-score, there was marked variability in the individual response to steroid exposure. BMD Z-score was marginally inversely correlated with 25(OH)D concentration by Pearson correlation (p=0.08) but not by Spearman rank correlation (p=0.16). Patients with SLE/MCTD and vasculitis had slighter higher prior year GC exposure (p<0.001), however, both of these groups included a patient with unusually high exposure (Table 4). Exclusion of these two patients demonstrated lower GC exposure for patients with sJRA (P=0.01). BMD Z-score was not associated with age, gender, ethnicity, disease duration, vitamin D dose, or PTH concentration (p> 0.10 for each). After adjustment for geographic site, gender, ethnicity, skeletal delay and underlying diagnosis in the final multivariable model for predicting spine BMD Z-score, lower BMD Z-scores were associated with increased prior year average daily GC dose (p=0.03), reduced height Z-score (p= 0.003), and lower 25(OH)D concentrations (p=0.03) (Table 5). Skeletal delay was highly correlated with height Z-score (Spearman rank correlation coefficient = 0.57 [p<0.001]) and was significantly associated with BMD Z-score in the multivariable model (p=0.02) if height Z-score was removed. The overall model explained between one-quarter and one-third of the variability in spinal BMD Z-score (adjusted R2=0.29). We subsequently added the number of high dose “pulse” methylpredisolone infusions to the model to explore the effect of different GC preparations, however this measure was not significant and there was no change in the overall model fit. We assessed for interactions between GC exposure and ethnicity and underlying diagnosis, respectively; however none were significant (P<0.05).
Table 3.
Spearman rank correlation coefficients (R) for the association between clinical parameters and both spinal BMD Z-score and 25(OH) D concentration †
| BMD Z-score | 25(OH)D (ng/mL) | |||
|---|---|---|---|---|
| Variable | R | p-value | R | p-value |
| Age (yrs) | −0.15 | 0.16 | −0.32 | 0.003 |
| Disease duration (yrs) | −0.03 | 0.78 | −0.19 | 0.08 |
| Skeletal delay (yrs) | 0.31 | 0.006 | 0.07 | 0.55 |
| Height Z-score | 0.39 | 0.0002 | 0.18 | 0.10 |
| Vit D supplement dose (IU/d) | −0.17 | 0.11 | 0.29 | 0.007 |
| 25(OH) D concentration (ng/mL)‡ | 0.15 | 0.16 | - | - |
| PTH (pg/mL) | 0.07 | 0.53 | −0.19 | 0.08 |
| Current GC dose (mg/kg/d) | −0.19 | 0.07 | −0.09 | 0.42 |
| Prior Year GC exposure | ||||
| Duration (days) | −0.13 | 0.22 | −0.37 | <0.001 |
| Cumulative dose (mg/kg) | −0.27 | 0.01 | −0.15 | 0.18 |
| Average daily dose (mg/kg/d) | −0.28 | 0.008 | 0.01 | 0.92 |
| Lifetime GC exposure | ||||
| Duration (days) | 0.005 | 0.98 | −0.30 | 0.04 |
| Cumulative dose (mg/kg) | −0.16 | 0.29 | −0.07 | 0.64 |
| Average daily dose (mg/kg/d) | −0.03 | 0.85 | −0.01 | 0.95 |
N=86 for all comparisons except 25(OH)D (N=85), PTH (N=83), skeletal delay (N=75) and lifetime GC exposures (N=45).
By Pearson correlation coefficient R=0.19, p=0.08.
Table 4.
Comparison of mean (SD) spine BMD Z-scores and prior year average daily GC exposure across the disease groups.
| SLE/MCTD | JDM | IBD | vasculitis | sJRA | |
|---|---|---|---|---|---|
| N (%) | 27 (31) | 24 (28) | 14 (16) | 11 (13) | 10 (12) |
| BMD Z-score (SD) | −1.5 (1.1) | −0.9 (1.0) | −1.3 (1.4) | −0.8 (1.3) | −0.5 (1.0) |
| GC exposure (mg/kg/d) | 0.9 (2.9)* | 0.4 (0.5) | 0.4 (0.3) | 0.9 (1.6)* | 0.2 (0.2) |
There were two subjects with very high prior year average GC exposure. They included one subject with vasculitis whose GC exposure was 5.7 mg/kg/d and BMD Z-score was −1.5 SD, and another with SLE whose GC exposure was 15.4 mg/kg/d and BMD Z-score −1.9 SD.
