Abstract
The autosomal Sry-related gene, Sox9, encodes a transcription factor, which performs an important role in testis differentiation in mammals. In several reptiles, Sox9 is differentially expressed in gonads, showing a significant upregulation during the thermo-sensitive period (TSP) at the male-promoting temperature, consistent with the idea that SOX9 plays a central role in the male pathway. However, in spite of numerous studies, it remains unclear how SOX9 functions during this event. In the present work, we developed an RNAi-based method for silencing Sox9 in an in vitro gonad culture system for the sea turtle, Lepidochelys olivacea. Gonads were dissected as soon as the embryos entered the TSP and were maintained in organ culture. Transfection of siRNA resulted in the decrease of both Sox9 mRNA and protein. Furthermore, we found coordinated expression patterns for Sox9 and the anti-Müllerian hormone gene, Amh, suggesting that SOX9 could directly or indirectly regulate Amh expression, as it occurs in mammals. These results demonstrate an in vitro method to knockdown endogenous genes in gonads from a sea turtle, which represents a novel approach to investigate the roles of important genes involved in sex determination or differentiation pathways in species with temperature-dependent sex determination.
Keywords: Sox9, Amh, RNAi, Lepidochelys olivacea, gonad culture
1. Introduction
Several species of reptiles apparently lack sex chromosomes and display temperature-dependent sex determination (TSD). In these species, the differentiation of gonads into ovaries or testes depends on the incubation temperature of the eggs during a critical period of embryonic development known as the thermo-sensitive period (TSP) [1,2]. In mammals, sex determination depends on the Sry gene [3], but no Sry homologue has been found in reptiles. Nevertheless homologues of several other mammalian sex-determining genes have been identified [4,5,6]. Among them, the autosomal Sry-related gene, Sox9, has been implicated in testis differentiation in birds [7,8] and reptiles [9,10,11,12,13]. Sox9 encodes a transcriptional activator necessary for mammalian testis development [14,15] and is a direct target of SRY [16]; since SOX9 has the ability to virtually replace the action of SRY in mammals [17], it may play a critical role in testis development in organisms displaying TSD.
In the American alligator, Alligator mississippiensis, basal levels of Sox9 expression have been observed early in the TSP at male-promoting temperature (MPT); expression was then upregulated at the end of the TSP, during testis differentiation, whereas at female-promoting temperature (FPT), Sox9 was expressed at basal levels at all stages [9]. Since upregulation occurred after sex determination and coincided with the differentiation of the testis, the authors concluded that this gene plays an important role in testis differentiation, but not in sex determination. In contrast, in the red-eared slider turtle, Trachemys scripta, Sox9 is expressed early in undifferentiated gonads at both MPT and FPT and then becomes restricted to the developing testis at the end of the TSP [11,12]. In the olive ridley sea turtle, Lepidochelys olivacea, SOX9 protein is present during the TSP in undifferentiated gonads of embryos incubated at both MPT and FPT. However, at the middle of the TSP, SOX9 remains present at MPT, while at FPT, it is downregulated. At the onset of gonadal differentiation, SOX9 protein is still present at MPT, but is not detected at FPT [10,18,19]. It has also been shown that SOX9 responds to temperature in cultured gonads exposed to sex-reversing temperature shifts in either direction (MPT to FPT and FPT to MPT) in both L. olivacea [20] and T. scripta [21].
In humans and mice, SOX9 is known to activate transcription of the anti-Müllerian hormone gene (Amh), which is critical for regression of the female structures derived from the Müllerian ducts [22], but not necessary for sex determination [23]. Consistent with this role, in T. scripta [11,12] and L. olivacea [24], it has been found that Sox9 expression precedes that of Amh. In contrast, in chicken and alligator, Sox9 follows the expression of Amh, suggesting that SOX9 is not required for the activation of Amh [25,26]. Thus, the role of SOX9 in sex determination/differentiation in reptiles remains elusive.
Gene silencing has been used to study the function of particular genes in different biological systems or backgrounds. Post-transcriptional gene silencing (PTGS) involves the repression of gene expression through specific mRNA degradation, representing an attractive strategy, because it has the potential to silence gene expression of any gene at any developmental stage. RNA interference (RNAi) has been widely used in PTGS approaches to elucidate gene function in several species [27]. While RNAi studies typically have been conducted in monolayer cell culture and in vivo, recently, the RNAi methodology has been adapted for use in organ culture of mouse kidney rudiments and ovaries [28,29].
In the present study, we developed an RNAi-based method to silence Sox9 in a turtle gonad culture system for L. olivacea. We hypothesized that if SOX9 is required for testis determination/differentiation, then Sox9 knockdown at MPT (26 °C) would result in gonad feminization evidenced by the expression of female-specific genes. Also, if SOX9 acts upstream of Amh, the latter should be downregulated after Sox9 knockdown. To evaluate the expression of a female-specific gene, we chose P-450 Aromatase (Cyp19a) as a potential candidate, since it is upregulated in the female gonad at the end of the TSP. The present study represents a novel approach to elucidate the function of genes involved in sex determination or differentiation in organisms displaying TSD.
