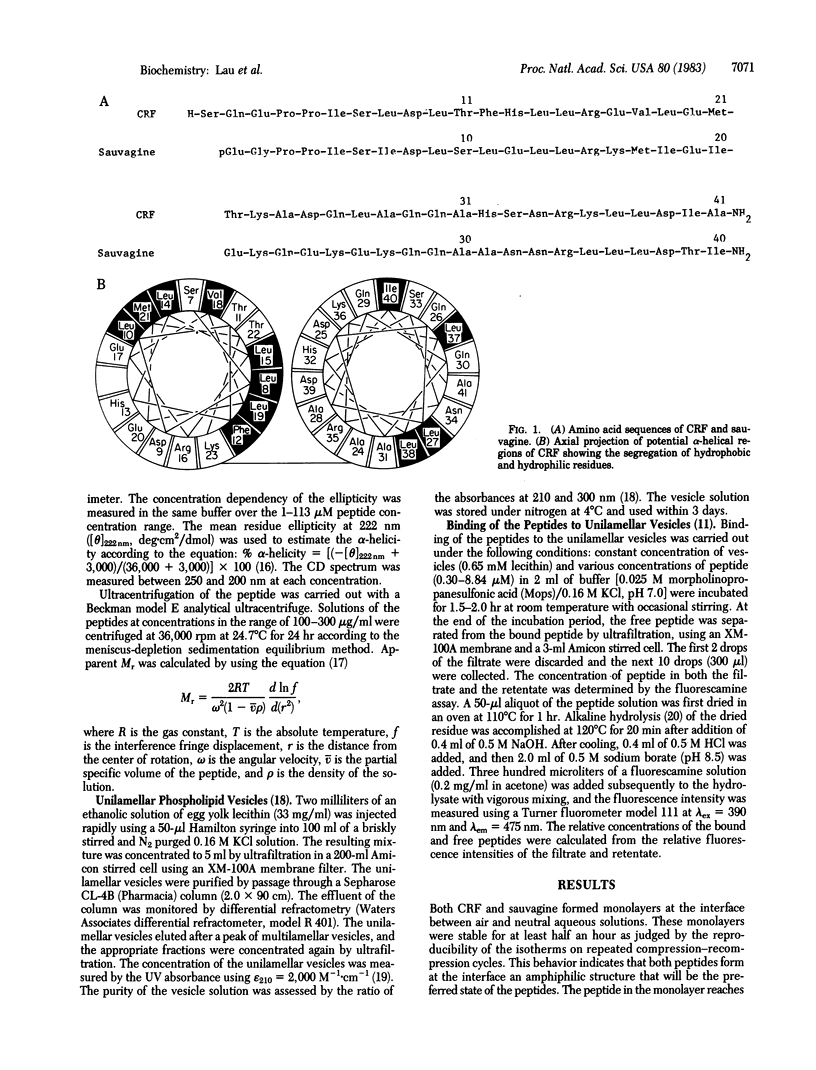
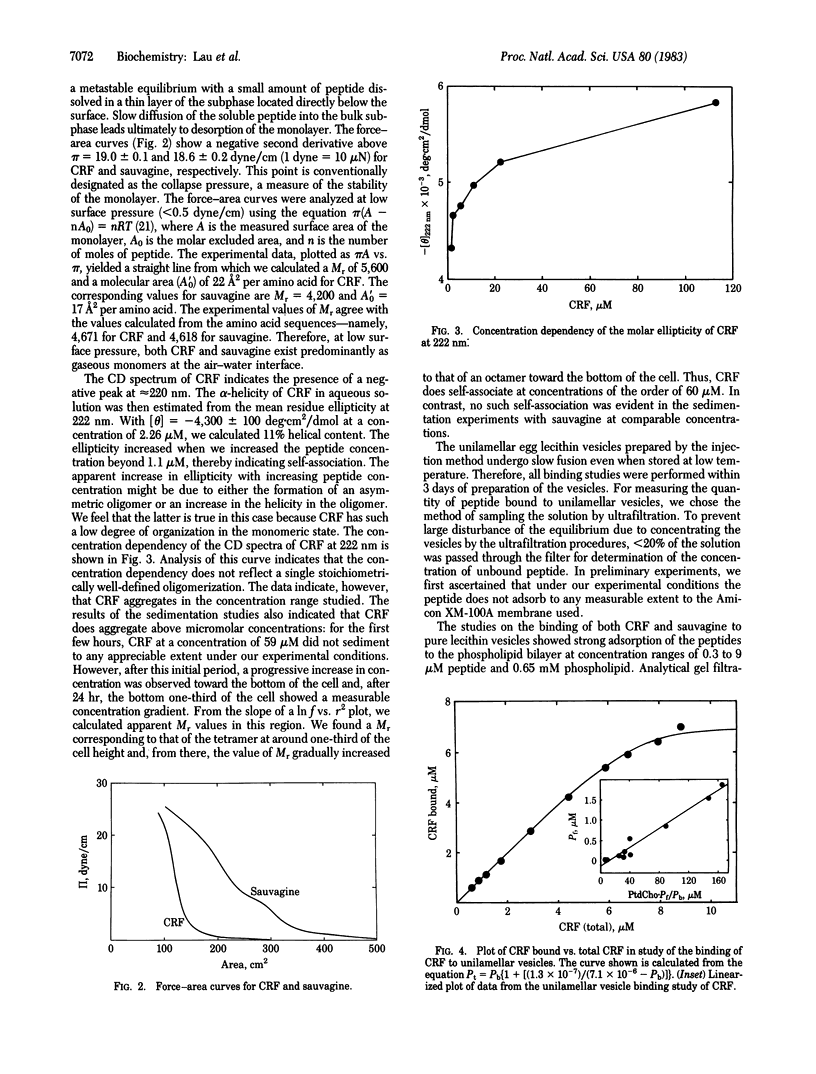
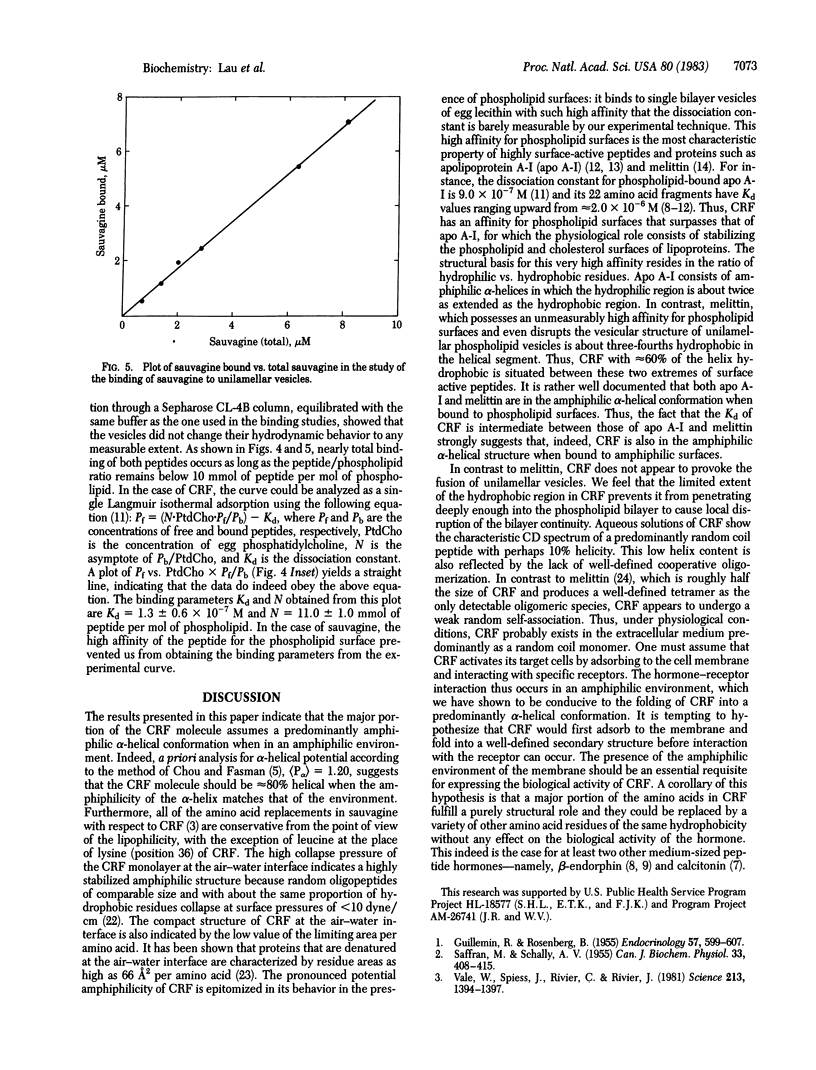
Abstract
Synthetic corticotropin (adrenocorticotropic hormone)-releasing factor [CRF; for the sequence, see Vale, W., Spiess, J., Rivier, C. & Rivier, J. (1981) Science 213, 1394-1397] in aqueous solution exists predominantly as a random coil. At concentrations greater than 1 microM, the peptide shows a tendency to self-aggregate with a concurrent slight increase in the apparent alpha-helical content as measured by the CD spectrum. The alpha-helix formed by this molecule is highly amphiphilic--i.e., the hydrophilic and hydrophobic regions are segregated on opposite faces of the helix. As predicted from the potential amphiphilic structure, CRF binds avidly to the surface of single bilayer egg phosphatidylcholine vesicles. This binding appears to obey a simple Langmuir isotherm with the following parameters: Kd = 1.3 +/- 0.6 X 10(-7) M and capacity at saturation (N) = 11.0 +/- 1.0 mmol of peptide per mol of phospholipid. CRF also readily forms an insoluble monolayer at the air-water interface. The monolayer is composed of monomers of the hormone with molecular areas, A'0 = 22 A2 per amino acid, suggesting a compact secondary structure. Judged from the collapse pressure (19.0 +/- 0.1 dyne/cm; 1 dyne = 10 microN) of the monolayer, the amphiphilicity of CRF approximates that of plasma apolipoproteins, a class of proteins of the most pronounced amphiphilic character. These results suggest that the binding of CRF to the cell membrane is accompanied by the induction of an alpha-helical secondary structure and it is this predominantly helical form that is the biologically active form of the peptide.