Figure 1.
Relationship between prior year average daily GC dose and PA spine BMD Z-score †
† Scatter plot of PA spine BMD Z-score versus prior year average daily GC dose overlaid with fitted regression line and 95% CI. Data shown for 84 subjects. Two subjects with very high prior year average daily GC dose were excluded; they had prior year average daily GC dose and BMD Z-scores of 5.7 mg/kg/d and −1.5 SD, and 15.4 mg/kg/d and −1.9 SD, respectively.
* R= Pearson correlation coefficient.
Table 5.
Results of multivariable model examining predictors of PA spine BMD Z-score after adjustment for geographic site, gender, ethnicity, diagnosis and skeletal delay † ‡ *
| Parameter | β- coefficient | 95% CI | p-value |
|---|---|---|---|
| Prior year average GC dose (per mg/kg/d) | −0.78 | −1.49 to −0.10 | 0.03 |
| Height Z-score (per SD) | 0.37 | 0.13 to 0.60 | 0.003 |
| 25(OH) D concentration (per ng/mL) | 0.05 | −0.005 to 0.09 | 0.03 |
The model includes 72 subjects, two subjects with very high prior year average daily GC dose were excluded. These subjects had prior year average daily GC dose and BMD Z-scores of 5.7 mg/kg/d and −1.5 SD, and 15.4 mg/kg/d and −1.9 SD, respectively.
The model adjusted R2 was 0.29.
Geographic site, gender, ethnicity and skeletal delay were not significantly associated with spine BMD Z-score in the multivariable model (P>0.05).
Vitamin D, calcium and PTH homeostasis
Forty-five percent of subjects had serum 25(OH)D concentrations below 20 ng/mL. The mean 25(OH)D concentration was 21.6 ± 8.8 ng/mL (range <5-38). Vitamin D dose among the 33 subjects taking supplements was 391 ± 142 IU/d (range 100-800). Dietary intake of vitamin D ranged from 23 to 477 IU/d (median 129). All serum concentrations of ionized calcium were within normal range (mean 1.2 ± 0.06 mmol/L) and dietary intake of calcium among those subjects who entered the treatment phase ranged from 359-2,278 mg/d (median 798 mg/d). PTH concentrations ranged from <10 to 103 pg/mL (mean 32.7 ± 14.7). There was a trend towards higher serum PTH concentrations among subjects with lower 25(OH)D concentrations (R= −0.19, p=0.08).
Factors associated with lower 25(OH)D concentrations (p≤0.1) in univariate analysis included increased age (p=0.003), increased disease duration (p=0.08), non-Caucasian ethnicity (overall p<0.001), lower vitamin D supplement dose (p=0.007), higher PTH concentration (p=0.08), increased number of days of GC exposure during the prior year (p<0.001) and over the lifetime (p=0.04) (Table 3). There was a marginal association (p=0.053) between underlying diagnosis and 25(OH)D concentration (17.9 ± 6.4 for SLE/MCTD, 21.5 ± 6.7 for vasculitis, 22.8 ± 8.0 for JDM, 23.6 ± 9.2 for sJRA, and 24.1 ± 6.9 for IBD). Geographic site was significantly associated (p=0.009) with 25(OH)D concentration (8.6 ± 7.2 for Boston, 19.6 ± 7.7 for San Francisco, 24.1 ± 7.4 for Los Angeles, and 25.2 ± 6.3 for Palo Alto). 25(OH)D concentration was not associated with gender (p>0.1). In the final multivariable model, lower 25(OH)D concentrations were associated with non-Caucasian ethnicity (overall p<0.001), increased age (p=0.004), increased PTH concentration (p=0.03), and living in the Boston area (overall p<0.001) (Table 6). The multivariable model explained almost one-half of the variability of vitamin D concentrations (adjusted R2= 0.46).
