Abstract
Objective: To develop a closed, automated system that standardizes the processing of human adipose tissue to obtain and concentrate regenerative cells suitable for clinical treatment of thermal and radioactive burn wounds.
Approach: A medical device was designed to automate processing of adipose tissue to obtain a clinical-grade cell output of stromal vascular cells that may be used immediately as a therapy for a number of conditions, including nonhealing wounds resulting from radiation damage.
Results: The Celution® System reliably and reproducibly generated adipose-derived regenerative cells (ADRCs) from tissue collected manually and from three commercial power-assisted liposuction devices. The entire process of introducing tissue into the system, tissue washing and proteolytic digestion, isolation and concentration of the nonadipocyte nucleated cell fraction, and return to the patient as a wound therapeutic, can be achieved in approximately 1.5 h. An alternative approach that applies ultrasound energy in place of enzymatic digestion demonstrates extremely poor efficiency cell extraction.
Innovation: The Celution System is the first medical device validated and approved by multiple international regulatory authorities to generate autologous stromal vascular cells from adipose tissue that can be used in a real-time bedside manner.
Conclusion: Initial preclinical and clinical studies using ADRCs obtained using the automated tissue processing Celution device described herein validate a safe and effective manner to obtain a promising novel cell-based treatment for wound healing.

John K. Fraser, PhD
Introduction
Acute cutaneous radiation syndrome is associated with unpredictable waves of inflammation that lead to progressive expansion of the necrotic zone often creating what has been described as insurmountable technical difficulties in application of conventional surgical remedies such as skin flaps and grafts.1 Local tissue ischemia, telangiectasia, and progressive fibrosis can lead to development of chronic cutaneous sequelae, including the development of open wounds. Indeed, cutaneous sequelae were the primary cause of death in a substantial number of patients after radiation release during the accident at the Chernobyl nuclear reactor.2 In recent years, it has become apparent that mesenchymal stem cells (MSCs), a population of adherent cells obtained following culture of bone marrow, have characteristics that may provide benefit in cutaneous radiation syndrome. In particular, these cells have the capacity to modulate the immune system and to express growth factors involved in angiogenesis and wound healing.3 This has led to their clinical use in a small number of patients with cutaneous wounds, including those associated with radiation exposure.1,4 However, this approach is limited by the time and cost required to expand these cells in culture.
Approximately 14 years ago, it was discovered that human adipose tissue contains a population of cells that shares many of the key properties of MSCs.5,6 These cells, referred to as adipose-derived stem cells (or adipose-derived stromal cells; ADSCs), are prepared by first obtaining the stromal vascular fraction (SVF) of adipose tissue by enzymatic digestion and then placing the SVF cells in culture conditions similar to those used to expand MSCs. However, subsequent work has shown that the cells that expand to form ADSCs are two or more orders of magnitude more frequent in adipose tissue than the equivalent cells in marrow.7,8 The high frequency of these cells within the SVF population suggests that cell culture might not be necessary when employing adipose tissue as the source of starting material. This suggestion has now been confirmed in a wide range of preclinical and clinical studies, including acute and chronic myocardial ischemia,9,10 renal ischemia,11 pulmonary hypertension,12 bone,13 intervertebral disc injury,14 peripheral nerve regeneration,15 and wound healing.16 These cells have also been applied clinically in patients with heart attack,17 breast reconstruction,18 bone healing,19,20 and wound healing.21,22 In addition to eliminating the time and costs associated with clinical-grade cell culture, this approach generates a population of cells that is not comprised solely of ADSCs, but also includes a substantial number of other cell types with therapeutic potential, including vascular endothelial cells, tissue macrophages, and so forth.23
This work has led to the use of adipose-derived SVF in patients with refractory cutaneous ulcers arising from local radiation exposure.21,24,25 This approach has a number of important advantages; removal of the need for cell culture eliminates delay by allowing tissue harvest, processing, and treatment to occur within the same surgical procedure, while also reducing costs associated with expensive clinical-grade tissue culture reagents and systems.26,27
Clinical Problem Addressed
Clinical application of SVF cells requires that they be prepared using a clinical-grade processing system and reagents within a standardized methodology. Ideally, the system, reagents, and methods used will generate a consistent, reproducible cell product rich in SVF cells. We refer to cells processed in such a manner as adipose-derived regenerative cells (ADRCs) as a means to distinguish them from the cell population obtained using nonclinical-grade enzyme reagents, open nonautomated system processes, or where consistent cell product composition has not been validated. The Celution® System provides for real-time adipose tissue processing at the patient's bedside or in a cell processing facility using an automated, functionally closed sterile fluid pathway system clinical-grade reagent, and a sterile, single-use consumable to generate high-quality ADRCs, which can be applied in the treatment of a number of disorders, including radiation-related injuries.