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batzri S., Korn E. D. Single bilayer liposomes prepared without sonication. Biochim Biophys Acta. 1973 Apr 16;298(4):1015–1019. doi: 10.1016/0005-2736(73)90408-2. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Conformational parameters for amino acids in helical, beta-sheet, and random coil regions calculated from proteins. Biochemistry. 1974 Jan 15;13(2):211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- DeGrado W. F., Musso G. F., Lieber M., Kaiser E. T., Kézdy F. J. Kinetics and mechanism of hemolysis induced by melittin and by a synthetic melittin analogue. Biophys J. 1982 Jan;37(1):329–338. doi: 10.1016/S0006-3495(82)84681-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima D., Yokoyama S., Kézdy F. J., Kaiser E. T. Binding of amphiphilic peptides to phospholipid/cholesterol unilamellar vesicles: a model for protein--cholesterol interaction. Proc Natl Acad Sci U S A. 1981 May;78(5):2732–2736. doi: 10.1073/pnas.78.5.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferberg J. P., Yokoyama S., Kézdy F. J. The kinetics of the phospholipase A2-catalyzed hydrolysis of Egg phosphatidylcholine in unilamellar vesicles. Product inhibition and its relief by serum albumin. J Biol Chem. 1981 Jun 25;256(12):6274–6281. [PubMed] [Google Scholar]
- Lai C. Y. Detection of peptides by fluorescence methods. Methods Enzymol. 1977;47:236–243. doi: 10.1016/0076-6879(77)47028-9. [DOI] [PubMed] [Google Scholar]