Table 6.
| Parameter | β- coefficient | 95% CI | p-value |
|---|---|---|---|
| Age (per year) | −0.6 | − 1.0 to −0.20 | 0.004 |
| Race (versus Caucasian) | |||
| African American | −8.1 | −12.9 to −3.2 | 0.001 |
| Asian | −7.1 | −11.2 to −3.0 | 0.001 |
| Hispanic | −6.4 | −9.8 to 3.0 | <0.001 |
| Mixed | −4.3 | −9.1 to 0.5 | 0.08 |
| PTH concentration (per pg/mL) | −0.1 | −0.2 to −0.01 | 0.03 |
| Site (versus Boston) | |||
| San Francisco | 2.7 | −1.0 to 6.3 | 0.15 |
| Palo Alto | 6.1 | 2.3 to 10.7 | 0.002 |
| Los Angeles | 8.4 | 4.1 to 12.8 | <0.001 |
Model includes 83 subjects.
The model adjusted R2 was 0.41.
Discussion
Among children with chronic inflammatory diseases and 6 months minimum lifetime exposure to GC therapy, we found highly variable deficits in bone mineral and a high prevalence of vitamin D insufficiency. Rates of reduced bone mass were substantially lower than expected for a population of subjects who had been identified for inclusion in an osteoporosis clinical trial. Only 60% of subjects expected to have reduced BMD were found to have BMD Z-scores ≤ −1.5 SD. These data suggest that in children, reduced BMD among individuals receiving chronic GC treatment may not be as common as expected.
Pediatric studies have previously reported variable associations between GC exposure and risk for reduced BMD. Some studies demonstrate a significant association between GC exposure and reduced BMD or fracture (6, 10-14), and others do not 21-23. Small sample sizes may have limited the ability to detect associations in some of these reports. Although our study found that each 1 mg/kg/d increase in the prior year average daily GC dose was associated with a 0.78 reduction of Z-score, the relationship was highly variable among subjects. Furthermore, the final multivariable model that combined GC exposure, height Z-score and 25(OH)D concentration explained only 29% of the variance in spinal BMD Z-score. Similar multivariable models have been reported in pediatric patients with IBD 11, suggesting that unmeasured factors, such as genetic determinants, disease, or other treatment-related variables may be important determinants of bone mass. We did not find a significant association for the common immunomodulatory drugs. However, considering our sample size among other factors, it was not feasible to control for all potential variables.
The lack of association between BMD and lifetime GC exposure may reflect the potential for bone mineral recovery, as previously described in children in remission from Cushing’s disease24. Alternatively, there could be inaccuracies in the lifetime GC exposure measurements. However, we observed a significant positive association between height Z-score and both lifetime duration of GC exposure and total cumulative dose (mg/kg) (data not shown), supporting the accuracy of the lifetime cumulative steroid measurements. These observations suggest that there may be a different effect of GC exposure on height compared to BMD in children.
Vitamin D insufficiency is frequently observed in healthy American adolescents25-26. In this sample of patients cared for in tertiary medical centers nearly 50% had vitamin D insufficiency. The geographic variability of 25(OH)D concentrations may reflect differences in latitude, with subjects living at the highest latitude (Boston) demonstrating on average the lowest vitamin D levels. The compensatory increase in PTH observed in our subjects may lead to increased bone resorption and reduced BMD. These findings suggest that patients receiving chronic GC therapy for inflammatory disorders should be systematically screened and supplemented with vitamin D to maximize vitamin D stores prior to considering more aggressive medications such as bisphosphonates.