2. Materials and Method
2.1. Egg Incubation
Freshly laid eggs of L. olivacea were obtained from La Escobilla beach in Oaxaca, Mexico, and transported to the Instituto de Investigaciones Biomédicas, UNAM, in Mexico City. Upon arrival, the eggs were divided into two batches and incubated at 26 °C (MPT) and 33 °C (FPT) in trays of moistened vermiculite.
2.2. Design of Turtle Specific Sox9 siRNAs
Using the Invitrogen siRNAs designer tool, two specific Sox9 siRNAs were designed from the transactivation domains of the L. olivacea Sox9 (GQ258676) sequence: Lo-siRNA(1) 5'-CAG CAU GAG UGA GGU UCA CUC UCC A-3', Lo-siRNA(2) 5'-UAG AGA CCU UUG ACG UAA AUG AGU U-3'. Both siRNAs were transfected together into the gonads. Additionally, we designed a scrambled control sequence: Lo-siRNA(cs) 5'-AGU CCA AGU UAU GCG UAA AGA GUU-3'.
2.3. Gonad Culture and Transfection
In order to develop a protocol to culture the gonads and knockdown Sox9, we worked first with T. scripta embryos as a preliminary approach in Florida Atlantic University, Boca Raton, FL, USA, according to Davies et al. [28] and Nayak et al. [30], and then, we adapted the protocol to L. olivacea. Since Sox9 expression has been detected prior the TSP at MPT [18], eggs were incubated until Miller’s stage (st) 23 [31]. Adrenal-kidney-gonad (AKG) complexes were dissected and floated in L-15 medium (Invitrogen, Life Technologies, Grand Island, NY, USA). The gonads were cultured at 26 °C (MPT), according to Moreno-Mendoza et al. [20]. We designed a dose curve with three different doses of siRNAs containing equimolar amounts of both siRNAs. Transfections were performed according to the Lipofectamine protocol from Invitrogen; briefly, the transfection mix was prepared using 100, 300 and 600 pmol of siRNA (both sequences) and 40 µL of Lipofectamine 2000 (Invitrogen, Life Technologies, Grand Island, NY, USA) in 600 µL of Opti-MEM (Invitrogen, Life Technologies, Grand Island, NY, USA). Similarly, the control transfection mix was prepared without siRNA and 40 µL of Lipofectamine 2000 in 600 µL of Opti-MEM. The supplemented media was removed from each well, and the transfection mix was applied directly to organ cultures. The transfected gonads were incubated at 26 °C for 72 hours.
Gonads incubated in ovo at the same temperature and stage (MPT st 23) were used as positive controls for Sox9 protein and mRNA expression assays. Since it is known that Sox9 is downregulated at FPT after st 27 [10,18], we decided to use gonads incubated in ovo at st 29 as negative controls. We also included three experimental controls in vitro (MPT st 23): CO (gonads cultured only with Opti-MEM); CL (gonads cultured with Lipofectamin and Opti-MEM); and CS (gonads transfected with the scrambled control sequence at a concentration of 300 pmol). Sox9 was examined at the protein and mRNA levels. Also, cell proliferation assays were performed by labeling with bromodeoxyuridine (BrdU), according to the manufacturer’s instructions (Roche Diagnostics, Mexico City, Mexico).
2.4. Immunofluorescence
SOX9 and cytokeratin (epithelial cells marker) were sequentially detected in 10 µm frozen sections from L. olivacea’s gonads. These were washed in PBS and incubated in 10 mM sodium citrate, pH 6, at 85 °C for 45 minutes for antigen retrieval. The slides were blocked with 5% inactivated horse serum in PBS-T (PBS/0.5% Triton) for 1 hour and subsequently incubated overnight first with rabbit anti-turtle SOX9 primary antibody diluted 1:150 at 4 °C [32]. After washing with PBS, sections were incubated with goat Cy5-conjugated anti-rabbit secondary antibody (Millipore, Mexico City, Mexico) (for 40 minutes at room temperature), then blocked with 1% horse serum in PBST for 30 minutes and incubated overnight with a mouse anti-Pan Cytokeratin Plus (AE1/AE3 + 8/18) antibody (Biocare Medical, Mexico City, Mexico) diluted 1:100 at 4 °C. Sections were then incubated with Alexa Fluor 488-conjugated anti-mouse secondary antibody (Invitrogen, Molecular Probes, Grand Island, NY, USA) for 40 minutes at room temperature. Cell nuclei were stained with TOTO-3iodide (Invitrogen, Molecular Probes, Grand Island, NY, USA). Frozen sections of gonads of embryos incubated at FPT (stage 29) and MPT (st 23) in ovo were included as negative and positive controls, respectively, for the presence of Sox9 protein. Additionally, proliferative cells were detected using an anti-BrdU antibody (Roche Diagnostics, Mexico City, Mexico); this immunofluorescence required pre-treatment with 2N HCl for 30 minutes at 37 °C prior to blocking. The images were captured and processed with a confocal Pascal LSM5-Zeiss microscope.
