Abstract
The peptide mating pheromone alpha-factor and the hydrolytic enzyme invertase (beta-D-fructofuranoside fructohydrolase, EC 3.2.1.26) are processed from larger precursor proteins during their secretion from yeast cells (Saccharomyces cerevisiae). An in-frame fusion of the structural genes for these two proteins was constructed by connecting the 5'-flanking region and prepro-leader portion of the coding sequence of the alpha-factor gene (MF alpha 1) to a large fragment of the invertase gene (SUC2) lacking its 5'-flanking region and the coding information for the first four amino acids of its signal sequence. Sites that have been implicated in normal proteolytic processing of the alpha-factor precursor have been retained in this construction. The chimeric gene directs synthesis of a high level of active invertase that is secreted efficiently into the periplasmic space, permitting cell growth on sucrose-containing media. This extracellular invertase appears to contain no prepro-alpha-factor sequences. The initial intracellular product is, however, a hybrid protein that can be detected either by treatment of the cells with the drug tunicamycin or by blockage of secretion in a temperature-conditional secretion-defective mutant (sec18). Therefore, prior to its efficient proteolytic removal, the alpha-factor portion of the hybrid protein apparently provides the necessary information for efficient export of the substantially larger protein invertase. Similar to MF alpha 1, the MF alpha 1-SUC2 fusion is expressed in alpha haploids at levels 65-75 times higher than in a haploids or in a/alpha diploids; also, high-level expression is eliminated in mat alpha 1 mutants but not in mat alpha 2 mutants. Unlike expression of SUC2, expression of the fusion is not affected by glucose concentration. Hence, the 5'-flanking region present in the fusion (about 950 base pairs) is sufficient to confer alpha cell-specific expression to the hybrid gene.
Full text
PDF

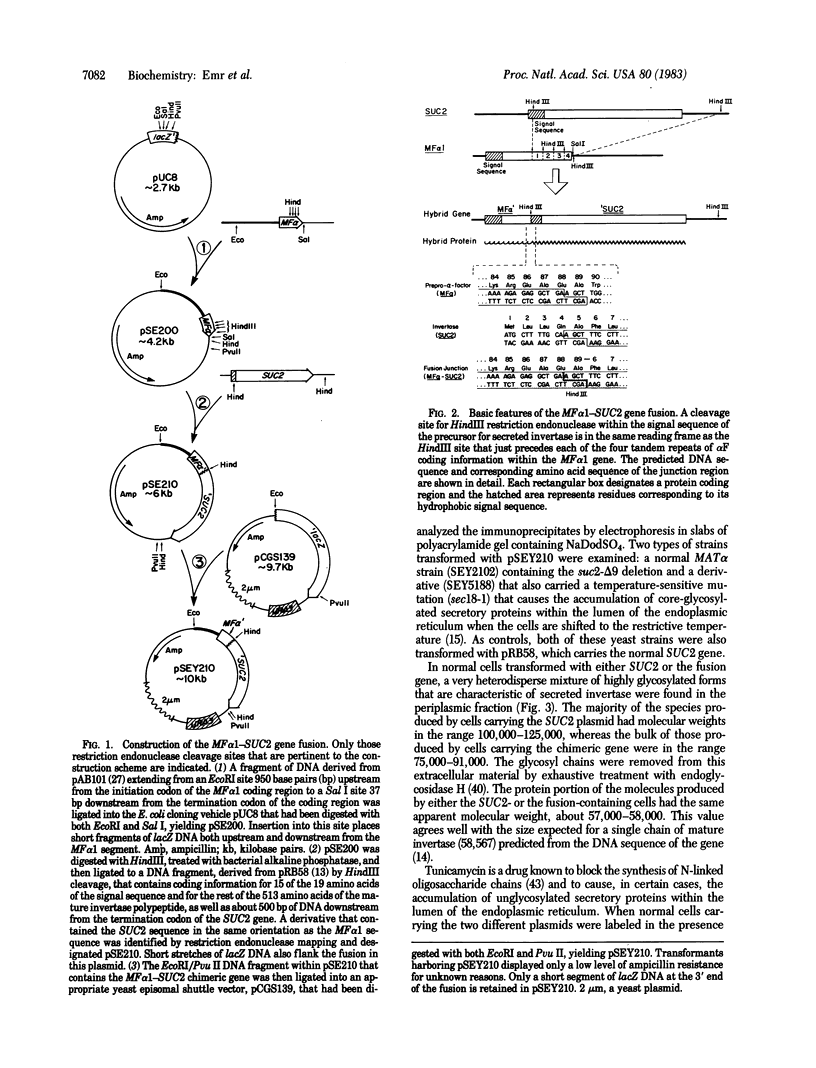


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassford P. J., Jr, Silhavy T. J., Beckwith J. R. Use of gene fusion to study secretion of maltose-binding protein into Escherichia coli periplasm. J Bacteriol. 1979 Jul;139(1):19–31. doi: 10.1128/jb.139.1.19-31.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith J. A genetic approach to characterizing complex promoters in E. coli. Cell. 1981 Feb;23(2):307–308. doi: 10.1016/0092-8674(81)90125-2. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Botstein D., Falco S. C., Stewart S. E., Brennan M., Scherer S., Stinchcomb D. T., Struhl K., Davis R. W. Sterile host yeasts (SHY): a eukaryotic system of biological containment for recombinant DNA experiments. Gene. 1979 Dec;8(1):17–24. doi: 10.1016/0378-1119(79)90004-0. [DOI] [PubMed] [Google Scholar]
- Brake A. J., Julius D. J., Thorner J. A functional prepro-alpha-factor gene in Saccharomyces yeasts can contain three, four, or five repeats of the mature pheromone sequence. Mol Cell Biol. 1983 Aug;3(8):1440–1450. doi: 10.1128/mcb.3.8.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M., Botstein D. Two differentially regulated mRNAs with different 5' ends encode secreted with intracellular forms of yeast invertase. Cell. 1982 Jan;28(1):145–154. doi: 10.1016/0092-8674(82)90384-1. [DOI] [PubMed] [Google Scholar]