Several limitations must be considered when interpreting our results. Spinal BMD Z-scores were determined from areal BMD measurements by DXA without adjustment for body size or skeletal maturity, which can result in overestimation of an apparent deficit in bone mass. However, in the absence of consensus regarding the best method of adjusting for these variables, we chose to use the age-adjusted BMD Z-score for this clinical trial, since this is the most commonly applied clinical measure in selecting patients for treatment, and to adjust for height Z-score and skeletal delay in the final multivariable model. Another limitation is the heterogeneity of the population. Heterogeneity of underlying pathologies was reduced in the study design by limiting subjects to those with inflammatory disorders, and univariate analysis did not reveal significant disease differences for BMD Z-score. However, the population is also heterogeneous with respect to ethnicity, gender, age and GC exposure, all variables known to influence BMD measures. To address this limitation we chose to control for all of these variables in the final multivariable model, even if not significant in univariate analysis, and we observed a persistent significant, although variable association between GC exposure and BMD Z-score. Of note, the current study sample was selected from a clinical trial which was not designed to yield an unbiased sample or to be of sufficient size to permit prevalence determinations. Thus, the observed associations are generalizable only to pediatric patients with similar inflammatory disorders and GC exposure. The authors acknowledge the controversy surrounding conversion of various GC preparations to prednisone-equivalents. We addressed this limitation by examining the significance of methylprednisolone in our final prediction model for BMD Z-score and did not identify an independent effect for methylprednisolone. Samples sizes were too small to examine dexamethasone and budesonide in a similar fashion. Despite these limitations, we feel that the unexpected prevalence of vitamin D insufficiency and apparent absence of significant skeletal deficits among many children with substantial prior GC exposure is important information for clinicians caring for similar populations. Finally, we recognize that bone mass is only one factor in determining bone strength and that GCs appear to increase bone fragility independent of BMD4.
The observation that only 60% of subjects had a BMD Z-score ≤ −1.5 suggests a need to re-examine our assumptions about the prevalence and severity of low bone mass in the setting of chronic disease and GC therapy. Of the 86 children evaluated, only 24% were eligible for the treatment phase of this trial. Fifty-two (40%) of at-risk patients had BMD Z-scores too high for randomization (inclusion criteria required BMD Z-score < −1.5) and 38 (45%) were excluded for vitamin D insufficiency (25(OH)D concentration < 20 ng/mL). Our findings suggest that even if GC exposure contributes to reduced BMD in some patients, there is considerable variability in skeletal response, beyond that attributed to background ethnicity and underlying disease. This observation, combined with the weak prediction model utilizing accepted clinical predictors, suggests that risk assessment is difficult in this population.
Our experience screening pediatric subjects with prior GC exposure for inclusion in a clinical trial provides valuable lessons for clinicians and investigators. We have demonstrated that the association between chronic GC exposure in the prior year and reduced BMD, although significant, is too variable to serve as an independent marker for children at risk for reduced BMD. Our experience also suggests that vitamin D insufficiency is a common problem in this patient population that may be overlooked even in chronically ill children receiving care in tertiary pediatric centers. These data suggest that risk-assessment for reduced BMD in children is complex and poorly understood. Therefore, in this era of increasing attention to childhood bone health and rising use of bisposphonates, we recommend that further studies be conducted to define the appropriate therapeutic indications for bisphosphonates and other aggressive osteoporosis therapies in children.
Acknowledgments
This study was supported by the Elizabeth Glaser Pediatric Research Foudnation. Drug and placebo were supplied by Merck & Co. Partial support was also provided by the general clinical research centers at Baylor College of Medicine (M01-RR-00188), Children’s Hospital Boston (M01-RR-02172), Stanford University (M01-RR-000070), UCLA (M01-RR-00865), and the pediatric clinical research center at UCSF (M01-RR-01271).
In addition to the participants and their families, the authors thank the following persons for their contributions to this project: Data and Safety Monitoring Board: A. Griffiths, P. Lavori, M. Leonard, C. Egla Rabinovich; Management Committee: C. Prober (Chair), S. Berg, L. Bomgaars, H. Cohen, C. Duggan, T. Moore, I. Salusky, E. von Scheven, D. Wilson; Nurse Coordinators and Research Assistants: H. Cohen, J. Davis, L. Howard, E. Khanukhova, T. Lihatsh, K. McNeil, J. Mooney, C. Sweeney, M. Wertz, K. Wilson, K. Zhang; UCSF Department of Radiology: J. Shepherd, M. Sherman; Central Office: C Crabtree, A. Kelley, A. Kim, K. Urbanek; and Westat.
Footnotes
Children’s Hospital Boston and Harvard Medical School; Children’s Medical Center, University of California San Francisco; Lucile S. Packard Children’s Hospital, Stanford University (Palo Alto, California); and Mattel Children’s Hospital, University of California Los Angeles
There are no conflict of interest issues for any of the authors.
Contributor Information
Emily von Scheven, Box 0107 Children’s Hospital, University of California at San Francisco San Francisco, CA 94143.