Materials and Methods
Adipose tissue collection
Adult human subcutaneous adipose tissue was obtained after informed consent from donors undergoing elective cosmetic liposuction. Tissue harvest was performed following infiltration of a wetting solution (usually a lactated Ringers solution supplemented with lidocaine and epinephrine) through a 0.5 cm stab incision at a volume of one volume of wetting solution for each volume of tissue to be aspirated. Tissue was then aspirated in accordance with the surgeon's standard practices (details below). Once sufficient tissue was obtained, the stab incisions were closed with a simple suture and pressure was applied.
Tissue harvest comparison studies were performed by collecting tissue from the same donor with simple aspiration using a handheld 60-mL Toomey syringe with a 3-mm cannula and with waterjet-assisted aspiration (BodyJet™; Human Med AG, Schwerin, Germany) with either a 3- or 4-mm two-hole Mercedes tip cannula applying aspiration at a pressure of ∼15–20 inHg (∼301–508 mmHg).
Adipose tissue processing
The Celution System (Cytori Therapeutics, San Diego, CA) was used to obtain ADRCs according to the manufacturer's instructions. Tissue processing within the Celution device uses a single-use sterile disposable set and the Celase® processing enzyme reagent (a proprietary proteolytic reagent optimized for isolation of cells from aspirated adipose tissue). The Celase reagent is validated to be free of risk of contamination with bovine spongiform encephalitis and is provided as a sterile USP Class VI product. Once the Celution disposable is placed within the device, the system performs a wet test to ensure the integrity of the closed system. Tissue is then introduced into the processing canister where it is weighed and then washed with the lactated Ringers solution to remove the residual wetting solution and extravasated blood. The Celution device calculates the amount of Celase reagent to be used (based on tissue weight) and then, at the appropriate stage, prompts the operator to add the required volume of Celase. The tissue is continuously agitated during enzymatic digestion of the connective tissue. Once digestion is complete, the ADRC fraction is pumped into a centrifuge chamber where it is washed and concentrated. The final cell product can then be aspirated from the chamber in a volume of 5 mL. The Celution 800 disposable and device are shown in Fig. 1.
Figure 1.
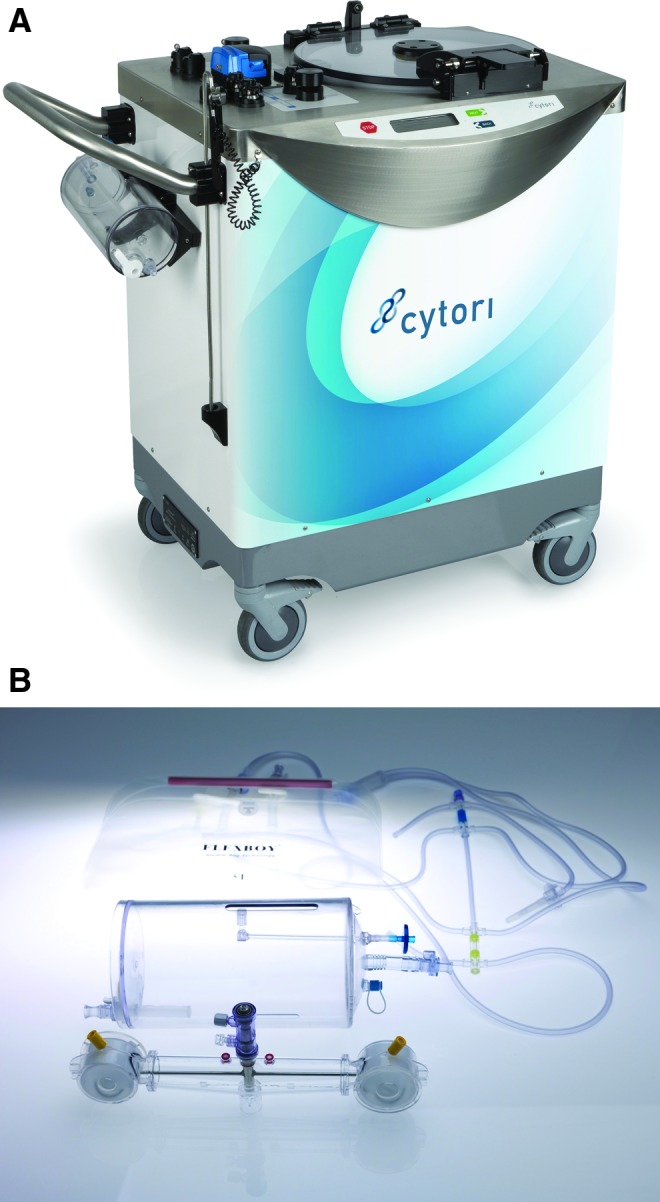
(A) The Celution processing device. (B) Single-use disposable processing set in which adipose is enzymatically processed to release and concentrate adipose-derived regenerative cells (ADRCs). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
To evaluate donor-to-donor variability, tissue from a series of 31 donors (average age 44 years; range 20–81 years) was processed and the cell yield and viability were assessed. To evaluate the effect of tissue collection site on output, tissue from 12 different donors was collected at three different clinics (four patients/clinic). The tissue was processed within the Celution System and evaluated by flow cytometry to compare the composition of cell types within the ADRC population.28
Ultrasonic energy processing