2.5. Quantitative PCR
Total RNA was extracted using Trizol reagent (Invitrogen, Accesolab, Mexico City, Mexico), followed by DNAse I treatment. cDNA was reverse-transcribed using random primers and the MMLV reverse transcriptase (Promega, Uniparts, Mexico City, Mexico). Primers used to assay gene expression were designed from the transactivation domain of L. olivacea Sox9 (Forward: 5'-GGG AAG ACA ACC GCC ACA CAT TG-3'; Reverse: 5'-GGC GTG CTG CTG ATC CCA TA-3'), from L. olivacea Amh (Forward: 5'-AGA AGT TCC AGG CTG TCA TCC A-3'; Reverse: 5'-ACC TTC CTC TTC TCC TGC CAG T-3') and from L. olivacea Aromatase (Forward: 5'-TGA AAA ACT GGA AGA CCA CAT GGA-3'; Reverse: 5'-TTC CAC TTT AGG GTG CTC TGC AAT-3'), amplifying fragments of 200, 217 and 181 bp, respectively. Gene expression was quantified with a SmartCycler (Cepheid, BioSelec, Mexico City, Mexico) using EvaGreen® (BioRad, Mexico City, Mexico). Individual samples were analyzed in duplicate, and β-actin was used as internal control [18]. PCR conditions were as follows: one cycle at 95 °C 30 seconds, 40 cycles at 95 °C, 5 seconds, and 60 °C, 20 seconds. Relative gene expression levels were calculated through the comparative ΔCT method according to Livak and Schmittgen [33]. ΔCT values were converted to the linear form using the 2−ΔCT equation [33]. One-way ANOVA coupled to an all pairwise multiple comparison analysis (Holm-Sidak test) was performed using 2−ΔCT data to find statistical differences in gene expression between groups (p < 0.05).
3. Results and Discussion
In order to develop a protocol to knockdown Sox9 in a gonad organ culture system, we worked first with the non-endangered species, T. scripta, based on previous reports [28,30], as a preliminary approach. To optimize conditions, we performed different assays, varying the amount of siRNA and Lipofectamine; also, we tested different culture media and incubation times with siRNA. Once the protocol was established, we adapted it to L. olivacea.
3.1. Sox9 Protein and mRNA Expression
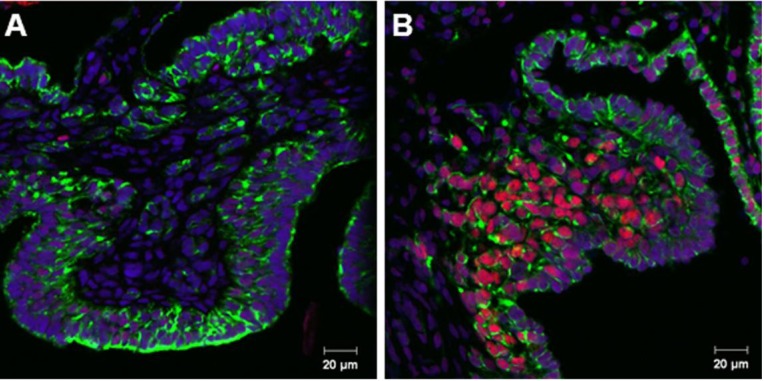
Consistent with previous reports of SOX9 expression in L. olivacea gonads [10], female gonads (st 29) did not show a SOX9 signal, neither in medullar cords or in the surface epithelium (Figure 1A), whereas male gonads (st 23) showed a positive and specific SOX9 signal in medullar cords, but not in the surface epithelium (Figure 1B).
Figure 1.
Double immunofluorescence of SOX9 (red) and cytokeratin (green) in gonads from L. olivacea in ovo. (A) Gonads of embryos incubated at female-promoting temperature (FPT) Miller’s stage (st) 29 and (B) male-promoting temperature (MPT) st 23.