Catherine M. Gordon, Divisions of Adolescent Medicine and Endocrinology Children’s Hospital Boston and Harvard Medical School.
David Wypij, Clinical Research Program Children’s Hospital Boston, Harvard Medical School, and Harvard School of Public Health.
Marcia Wertz, Department of Pediatrics UCSF Children’s Hospital, University of California at San Francisco.
Kerry T. Gallagher, Division of Rheumatology Mattel Children’s Hospital at UCLA.
Laura Bachrach, Department of Pediatrics-Endocrinology Lucile Packard Children’s Hospital at Stanford University Medical Center.
References
- 1.Canalis E, Bilezikian JP, Angeli A, Giustina A. Perspectives on glucocorticoid-induced osteoporosis. Bone. 2004;34(4):593–8. doi: 10.1016/j.bone.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 2.Saag KG, Koehnke R, Caldwell JR, Brasington R, Burmeister LF, Zimmerman B, et al. Low dose long-term corticosteroid therapy in rheumatoid arthritis: an analysis of serious adverse events. Am J Med. 1994;96(2):115–23. doi: 10.1016/0002-9343(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 3.Lukert BP. Glucocorticoid-induced osteoporosis. South Med J. 1992;85(8):2S48–51. doi: 10.1097/00007611-199208001-00009. [DOI] [PubMed] [Google Scholar]
- 4.Van Staa TP, Laan RF, Barton IP, Cohen S, Reid DM, Cooper C. Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum. 2003;48(11):3224–9. doi: 10.1002/art.11283. [DOI] [PubMed] [Google Scholar]
- 5.Kanis JA, Johansson H, Oden A, Johnell O, de Laet C, Melton IL, et al. A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res. 2004;19(6):893–9. doi: 10.1359/JBMR.040134. [DOI] [PubMed] [Google Scholar]
- 6.van Staa TP, Cooper C, Leufkens HG, Bishop N. Children and the risk of fractures caused by oral corticosteroids. J Bone Miner Res. 2003;18(5):913–8. doi: 10.1359/jbmr.2003.18.5.913. [DOI] [PubMed] [Google Scholar]
- 7.Recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis: 2001 update. American College of Rheumatology Ad Hoc Committee on Glucocorticoid-Induced Osteoporosis. Arthritis Rheum. 2001;44(7):1496–503. doi: 10.1002/1529-0131(200107)44:7<1496::AID-ART271>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 8.Eastell R, Reid DM, Compston J, Cooper C, Fogelman I, Francis RM, et al. A UK Consensus Group on management of glucocorticoid-induced osteoporosis: an update. J Intern Med. 1998;244(4):271–92. doi: 10.1046/j.1365-2796.1998.00408.x. [DOI] [PubMed] [Google Scholar]
- 9.Robinson IC, Gabrielsson B, Klaus G, Mauras N, Holmberg C, Mehls O. Glucocorticoids and growth problems. Acta Paediatr Suppl. 1995;411:81–6. doi: 10.1111/j.1651-2227.1995.tb13870.x. [DOI] [PubMed] [Google Scholar]
- 10.Alsufyani KA, Ortiz-Alvarez O, Cabral DA, Tucker LB, Petty RE, Nadel H, et al. Bone mineral density in children and adolescents with systemic lupus erythematosus, juvenile dermatomyositis, and systemic vasculitis: relationship to disease duration, cumulative corticosteroid dose, calcium intake, and exercise. J Rheumatol. 2005;32(4):729–33. [PubMed] [Google Scholar]
- 11.Boot AM, Bouquet J, Krenning EP, de Muinck Keizer-Schrama SM. Bone mineral density and nutritional status in children with chronic inflammatory bowel disease. Gut. 1998;42(2):188–94. doi: 10.1136/gut.42.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cowan FJ, Warner JT, Dunstan FD, Evans WD, Gregory JW, Jenkins HR. Inflammatory bowel disease and predisposition to osteopenia. Arch Dis Child. 1997;76(4):325–9. doi: 10.1136/adc.76.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falcini F, Trapani S, Civinini R, Capone A, Ermini M, Bartolozzi G. The primary role of steroids on the osteoporosis in juvenile rheumatoid patients evaluated by dual energy X-ray absorptiometry. J Endocrinol Invest. 1996;19(3):165–9. doi: 10.1007/BF03349860. [DOI] [PubMed] [Google Scholar]