- Lederis K., Letter A., McMaster D., Moore G., Schlesinger D. Complete amino acid sequence of urotensin I, a hypotensive and corticotropin-releasing neuropeptide from Catostomus. Science. 1982 Oct 8;218(4568):162–165. doi: 10.1126/science.6981844. [DOI] [PubMed] [Google Scholar]
- Morrisett J. D., David J. S., Pownall H. J., Gotto A. M., Jr Interaction of an apolipoprotein (apoLP-alanine) with phosphatidylcholine. Biochemistry. 1973 Mar 27;12(7):1290–1299. doi: 10.1021/bi00731a008. [DOI] [PubMed] [Google Scholar]
- SAFFRAN M., SCHALLY A. V. The release of corticotrophin by anterior pituitary tissue in vitro. Can J Biochem Physiol. 1955 May;33(3):408–415. [PubMed] [Google Scholar]
- Schiffer M., Edmundson A. B. Use of helical wheels to represent the structures of proteins and to identify segments with helical potential. Biophys J. 1967 Mar;7(2):121–135. doi: 10.1016/S0006-3495(67)86579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B. W., Scanu A. M. Properties of human apolipoprotein A-I at the air-water interface. Biochemistry. 1980 Aug 5;19(16):3643–3650. doi: 10.1021/bi00557a001. [DOI] [PubMed] [Google Scholar]
- Taylor J. W., Miller R. J., Kaiser E. T. Structural characterization of beta-endorphin through the design, synthesis, and study of model peptides. Mol Pharmacol. 1982 Nov;22(3):657–666. [PubMed] [Google Scholar]
- Vale W., Spiess J., Rivier C., Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981 Sep 18;213(4514):1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- Yokoyama S., Fukushima D., Kupferberg J. P., Kézdy F. J., Kaiser E. T. The mechanism of activation of lecithin:cholesterol acyltransferase by apolipoprotein A-I and an amphiphilic peptide. J Biol Chem. 1980 Aug 10;255(15):7333–7339. [PubMed] [Google Scholar]