- Carlson M., Taussig R., Kustu S., Botstein D. The secreted form of invertase in Saccharomyces cerevisiae is synthesized from mRNA encoding a signal sequence. Mol Cell Biol. 1983 Mar;3(3):439–447. doi: 10.1128/mcb.3.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R. K., Melnick L. M., Blair L. C., Thorner J. Extracellular suppression allows mating by pheromone-deficient sterile mutants of Saccharomyces cerevisiae. J Bacteriol. 1983 Aug;155(2):903–906. doi: 10.1128/jb.155.2.903-906.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duksin D., Mahoney W. C. Relationship of the structure and biological activity of the natural homologues of tunicamycin. J Biol Chem. 1982 Mar 25;257(6):3105–3109. [PubMed] [Google Scholar]
- Emr S. D., Hall M. N., Silhavy T. J. A mechanism of protein localization: the signal hypothesis and bacteria. J Cell Biol. 1980 Sep;86(3):701–711. doi: 10.1083/jcb.86.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emr S. D., Silhavy T. J. Molecular components of the signal sequence that function in the initiation of protein export. J Cell Biol. 1982 Dec;95(3):689–696. doi: 10.1083/jcb.95.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon B., Novick P., Schekman R. Compartmentalized assembly of oligosaccharides on exported glycoproteins in yeast. Cell. 1981 Aug;25(2):451–460. doi: 10.1016/0092-8674(81)90063-5. [DOI] [PubMed] [Google Scholar]
- Frevert J., Ballou C. E. Yeast invertase polymorphism is correlated with variable states of oligosaccharide chain phosphorylation. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6147–6150. doi: 10.1073/pnas.79.20.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel O., Wang S. F. Determination of enzymatic activity in polyacrylamide gels. I. Enzymes catalyzing the conversion of nonreducing substrates to reducing products. Anal Biochem. 1969 Mar;27(3):545–554. doi: 10.1016/0003-2697(69)90068-2. [DOI] [PubMed] [Google Scholar]
- Goldstein A., Lampen J. O. Beta-D-fructofuranoside fructohydrolase from yeast. Methods Enzymol. 1975;42:504–511. doi: 10.1016/0076-6879(75)42159-0. [DOI] [PubMed] [Google Scholar]
- Guarente L., Ptashne M. Fusion of Escherichia coli lacZ to the cytochrome c gene of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2199–2203. doi: 10.1073/pnas.78.4.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L., Yocum R. R., Gifford P. A GAL10-CYC1 hybrid yeast promoter identifies the GAL4 regulatory region as an upstream site. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7410–7414. doi: 10.1073/pnas.79.23.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert E., Uhler M. Biosynthesis of polyprotein precursors to regulatory peptides. Cell. 1982 Aug;30(1):1–2. doi: 10.1016/0092-8674(82)90002-2. [DOI] [PubMed] [Google Scholar]
- Julius D., Blair L., Brake A., Sprague G., Thorner J. Yeast alpha factor is processed from a larger precursor polypeptide: the essential role of a membrane-bound dipeptidyl aminopeptidase. Cell. 1983 Mar;32(3):839–852. doi: 10.1016/0092-8674(83)90070-3. [DOI] [PubMed] [Google Scholar]
- Kurjan J., Herskowitz I. Structure of a yeast pheromone gene (MF alpha): a putative alpha-factor precursor contains four tandem copies of mature alpha-factor. Cell. 1982 Oct;30(3):933–943. doi: 10.1016/0092-8674(82)90298-7. [DOI] [PubMed] [Google Scholar]