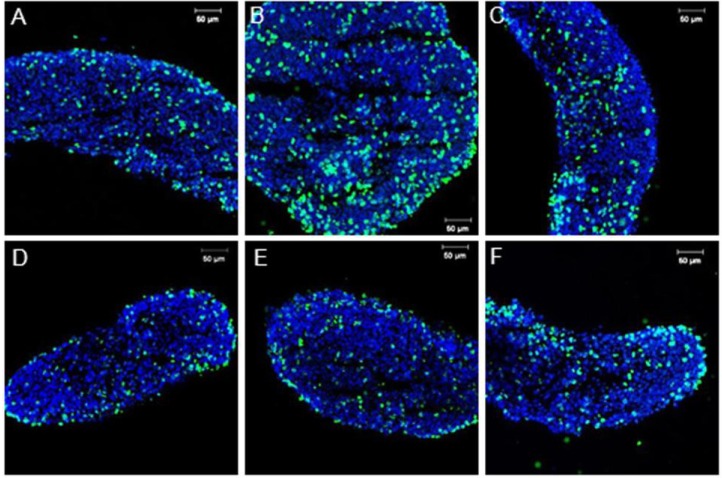
Similar results were shown in vitro with the experimental controls in which CO, CL and CS showed a positive SOX9 signal only in medullar cords (Figure 2A–C, respectively). These results suggest that neither the in vitro culture media nor the Lipofectamine or the control scrambled siRNA affected SOX9 protein expression. siRNA transfection in L. olivacea cultured gonads resulted in a decrease of SOX9 protein in the three concentrations tested (Figure 2D–F). These results, although not quantitative, suggest that Sox9 expression was (at least partially) blocked. BrdU incorporation in experimental controls (Figure 3A–C) and transfected gonads (Figure 3D–F) seemed similar; although cell death due to toxicity was not assessed in this study, these results (though not quantitative) showed comparable cell proliferation of Sox9-expressing cells in all experiments.
Figure 2.
Double immunofluorescence of SOX9 (red) and cytokeratin (green) in gonads from L. olivacea cultured with (A) Opti-MEM, CO, (B) Lipofectamin and Opti-MEM, CL, (C) transfected with the scrambled control sequence, CS, and (D–F) transfected with 100, 300 and 600 pmol of siRNA, respectively. Scale bar = 20 µm.
Figure 3.
Immunofluorescence of BrdU in experimental controls (A) CO, (B) CL, (C) CS; and (D–F) gonads transfected with 100, 300 and 600 pmol of siRNA, respectively. Scale bar = 20 µm.
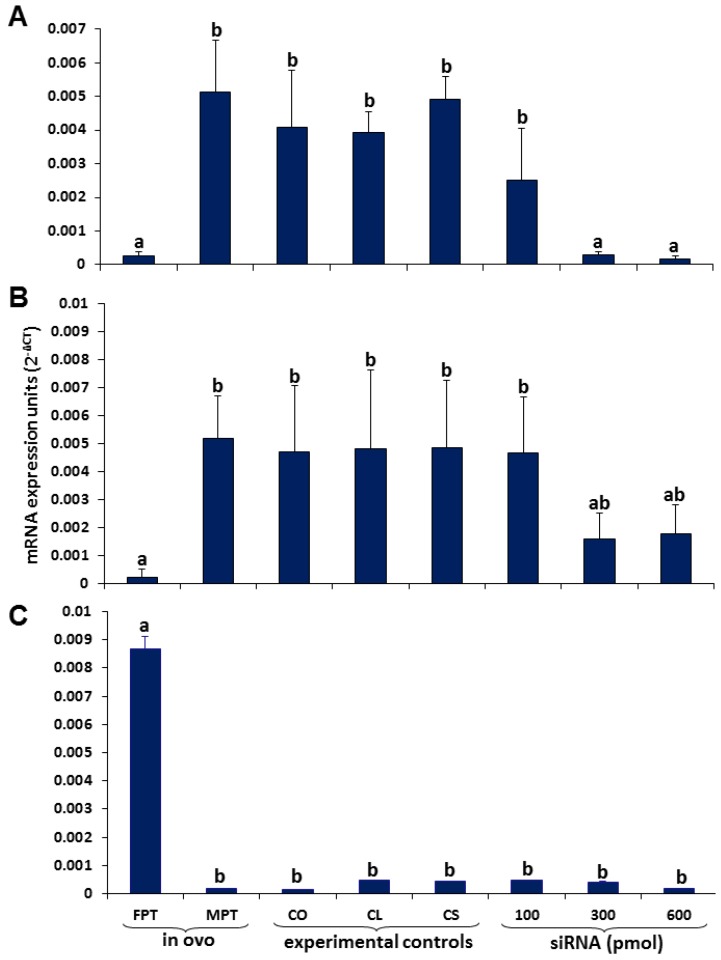
Consistent with our results and with a previous report [18], qPCR analysis showed significantly higher mRNA levels of Sox9 in gonads incubated in ovo at MPT (st 23) than in gonads at FPT (st 29; p < 0.001; Figure 4A). Sox9 expression levels in two of the experimental controls (CO and CL) were slightly lower than those in the in vivo control at MPT; nevertheless, the differences were not significant (p = 0.22 and 0.442 for CO and CL, respectively); the experimental control, CS, showed similar expression levels to the in vivo control incubated at MPT (p = 0.819) (Figure 4A). We observed a decrease of Sox9 mRNA levels in transfected gonads. The decrease observed with the lowest siRNA concentration (100 pmol) was not significant; however, higher concentrations of siRNAs (300 and 600 pmol) were significantly lower than the in vivo control at MPT and the three experimental controls (CO, CL and CS) (p < 0.001, Figure 4A). Yet, they did not show significant differences between each other (p = 0.966), suggesting that 300 pmol was enough to knockdown Sox9. Off-target effects were not detected.