To evaluate the ability of ultrasound energy as a substitute for enzymatic digestion, adipose tissue from five healthy, normal, human donors was obtained by standard vacuum-assisted lipoaspirate and allowed to gravity settle. Tissue was then transferred into 50-mL sterile, polypropylene conical centrifuge tubes (BD Falcon, BD Biosciences, San Jose, CA). Ultrasonic energy was applied using a Hielscher UP200S ultrasonic processor with a 14 mm sonotrode placed at each of the three different depths within the tube (15, 30, and 45 mL marks) to ensure distribution of energy throughout the tissue. Following application of the ultrasound energy, samples were diluted with the Lactated Ringer's solution (LR) at 1:1 volume, transferred into 50-mL conical tubes, and then centrifuged at 400 g for 10 min. An initial assessment of higher ultrasound energy settings in pilot studies resulted in significant tissue temperature elevation and cellular disruption leading to a cell death rate exceeding 90%. Therefore, only three settings were used for further studies: low ultrasonic energy (35% amplitude, with an energy disbursement pattern of 5 s on/10 s off for 1 min); medium energy (55% amplitude, with an energy disbursement pattern of 5 s on/10 s off for 1 min); and a negative control in which tissue was processed as above, but with zero ultrasound energy (device not powered on). Tissue from the same donors was also processed using the Celution System.
ADRC analysis
The number and relative viability of ADRCs recovered from tissue processing samples in each study were determined using a Semiautomated Cell Counter (NucleoCounter Mammalian Cell Counter; New Brunswick Scientific, Enfield, CT) according to the manufacturer's instructions. In our hands, the NucleoCounter provides an accurate and reproducible enumeration of viable nucleated cell count that avoids the potentially confounding effects of debris, red blood cells, and lipid droplets.28 The assessment of adipose-derived stromal cell frequency within ADRCs was determined using the standard fibroblast colony-forming unit assay (CFU-F) method.28
Results
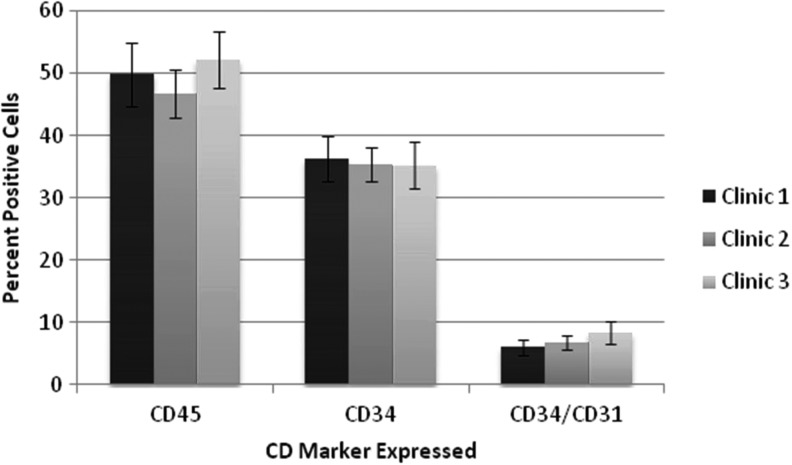
Reliability and reproducibility of viability, yield, and composition were established by processing tissue from 31 donors (average age 44 years; range 20–81 years). Results showed an average cell yield of 3.6±1.8×105 cells/g of tissue processed (range 1.5–7.7×105/g) with viability of 84.7% (range 71.3–95.3%). Consistent with this, a smaller study evaluating ADRCs from tissue collected from 12 donors at three different clinics showed that the viable nucleated cell yield ranged from 3.32×105 to 5.63×105 with cell viability of 82.4–88.0%. Most importantly, flow cytometric analysis of the cells obtained from each process demonstrated that all major cell populations (populations that comprise ∼90% of the ADRCs) were present at essentially the same frequency in the ADRC population irrespective of the clinic in which the tissue was collected (Fig. 2). These findings are consistent with published data for the Celution System.23
Figure 2.
Chart demonstrating the interlaboratory reproducibility of the Celution System to generate autologous human ADRCs of equivalent identity based upon flow cytometric assessment of cell surface protein expression. Each column denotes the percentage of cells expressing that marker or combination of markers.