- 14.Lien G, Selvaag AM, Flato B, Haugen M, Vinje O, Sorskaar D, et al. A two-year prospective controlled study of bone mass and bone turnover in children with early juvenile idiopathic arthritis. Arthritis Rheum. 2005;52(3):833–40. doi: 10.1002/art.20963. [DOI] [PubMed] [Google Scholar]
- 15.Trapani S, Civinini R, Ermini M, Paci E, Falcini F. Osteoporosis in juvenile systemic lupus erythematosus: a longitudinal study on the effect of steroids on bone mineral density. Rheumatol Int. 1998;18(2):45–9. doi: 10.1007/s002960050056. [DOI] [PubMed] [Google Scholar]
- 16.Varonos S, Ansell BM, Reeve J. Vertebral collapse in juvenile chronic arthritis: its relationship with glucocorticoid therapy. Calcif Tissue Int. 1987;41(2):75–8. doi: 10.1007/BF02555248. [DOI] [PubMed] [Google Scholar]
- 17.Leonard MB, Feldman HI, Zemel BS, Berlin JA, Barden EM, Stallings VA. Evaluation of low density spine software for the assessment of bone mineral density in children. J Bone Miner Res. 1998;13(11):1687–90. doi: 10.1359/jbmr.1998.13.11.1687. [DOI] [PubMed] [Google Scholar]
- 18.Faulkner RA, Bailey DA, Drinkwater DT, McKay HA, Arnold C, Wilkinson AA. Bone densitometry in Canadian children 8-17 years of Age. Calcif Tissue Int. 1996;59(5):344–51. doi: 10.1007/s002239900138. [DOI] [PubMed] [Google Scholar]
- 19.Punthakee Z, Legault L, Polychronakos C. Prednisolone in the treatment of adrenal insufficiency: a re-evaluation of relative potency. J Pediatr. 2003;143(3):402–5. doi: 10.1067/S0022-3476(03)00294-4. [DOI] [PubMed] [Google Scholar]
- 20.Rockett HR, Breitenbach M, Frazier AL, Witschi J, Wolf AM, Field AE, et al. Validation of a youth/adolescent food frequency questionnaire. Prev Med. 1997;26(6):808–16. doi: 10.1006/pmed.1997.0200. [DOI] [PubMed] [Google Scholar]
- 21.Daniels MW, Wilson DM, Paguntalan HG, Hoffman AR, Bachrach LK. Bone mineral density in pediatric transplant recipients. Transplantation. 2003;76(4):673–8. doi: 10.1097/01.TP.0000076627.70050.53. [DOI] [PubMed] [Google Scholar]
- 22.Castro TC, Terreri MT, Szejnfeld VL, Len C, Fonseca AS, Hilario MO. Bone mineral density of Brazilian girls with juvenile dermatomyositis. Braz J Med Biol Res. 2005;38(2):309–13. doi: 10.1590/s0100-879x2005000200020. [DOI] [PubMed] [Google Scholar]
- 23.Leonard MB, Feldman HI, Shults J, Zemel BS, Foster BJ, Stallings VA. Long-term, high-dose glucocorticoids and bone mineral content in childhood glucocorticoid-sensitive nephrotic syndrome. N Engl J Med. 2004;351(9):868–75. doi: 10.1056/NEJMoa040367. [DOI] [PubMed] [Google Scholar]
- 24.Di Somma C, Pivonello R, Loche S, Faggiano A, Klain M, Salvatore M, et al. Effect of 2 years of cortisol normalization on the impaired bone mass and turnover in adolescent and adult patients with Cushing’s disease: a prospective study. Clin Endocrinol (Oxf) 2003;58(3):302–8. doi: 10.1046/j.1365-2265.2003.01713.x. [DOI] [PubMed] [Google Scholar]
- 25.Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30(5):771–7. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 26.Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158(6):531–7. doi: 10.1001/archpedi.158.6.531. [DOI] [PubMed] [Google Scholar]