Figure 4.
Relative expression of (A) Sox9, (B) Amh and (C) Aromatase in L. olivacea showing a decreased expression of Sox9 and Amh in gonads transfected with 300 and 600 pmol of siRNA. Aromatase expression levels remained low in all treatments, except at FPT. Different letters indicate significant differences in expression levels. n = 3 for each group.
Previously, Davies et al. [28] adapted this technique in an organ culture system of kidney rudiments of mice to investigate a regulator of renal development, Wt1. A similar study was conducted more recently by Wang et al. [29] in which they reduced Hnrnpk mRNA levels using siRNAs in neonatal ovaries of mice in culture. In both studies, the authors not only showed a decrease in the target protein, but also showed a phenotypic effect. Unfortunately, in the present study, we were not able to observe any morphological effect in cultured gonads, probably due to the short time the gonads were maintained in culture; in 72 hours, the gonads must have changed at most from st 23 to st 24 at MPT. Nevertheless, in an effort to detect any effect of Sox9 silencing in gonad feminization, we decided to evaluate the expression of a presumably SOX9-regulated gene, the Anti-Müllerian Hormone (Amh), which is responsible for the regression of the Müllerian duct in the male gonad, and a female-specific gene, P-450 Aromatase, even if previous works indicate that expression levels of the latter increase in the female gonads from other reptiles at the end of the TSP [34], which in L. olivacea would be st 25–26 at MPT and st 26–27 at FPT [23].
3.2. Coordinated Expression of Sox9 and Amh
It has been suggested that in T. scripta and L. olivacea, SOX9 regulates the expression of Amh as it occurs in mammals [22]. This has been suggested, due to the expression profiles of both genes, higher at MPT than at FPT, during and after the TSP [11,12,18], and, also, due to a pattern observed in their expression levels, in which Sox9 expression precedes that of Amh [12,23]. Consistent with these observations and our results regarding Sox9 expression, we found higher expression levels of Amh in gonads incubated at MPT (st 23) than gonads incubated at FPT (st 29) (p < 0.001, Figure 4B) in ovo. In the experimental controls, in vitro Amh expression levels were similar to those observed in the in vivo control incubated at MPT (p = 0.582, 0.518 and 0.235 in CO, CL and CS, respectively). Interestingly, we found a decrease of Amh mRNA levels in response to Sox9 siRNA in gonads transfected with 300 and 600 pmol; although maximal decreases were detected at 300 pmol, no significant differences were detected between this concentration and experimental controls (p = 0.073, 0.089 and 0.068 for CO, CL and CS, respectively) or between 300 and 600 pmol (p = 0.908, Figure 4B). Further investigations are required to determine whether SOX9, directly or indirectly, influences Amh expression.
It has been shown that in L. olivacea, AMH protein and its transcript are detected in medullar cords of embryos (st 27) incubated at MPT [23], coinciding with the site of SOX9 protein detection [10] and also with Sox9 mRNA localization [23]. These observations suggest that in L. olivacea, the mechanisms mediating male reproductive tract development (including Sertoli cell differentiation and Müllerian duct regression) involve the coordinated action of both genes. First, SOX9 triggers Sertoli cell differentiation and then activates Amh expression, leading to the regression of the Müllerian duct as it occurs in mammals [35]. It is worth mentioning that in mammals, AMH is not required for Sertoli cell differentiation, since human individuals harboring a homozygous mutation for the AMH gene show Müllerian duct persistence, but normal Sertoli cells [35].
3.3. Aromatase Expression
Aromatase, a CYP450 enzyme, is responsible for the conversion of androgens into estrogens. Previously, it has been shown that both aromatase expression and activity increase at the end of the TSP in gonads incubated at FPT, while at MPT, they remain low, suggesting a role for differential estrogen production in ovarian determination/differentiation in marine and fresh water turtles, such as Emys orbicularis [36], Dermochelys coriacea [37], Malaclemys terrapin [38], Chelydra serpentina [39], T. scripta [40] and Chrysemys picta [41]. Since aromatase expression increases in the female gonad, we expected even a mild upregulation after Sox9 knockdown, suggesting a possible feminization of the gonads. However, aromatase expression levels did not significantly change in gonads treated with the three different doses of siRNA (Figure 4C); instead, they remained low, as in the in ovo control incubated at MPT (st 23) and experimental controls, CO, CL and CS. This is consistent with the absence of morphological effects, since the role of aromatase has been linked to ovarian differentiation. As explained above, this could be due to the short time the gonads were maintained in culture (72 hours). It is known that in L. olivacea, the TSP at MPT lasts approximately six days [23], and also, it has been shown that in several turtles, aromatase upregulation is presented at the end of the TSP at FPT [36,37,38,39,40,41]. Thus, in the present work, it was not possible to reach the onset of aromatase upregulation; therefore, the potential effect of Sox9 silencing on aromatase expression in cultured gonads was not detected.