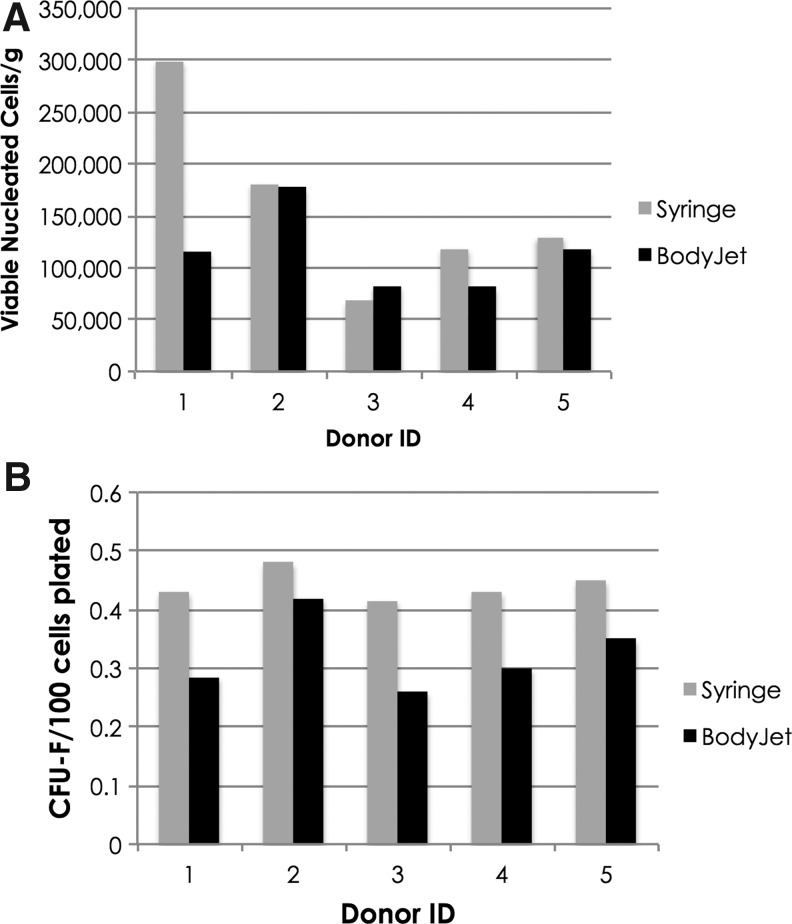
Processing tissue collected from the same donors using both syringe harvest and the BodyJet System showed no significant difference in the ADRC yield (viable nucleated cells per gram of tissue processed; Fig. 3). However, the frequency of CFU-F cells in samples collected by BodyJet was consistently lower compared with the syringe.
Figure 3.
Yield and composition of cells processed within the Celution System from adipose tissue collected using either the BodyJet™ System or manual syringe aspiration. (A) Yield of viable nucleated cells per gram of tissue processed; (B) fibroblast colony-forming unit (CFU-F) frequency (number of CFU-F colonies per 100 viable nucleated cells plated). Gray bars denote tissue collected using a syringe, black bars denote tissue collected using BodyJet.
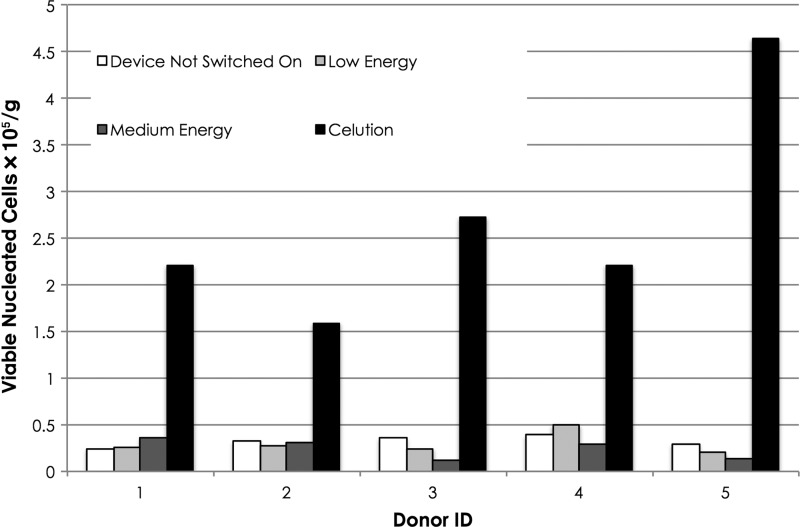
Processing tissue using ultrasound energy yielded a product that was significantly inferior to that obtained using enzymatic digestion. Indeed, the results indicate that the application of low and medium ultrasound energy is no better and may be worse than the negative control in which no ultrasound energy was applied (Figs. 4 and 5). As noted above, the use of higher energy settings resulted in substantial cell death due to heating and cavitation. On average, viable nucleated cell yield obtained with the ultrasound device switched off and with low and medium ultrasound energy was 13.7%±4.9%, 13.0%±6.3%, and 11.2%±6.5%, respectively, compared with the positive control processed using the Celution System. The difference between each of the ultrasound samples and the enzyme control was statistically significant (p<0.02). There was no significant difference between the low or medium energy levels and the negative control.
Figure 4.
Yield of cells in output of adipose tissue processed within the Celution System or using ultrasound energy. Recovery of viable nucleated cells×105 per gram of tissue processed.