- Novick P., Schekman R. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1858–1862. doi: 10.1073/pnas.76.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osley M. A., Hereford L. Identification of a sequence responsible for periodic synthesis of yeast histone 2A mRNA. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7689–7693. doi: 10.1073/pnas.79.24.7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O., Cannon L. E. Presecretory and cytoplasmic invertase polypeptides encoded by distinct mRNAs derived from the same structural gene differ by a signal sequence. Proc Natl Acad Sci U S A. 1982 Feb;79(3):781–785. doi: 10.1073/pnas.79.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M., Casadaban M. J., Botstein D. Yeast genes fused to beta-galactosidase in Escherichia coli can be expressed normally in yeast. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2460–2464. doi: 10.1073/pnas.78.4.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. H., Schekman R. Lyticase: endoglucanase and protease activities that act together in yeast cell lysis. J Bacteriol. 1980 May;142(2):414–423. doi: 10.1128/jb.142.2.414-423.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy T. J., Benson S. A., Emr S. D. Mechanisms of protein localization. Microbiol Rev. 1983 Sep;47(3):313–344. doi: 10.1128/mr.47.3.313-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman S. J., Rose M., Botstein D., Fink G. R. Regulation of HIS4-lacZ fusions in Saccharomyces cerevisiae. Mol Cell Biol. 1982 Oct;2(10):1212–1219. doi: 10.1128/mcb.2.10.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague G. F., Jr, Jensen R., Herskowitz I. Control of yeast cell type by the mating type locus: positive regulation of the alpha-specific STE3 gene by the MAT alpha 1 product. Cell. 1983 Feb;32(2):409–415. doi: 10.1016/0092-8674(83)90460-9. [DOI] [PubMed] [Google Scholar]
- Stevens T., Esmon B., Schekman R. Early stages in the yeast secretory pathway are required for transport of carboxypeptidase Y to the vacuole. Cell. 1982 Sep;30(2):439–448. doi: 10.1016/0092-8674(82)90241-0. [DOI] [PubMed] [Google Scholar]
- Strathern J., Hicks J., Herskowitz I. Control of cell type in yeast by the mating type locus. The alpha 1-alpha 2 hypothesis. J Mol Biol. 1981 Apr 15;147(3):357–372. doi: 10.1016/0022-2836(81)90488-5. [DOI] [PubMed] [Google Scholar]
- Taussig R., Carlson M. Nucleotide sequence of the yeast SUC2 gene for invertase. Nucleic Acids Res. 1983 Mar 25;11(6):1943–1954. doi: 10.1093/nar/11.6.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble R. B., Maley F. Subunit structure of external invertase from Saccharomyces cerevisiae. J Biol Chem. 1977 Jun 25;252(12):4409–4412. [PubMed] [Google Scholar]
- Trimble R. B., Maley F. The use of endo-beta-N-acetylglucosaminidase H in characterizing the structure and function of glycoproteins. Biochem Biophys Res Commun. 1977 Oct 10;78(3):935–944. doi: 10.1016/0006-291x(77)90512-5. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Zimmermann F. K., Khan N. A., Eaton N. R. Identification of new genes involved in disaccharide fermentation in yeast. Mol Gen Genet. 1973;123(1):29–41. doi: 10.1007/BF00282986. [DOI] [PubMed] [Google Scholar]