It has been previously shown that female specific genes, such as FoxL2, are upregulated after Sox9 knockdown in mouse gonads maintained in culture for three days [42]; moreover, it is known that aromatase is positively regulated by FOXL2 in the fetal goat ovary [43]. It is likely that in reptiles, a similar gene network is participating in the developing ovary, where a repression of Sox9 could lead to a concomitant upregulation of FoxL2 and aromatase; therefore, it is recommended to maintain the gonads in culture for a longer period of time (evaluating at the same time the duration of the silencing effect), taking into account the slow rate of development that has been shown in L. olivacea [20].
4. Conclusions
We have established a gene-specific knockdown method to investigate if SOX9 is required for testis determination/differentiation; this question remains to be fully answered, since gonads need to be maintained in culture for a longer period of time in order to differentiate, and expression of female-specific genes should be measured. Nevertheless, we were able to measure Amh expression under a Sox9 knockdown environment. The regression of the Müllerian duct by AMH is an important event in the male reproductive system differentiation pathway. This first report of Sox9 gene silencing by RNAi in a gonad culture system of turtles displaying TSD shows a coordinated expression of both genes, supporting the hypothesis that SOX9 directly or indirectly regulates Amh. Although possible off-target effects and the viability of the transfected organs remain to be further investigated, the demonstrated efficacy of this technique in turtle gonadal cultures expands our toolbox to address critical questions of sex determination pathways in TSD.
Acknowledgments
We thank Thane Wibbels and Jeanette Wyneken for their help in obtaining the T. scripta nests; to Martha Harfush, Cuauhtémoc Peñaflores and Elpidio López for assistance in collecting the L. olivacea nest; to DGVS-SEMARNAT for capture permits; to Demetri Spyropoulos, the five anonymous reviewers and the guest editor, Célia Baroux, for their constructive comments to this manuscript; and to Rubí Hernández and Alejandro Marmolejo for technical assistance. We also thank CIAD and CONACYT (Mexico) and PAPIIT-UNAM (IN205213) for the financial support granted to ISR and HML.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Wibbels T. Critical approaches to sex determination in Turtles. In: Lutz P.L., Musik J.A., Wineken J., editors. The Biology of Sea Turtles. Volume 2. CRC Press Inc; Boca Ratón, FL, USA: 2003. pp. 103–134. [Google Scholar]
- 2.Pieau C., Dorizzi M. Oestrogens and temperatura-dependent sex determination in reptiles: All is in the gonads. J. Endocrinol. 2004;181:367–377. doi: 10.1677/joe.0.1810367. [DOI] [PubMed] [Google Scholar]
- 3.Koopman P., Gubbay J., Vivian N., Goodfellow P., Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351:117–121. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- 4.Shoemaker C., Crews D. Analyzing the coordinated gene network underlying temperature-dependent sex determination in Reptiles. Semin. Cell. Dev. Biol. 2009;20:293–303. doi: 10.1016/j.semcdb.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhen T., Schroeder A. Molecular mechanisms of sex determination in reptiles. Sex. Dev. 2010;4:16–28. doi: 10.1159/000282495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chue J., Smith C.A. Sex determination and sexual differentiation in the avian model. FEBS J. 2011;278:1027–1034. doi: 10.1111/j.1742-4658.2011.08032.x. [DOI] [PubMed] [Google Scholar]
- 7.Kent J., Wheatley S.C., Andrews J.E., Sinclair A.H., Koopman P. A male-specific role for SOX9 in vertebrate sex determination. Development. 1996;122:2813–2822. doi: 10.1242/dev.122.9.2813. [DOI] [PubMed] [Google Scholar]
- 8.Morais da Silva S., Hacker A., Harley V., Goodfellow P., Swain A., Lovell-Badge R. Sox9 expression during gonadal development implies a conserved role for gene in testis differentiation in mammals and birds. Nat. Genet. 1996;14:62–68. doi: 10.1038/ng0996-62. [DOI] [PubMed] [Google Scholar]