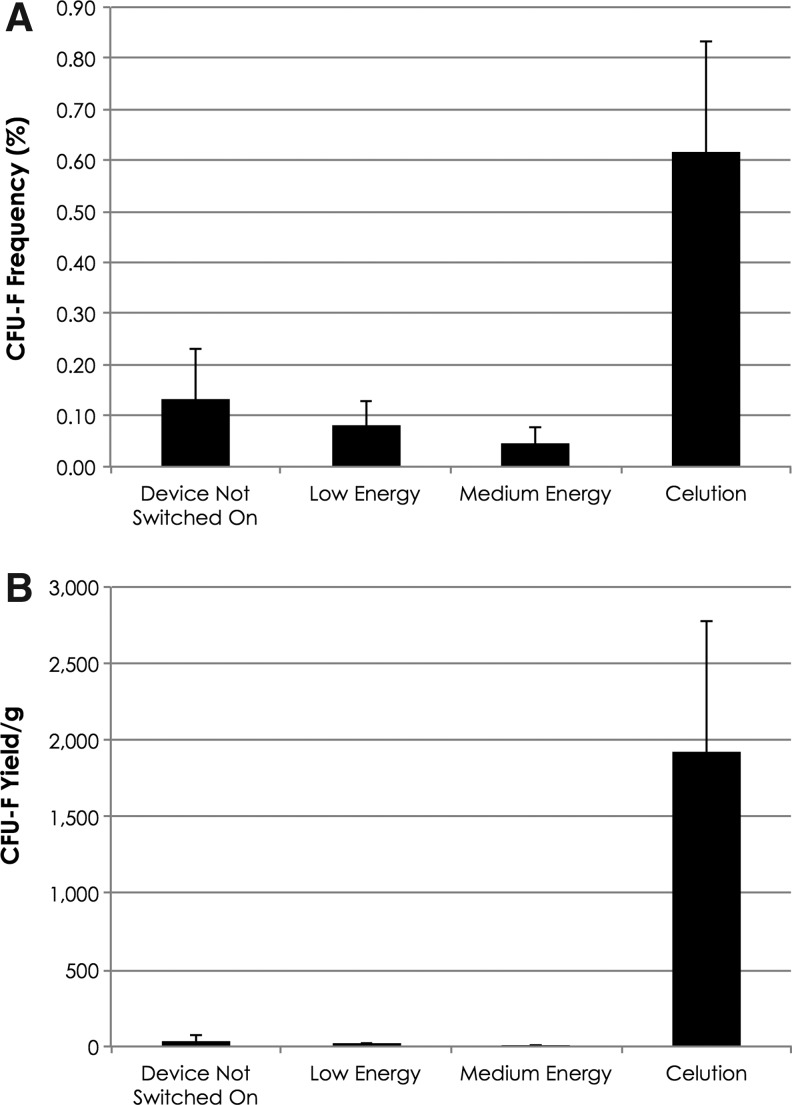
Figure 5.
Frequency and yield of CFU-F in output of adipose tissue processed within the Celution System or using ultrasound energy. (A) Average number of CFU-F per 100 viable nucleated cells plated. (B) Number of CFU-F recovered per gram of adipose tissue processed.
Similarly, the frequency of CFU-F for the negative control arm, the low energy and medium energy ultrasound groups was very similar and substantially lower than that observed with Celution (Fig. 5). On average, the CFU-F yield for the negative control arm, the low energy and medium energy ultrasound groups was 41±29 CFU-F/g, 18±9 CFU-F/g, and 6±5 CFU-F/g, respectively (Fig. 5B). By contrast, the yield following enzymatic processing in the Celution System was 1,919±853 CFU-F/g of adipose tissue. On average, the negative control, low and medium energy groups contained 1.8%±0.6%, 1.5%±0.7%, and 0.25%±0.14%, respectively, of the stem cells obtained by enzymatic processing with the Celution System. The yield with medium ultrasound energy was significantly less compared with the negative control, where zero energy was applied (p<0.05). All ultrasound arms were significantly less than the enzymatic arm (p<0.001).
Similar results were seen for the frequency and yield of CD34+/CD31+ cells and CD34+/CD31− cells (data not shown).
Discussion
The Celution System is regulated as a medical device and is, at this time, the only system with the CE Mark approval as a medical device for processing ADRCs. Indications for use within the CE Mark include use in soft tissue defects and general surgical procedures to facilitate healing. The Celution System is also regulated as a medical device by the U.S. Food and Drug Administration (FDA). In this respect, the manufacturer has announced initiation of enrollment of a clinical trial in patients with chronic heart disease executed in the United States under an Investigational Device Exemption granted by the FDA. As part of obtaining CE Mark clearance and permission to initiate USA-based clinical activity, the Celution System (including the device, consumable, and Celase) has been subjected to and passed a broad range of testing and validation relevant to medical devices, including sterilization validation, electrical safety, medical device software evaluation, and the ISO 10993 battery of tests that includes cytotoxicity, systemic toxicity, pyrogenicity, hemolysis, sensitization, and genotoxicity.
The combination of the Celution device and consumable creates a functionally closed, sterile system in which human adipose tissue collected by aspiration can be processed to yield an ADRC population with well-defined characteristics capable of being used within the confines of the same surgical procedure. Preclinical and clinical studies with these cells have shown improvement in angiogenesis,9 reduced inflammation,10,11 and in other parameters associated with mechanism by which healing can be improved.12,16 Recently, the United States Government has awarded Cytori Therapeutics, Inc., the developer of the Celution System, with a large research contract for evaluation of the system for potential use in patients with acute radiation exposure with concomitant thermal burn injury.29 Specifically, the Biomedical Advanced Research and Development Authority (BARDA; a branch of the U.S. Department of Health and Human Services) awarded Cytori a research contract valued at up to US$106 million over 5 years. This contract will support preclinical and clinical research and device development related to use of the Celution System in patients with concomitant thermal burn injury and radiation exposure following a mass casualty event. In such circumstances, the Celution System could be deployed at tertiary care facilities, where its ease of use, automation, and reproducibility could be applied as either a part of primary treatment of the injury or as a bridge to definitive care.
The current study showed considerable consistency in the yield and composition of the SVF population prepared using the Celution System even when the tissue was harvested by different clinics. The use of a powered tissue collection system (BodyJet) showed a similar yield although with slightly reduced recovery of stem cells as assessed by the CFU-F assay. These cells are believed to be perivascular30 and it may be that the high volume of fluid applied with the BodyJet system during tissue harvest and collection washes away vascular fragments thereby reducing the CFU-F content. Ongoing studies with other powered approaches, including the PAL Liposculptor™ system (Microaire, Charlottesville, VA) and the VASER® System (Sound Surgical, Boulder, CO), operated at 50% amplitude and at 80% amplitude suggest that ADRCs prepared using the Celution System with tissue collected using these approaches are similar to those collected by manual aspiration (Schlaudraff et al.; manuscript in preparation).
As yet, there are no published studies evaluating different automated processing systems in a head-to-head fashion using tissue from the same donors. However, one recent publication has compared published data obtained with the Celution System23 with the output of tissue that had been digested using a manual process and then washed and concentrated using the Sepax™ system (Biosafe SA, Geneva, Switzerland), a product originally designed for processing umbilical cord blood.31 The results suggest comparability of output, within the limitations of the nonhead-to-head study design. However, with this approach, the digestion component of processing is neither automated nor standardized. That is, the Sepax System performs only cell washing and concentration; tissue digestion must be performed manually, meaning that, the user must develop and validate appropriate enzyme treatment procedures. Similar nonhead-to-head data have been published from a different automated system manufactured by Tissue Genesis, Inc. (Honolulu, HI).29 Flow cytometry data in the publication demonstrate that a substantial fraction of the cells express the CD34 cell surface marker protein indicating that the population isolated contains SVF cells. However, this device is limited to a maximum processing volume of 60 mL of tissue and prepares the cells in a large final volume (35 mL), which required the authors to perform an additional, manual centrifugation step to concentrate the cells before clinical use. By contrast, the Celution System has a maximum capacity of 360 mL of tissue and concentrates the cells to a final volume of only 5 mL, which can be applied to a wound without further manipulation.24
Others have suggested that enzymatic digestion, the gold standard for obtaining such cells for approximately 50 years,32,33 might be dispensed with by using ultrasound cavitation to generate a single cell suspension without compromising cell viability.34 In an effort to evaluate this hypothesis, we performed a head-to-head comparison of tissue processed using the approach shown in this article. As shown in the data above, ultrasound energy was an extremely inefficient means of extracting cells from the extracellular matrix of adipose tissue (Figs. 4 and 5). Indeed, the data indicate that application of ultrasound energy had no effect or even decreased yield of SVF cells such as CFU-F (Fig. 5).
These results show that adipose tissue can be processed within the closed sterile fluid pathway of the Celution consumable to yield a reproducible population of ADRCs at high efficiency. The data further show that, at least at the settings applied, ultrasound energy is considerably inferior to enzymatic digestion for generation of these cells. By providing surgeons with a reproducible means of preparing well-characterized, clinical-grade ADRCs with regenerative properties without the need for cell culture, the Celution System represents an important tool in the development of new therapeutic approaches to the treatment of the sequelae of radiation exposure.
Innovation
The Celution System is the first medical device validated and approved by multiple international regulatory authorities to generate autologous stromal vascular cells from adipose tissue that can be used in a real-time bedside manner. By generating clinical-grade instrumentation for adipose tissue harvest, automated processing, and cell delivery, the Celution System is the first comprehensive medical technology platform for the generation of adipose-derived autologous cell therapies.
KEY FINDINGS.
The Celution System
• …is a functionally closed automated system for processing human adipose tissue to generate ADRCs that can be delivered to the patient in the same surgical procedure in which the tissue was harvested.