- 9.Western P.S., Harry J.L., Graves J.A.M., Sinclair A.H. Temperature-dependent sex determination: Upregulation of SOX9 expression after commitment to male development. Dev. Dyn. 1999;214:171–177. doi: 10.1002/(SICI)1097-0177(199903)214:3<171::AID-AJA1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 10.Moreno-Mendoza N., Harley V.R., Merchant-Larios H. Differential expression of SOX9 in gonads of the sea turtle Lepidochelys olivacea at male- or female- promoting temperatures. J. Exp. Zool. 1999;284:705–710. doi: 10.1002/(SICI)1097-010X(19991101)284:6<705::AID-JEZ12>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 11.Shoemaker C.M., Queen J., Crews D. Response of candidate sex-determinig genes to changes in temperature reveals their involvement in the molecular network undelying temperature-dependent sex determination. Mol. Endocrinol. 2007;21:2750–2763. doi: 10.1210/me.2007-0263. [DOI] [PubMed] [Google Scholar]
- 12.Shoemaker C., Ramsey M., Queen J., Crews D. Expression of Sox9, Mis, and Dmrt1, in the gonad of a species with temperature-dependent sex determination. Dev. Dyn. 2007;236:1055–1063. doi: 10.1002/dvdy.21096. [DOI] [PubMed] [Google Scholar]
- 13.Barske L.A., Capel B. Estrogen represses SOX9 during sex determination in the red-eared slider turtle Trachemys scripta. Dev. Biol. 2010;341:305–314. doi: 10.1016/j.ydbio.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Harley V.R., Clarkson M.J., Argentaro A. The molecular action and regulation of the testis-determining factors, SRY (sex determining region on the Y chromosome) and SOX9 [SRY-related high-mobility group (HMG) box 9] Endocrinol. Rev. 2003;24:466–487. doi: 10.1210/er.2002-0025. [DOI] [PubMed] [Google Scholar]
- 15.Sim H., Argentaro A., Harley V.R. Boys, girls and shuttling of SRY and SOX9. Trends Endocrinol. Metab. 2008;19:213–222. doi: 10.1016/j.tem.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Sekido R., Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. 2008;453:930–934. doi: 10.1038/nature06944. [DOI] [PubMed] [Google Scholar]
- 17.Vidal V.P., Chaboissier M.C., de Rooji D.G., Schedl A. Sox9 induces testis development in XX transgenic mice. Nat. Genet. 2001;28:216–217. doi: 10.1038/90046. [DOI] [PubMed] [Google Scholar]
- 18.Torres-Maldonado L.C., Moreno-Mendoza N., Landa A., Merchant-Larios H. Timing of SOX9 downregulation and female sex determination in gonads of the sea turtle Lepidochelys olivacea. J. Exp. Zool. 2001;290:498–503. doi: 10.1002/jez.1093. [DOI] [PubMed] [Google Scholar]
- 19.Torres-Maldonado L.C., Landa-Piedra A., Moreno-Mendoza N., Marmolejo-Valencia A., Meza-Martínez A., Merchant-Larios H. Expression profiles of Dax1, Dmrt1, and Sox9 during temperature sex determination in gonads of the sea turtle Lepidochelys olivacea. Gen. Comp. Endocrinol. 2002;129:20–26. doi: 10.1016/S0016-6480(02)00511-7. [DOI] [PubMed] [Google Scholar]
- 20.Moreno-Mendoza N., Harley V.R., Merchant-Larios H. Temperature regulates SOX9 expression in cultured gonads of Lepidochelys olivacea, a species with temperature sex determination. Dev. Biol. 2001;229:319–326. doi: 10.1006/dbio.2000.9952. [DOI] [PubMed] [Google Scholar]
- 21.Shoemaker-Daly C.M., Jackson K., Yatsu R., Matsumoto Y., Crews D. Genetic network underlying temperature-dependent sex determination is endogenously regulated by temperature in isolated cultured Trachemys scripta gonads. Dev. Dyn. 2010;239:1061–1075. doi: 10.1002/dvdy.22266. [DOI] [PubMed] [Google Scholar]
- 22.De Santa Barbara P., Bonneaud N., Boizet B. Direct interaction of SRY-related protein SOX9 and Steroidogenic factor 1 regulates transcription of the human anti-Mullerian hormone gene. Mol. Cell Biol. 1998;18:6653–6718. doi: 10.1128/mcb.18.11.6653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Behringer R.R., Finegold M.J., Cate R.L. Müllerian-inhibiting substance, function during mammalian sexual development. Cell. 1994;79:415–425. doi: 10.1016/0092-8674(94)90251-8. [DOI] [PubMed] [Google Scholar]
- 24.Merchant-Larios H., Díaz-Hernández V., Marmolejo-Valencia A. Gonadal morphogenesis and gene expression in reptiles with temperature-dependent sex determination. Sex. Dev. 2010;4:50–61. doi: 10.1159/000276768. [DOI] [PubMed] [Google Scholar]
- 25.Oreal E., Pieau C., Mattei M.G., Josso N., Picard J.Y., Eusébe D.C., Magre S. Early expression of Amh in chicken embryonic gonads precedes testicular Sox9 expression. Dev. Dyn. 1998;212:522–532. doi: 10.1002/(SICI)1097-0177(199808)212:4<522::AID-AJA5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 26.Western P.S., Sinclair A.H. Sex, genes and heat: Triggers of diversity. J. Exp. Zool. 2001;290:624–631. doi: 10.1002/jez.1113. [DOI] [PubMed] [Google Scholar]