• …provides reproducible and reliable ADRC yield, viability, and composition with a range of different tissue collection approaches.
• …draws from a larger body of published clinical experience of any automated adipose tissue processing device, including areas as diverse as cardiology,17 stress urinary incontinence,35 breast reconstruction,18 cryptoglandular fistula-in-ano,36 and healing of refractory cutaneous wounds.24,25,37
Abbreviations and Acronyms
- ADSCs
adipose-derived stem cells (or adipose-derived stromal cells)
- ADRCs
adipose-derived regenerative cells
- MSCs
mesenchymal stem cells (or marrow stromal cells)
- SVF
stromal vascular fraction
Acknowledgments and Funding Sources
The authors would like to thank the many surgeons and more than 3,000 patients who have donated adipose tissue for Cytori's research and development activities. All studies described herein were funded by Cytori Therapeutics, Inc.
Author Disclosure and Ghostwriting
Dr. Scott Miller has no conflicts of interest with regard to this study. All other authors are paid employees and stock holders of Cytori Therapeutics, Inc., San Diego, CA. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
During the past decade, John K. Fraser and Douglas M. Arm have led Cytori's research and development team. Dr. Fraser currently serves as Cytori's Principal Scientist and head of Scientific Affairs. Dr. Arm is Cytori's Senior Vice President of Operations and oversees Cytori's biologics, engineering, manufacturing and quality assurance departments.
References
- 1.Benderitter M, Gourmelon P, Bey E, et al.: New emerging concepts in the medical management of local radiation injury. Health Phys 2010; 98:851. [DOI] [PubMed] [Google Scholar]
- 2.Gottlober P, Steinert M, Weiss M, et al.: The outcome of local radiation injuries: 14 years of follow-up after the Chernobyl accident. Radiat Res 2001; 155:409. [DOI] [PubMed] [Google Scholar]
- 3.Caplan AI, and Correa D: The MSC: an injury drugstore. Cell Stem Cell 2011; 9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bey E, Prat M, Duhamel P, et al.: Emerging therapy for improving wound repair of severe radiation burns using local bone marrow-derived stem cell administrations. Wound Repair Regen 2010; 18:50. [DOI] [PubMed] [Google Scholar]
- 5.Zuk PA, Zhu M, Ashjian P, et al.: Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 2002; 13:4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuk PA, Zhu M, Mizuno H, et al.: Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 2001; 7:211. [DOI] [PubMed] [Google Scholar]
- 7.Fraser JK, Wulur I, Alfonso Z, and Hedrick MH: Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol 2006; 24:150. [DOI] [PubMed] [Google Scholar]
- 8.Oedayrajsingh-Varma MJ, van Ham SM, Knippenberg M, et al.: Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy 2006; 8:166. [DOI] [PubMed] [Google Scholar]
- 9.Schenke-Layland K, Strem BM, Jordan MC, et al.: Adipose tissue-derived cells improve cardiac function following myocardial infarction. J Surg Res 2009; 153:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Premaratne GU, Ma LP, Fujita M, Lin X, Bollano E, and Fu M: Stromal vascular fraction transplantation as an alternative therapy for ischemic heart failure: anti-inflammatory role. J Cardiothorac Surg 2011; 6:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng Z, Ting J, Alfonso Z, et al.: Fresh and cryopreserved, uncultured adipose tissue-derived stem and regenerative cells ameliorate ischemia-reperfusion-induced acute kidney injury. Nephrol Dial Transplant 2010; 25:3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angelini A, Castellani C, Ravara B, et al.: Stem-cell therapy in an experimental model of pulmonary hypertension and right heart failure: Role of paracrine and neurohormonal milieu in the remodeling process. J Heart Lung Transplant 2011; 30:1281. [DOI] [PubMed] [Google Scholar]
- 13.Muller AM, Mehrkens A, Schafer DJ, et al.: Towards an intraoperative engineering of osteogenic and vasculogenic grafts from the stromal vascular fraction of human adipose tissue. Eur Cell Mater 2010; 19:127. [DOI] [PubMed] [Google Scholar]
- 14.Ganey T, Hutton WC, Moseley T, Hedrick M, and Meisel HJ: Intervertebral disc repair using adipose tissue-derived stem and regenerative cells: experiments in a canine model. Spine (Phila Pa 1976) 2009; 34:2297. [DOI] [PubMed] [Google Scholar]
- 15.Mohammadi R, Azizi S, Delirezh N, Hobbenaghi R, and Amini K: Transplantation of uncultured omental adipose-derived stromal vascular fraction improves sciatic nerve regeneration and functional recovery through inside-out vein graft in rats. J Trauma Acute Care Surg 2012; 72:390. [DOI] [PubMed] [Google Scholar]
- 16.Nambu M, Ishihara M, Nakamura S, et al.: Enhanced healing of mitomycin C-treated wounds in rats using inbred adipose tissue-derived stromal cells within an atelocollagen matrix. Wound Repair Regen 2007; 15:505. [DOI] [PubMed] [Google Scholar]
- 17.Houtgraaf JH, den Dekker WK, van Dalen BM, et al.: First experience in humans using adipose tissue-derived regenerative cells in the treatment of patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol 2012; 59:539. [DOI] [PubMed] [Google Scholar]
- 18.Perez-Cano R, Vranckx JJ, Lasso JM, et al.: Prospective trial of adipose-derived regenerative cell (ADRC)-enriched fat grafting for partial mastectomy defects: the RESTORE-2 trial. Eur J Surg Oncol 2012; 38:382. [DOI] [PubMed] [Google Scholar]
- 19.Lendeckel S, Jodicke A, Christophis P, et al.: Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg 2004; 32:370. [DOI] [PubMed] [Google Scholar]
- 20.Pak J: Autologous adipose tissue-derived stem cells induce persistent bone-like tissue in osteonecrotic femoral heads. Pain Physician 2012; 15:75. [PubMed] [Google Scholar]
- 21.Akita S, Akino K, Hirano A, Ohtsuru A, and Yamashita S: Noncultured autologous adipose-derived stem cells therapy for chronic radiation injury. Stem Cells Int 2010; 2010:532704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cervelli V, Gentile P, De Angelis B, et al.: Application of enhanced stromal vascular fraction and fat grafting mixed with PRP in post-traumatic lower extremity ulcers. Stem Cell Res 2011; 6:103. [DOI] [PubMed] [Google Scholar]
- 23.Lin K, Matsubara Y, Masuda Y, et al.: Characterization of adipose tissue-derived cells isolated with the Celution system. Cytotherapy 2008; 10:417. [DOI] [PubMed] [Google Scholar]
- 24.Akita S, Yoshimoto H, Akino K, et al.: Early experiences with stem cells in treating chronic wounds. Clin Plast Surg 2012; 39:281. [DOI] [PubMed] [Google Scholar]
- 25.Akita S, Yoshimoto H, Ohtsuru A, Hirano A, and Yamashita S: Autologous adipose-derived regenerative cells are effective for chronic intractable radiation injuries. Radiat Prot Dosim 2012; 151:656. [DOI] [PubMed] [Google Scholar]
- 26.Fekete N, Rojewski MT, Furst D, et al.: GMP-compliant isolation and large-scale expansion of bone marrow-derived MSC. PLoS One 2012; 7:e43255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sensebe L, and Bourin P: Producing MSC according GMP: process and controls. Biomed Mater Eng 2008; 18:173. [PubMed] [Google Scholar]
- 28.Hicok KC, and Hedrick MH: Automated isolation and processing of adipose-derived stem and regenerative cells. Methods Mol Biol 2011; 702:87. [DOI] [PubMed] [Google Scholar]
- 29.Doi K, Tanaka S, Iida H, et al.: Stromal vascular fraction isolated from lipo-aspirates using an automated processing system: bench and bed analysis. J Tissue Eng Regen Med 2012March22 [Epub ahead of print]; DOI: 10.1002/term.1478 [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto N, Akamatsu H, Hasegawa S, et al.: Isolation of multipotent stem cells from mouse adipose tissue. J Dermatol Sci 2007; 48:43. [DOI] [PubMed] [Google Scholar]
- 31.Guven S, Karagianni M, Schwalbe M, et al.: Validation of an automated procedure to isolate human adipose tissue-derived cells by using the Sepax(R) technology. Tissue Eng Part C Methods 2012; 18:575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hollenberg CH, and Vost A: Regulation of DNA synthesis in fat cells and stromal elements from rat adipose tissue. J Clin Invest 1969; 47:2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodbell M: Metabolism of Isolated Fat Cells. I. Effects of hormones on glucose metabolism and lipolysis. J Biol Chem 1964; 239:375. [PubMed] [Google Scholar]
- 34.Toscano N, Holtzclaw D, and Victor S: A prospective pilot study on the clinical application of stromal vascular fraction stem cells in the treatment of Miller Class I and II Gingival Recession Defects. J Implant Adv Clin Dentistry 2011; 3:23 [Google Scholar]
- 35.Yamamoto T, Gotoh M, Kato M, et al.: Periurethral injection of autologous adipose-derived regenerative cells for the treatment of male stress urinary incontinence: report of three initial cases. Int J Urol 2012; 19:652. [DOI] [PubMed] [Google Scholar]
- 36.Borowski DW, Gill TS, Agarwal AK, and Bhaskar P: Autologous adipose-tissue derived regenerative cells for the treatment of complex cryptoglandular fistula-in-ano: a report of three cases. BMJ Case Rep 2012; (Nov07_1):bcr2012006988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karaaltin MV, and Baghaki S: Adipose derived regenerative cell therapy for treating a diabetic wound: a case report. Wounds 2011; 23:1. [PubMed] [Google Scholar]