- 27.Sifuentes-Romero I., Milton L.S., García-Gasca A. Post-trasncriptional gene silencing by RNA interferance in non-mammalian vertebrate systems: Where do we stand? Mut. Res. 2011;728:158–171. doi: 10.1016/j.mrrev.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Davies J.A., Ladomery M., Hohenstein P., Michael L., Shafe A., Spraggon L., Hastie N. Development of an siRNA-based method for repressing specific genes in renal organ culture and its use to show that the Wt1 tumour suppressor is required for nephron differentiation. Hum. Mol. Genet. 2004;13:235–246. doi: 10.1093/hmg/ddh251. [DOI] [PubMed] [Google Scholar]
- 29.Wang N., Zhang P., Guo X., Zhou Z., Sha J. Hnrnpk, a protein differentially expressed in immature rat ovarian development, is required for normal primordial follicle assembly and development. Endocrinology. 2011;152:1024–1035. doi: 10.1210/en.2010-0797. [DOI] [PubMed] [Google Scholar]
- 30.Nayak G., Prentice H.M., Milton S.L. Role of neuroglobin in regulating reactive oxygen species in the brain of the anoxic-tolerant turtle Trachemys scripta. J. Neurochem. 2009;110:603–612. doi: 10.1111/j.1471-4159.2009.06157.x. [DOI] [PubMed] [Google Scholar]
- 31.Miller D. Embryology of marine Turtles. In: Gans C., Billet F., Maderson P.F.A., editors. Biology of the Reptilia. Wiley; New York, NY, USA: 1985. pp. 270–328. [Google Scholar]
- 32.Díaz-Hernández V., Marmolejo-Valencia A., Harfush M., Merchant-Larios H. Formation of the genital ridges is preceded by a domain of ectopic Sox9-expressing cells in Lepidochelys olivacea. Dev. Biol. 2012;361:156–166. doi: 10.1016/j.ydbio.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−2ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 34.Lance V.A. Is regulation of Aromatase expression in reptiles the key to understanding temperature-dependent sex determination? J. Exp. Zool. 2009;311:314–322. doi: 10.1002/jez.465. [DOI] [PubMed] [Google Scholar]
- 35.Josso N., Belville C., di Clemente N., Picard J.Y. AMH and AMH receptor defects in persistent Müllerian duct syndrome. Hum. Reprod. Update. 2005;11:351–356. doi: 10.1093/humupd/dmi014. [DOI] [PubMed] [Google Scholar]
- 36.Desvages G., Pieau C. Aromatase activity in gonads of turtle embryos as a function of the incubation temperature of eggs. J. Steroid Biochem. Mol. Biol. 1992;41:851–853. doi: 10.1016/0960-0760(92)90437-N. [DOI] [PubMed] [Google Scholar]
- 37.Desvages G., Girondot M., Pieau C. Sensitive stages for the effects of temperature on gonadal aromatase activity in embryos of marine turtle Dermochelys coriacea. Gen. Comp. Endocrinol. 1993;92:54–61. doi: 10.1006/gcen.1993.1142. [DOI] [PubMed] [Google Scholar]
- 38.Jeyasuria P., Roosenburg W.M., Place A.R. Role of P-450 Aromatase in sex determination of the diamondback terrapin, Malaclemys terrapin. J. Exp. Zool. 1994;270:95–111. doi: 10.1002/jez.1402700111. [DOI] [PubMed] [Google Scholar]
- 39.Rhen T., Metzger K., Schroeder A., Woodward R. Expression of putative sex-determining genes during the thermosensitive period of gonad development in the snapping turtle, Chelydra serpentina. Sex Dev. 2007;1:255–270. doi: 10.1159/000104775. [DOI] [PubMed] [Google Scholar]
- 40.Ramsey M., Crews D. Adrenal-kidney-gonad complex measurements may not predict gonad-specific changes in gene expression patterns during temperature-dependent sex determination in the red-eared slider turtle (Trachemys scripta elegans) J. Exp. Zool. A Ecol. Gen. Phys. 2007;307:463–470. doi: 10.1002/jez.399. [DOI] [PubMed] [Google Scholar]
- 41.Valenzuela N., Neuwald J.L., Literman R. Transcriptional evolution underlying vertebrate sexual development. Dev. Dyn. 2012;242:307–326. doi: 10.1002/dvdy.23897. [DOI] [PubMed] [Google Scholar]
- 42.Ryan J., Ludbrook L., Wilhelm D., Sinclair A., Koopman P., Bernard P., Harley V.R. Analysis of gene function in cultured embryonic mouse gonads using nucleofection. Sex Dev. 2011;5:7–15. doi: 10.1159/000322162. [DOI] [PubMed] [Google Scholar]
- 43.Veitia R.A. FoxL2 versus Sox9: A lifelong “battle of the sexes”. BioEssays. 2010;32:375–380. doi: 10.1002/bies.200900193. [DOI] [PubMed] [Google Scholar]