Abstract
Aims
Congenital human cytomegalovirus (HCMV) infection can lead to long-term neurodevelopmental sequelae, including mental retardation and sensorineural hearing loss. Preconception vaccine strategies relevant to prevention of HCMV-mediated injury to the newborn can be studied in the guinea pig cytomegalovirus (GPCMV) model. The objectives of this study were: 1) to assess in guinea pigs the protective efficacy against congenital infection and disease of a recombinant live, attenuated vaccine with a targeted deletion of the GPCMV homolog of the HCMV pUL83 tegument protein, GP83; and, 2) to compare the extent of placental infection in vaccine and control groups, using an in situ hybridization (ISH) assay.
Materials and methods
Outbred Hartley guinea pigs were vaccinated prior to pregnancy with a two-dose series of 5×104 pfu of vAM409, a GP83 deletion virus. Deletion of the GP83 gene resulted in an attenuated virus, and vAM409 vaccinated animals did not demonstrate evidence of DNAemia following vaccination, although ELISA antibody responses were comparable to those observed in natural infection. After mating, pregnant animals were challenged with salivary gland-adapted (SG) GPCMV (1×106 pfu) in the second trimester, and pregnancy outcomes were compared to controls.
Results
Compared to placebo-immunized controls, vaccination resulted in significantly reduced maternal DNAemia following SG challenge, and there was significantly decreased pup mortality in litters born to vaccinated dams (3/29; 10%), compared to control (35/50; 70%; p<0.001). By in situ hybridization study, recovered placentas in the vAM409 vaccine group demonstrated reduced infection and fewer infectious foci compared to the control group.
Conclusions
In summary, preconception immunization with a GP83 deletion vaccine reduced maternal DNAemia and results in protection against congenital GPCMV-associated pup mortality compared to unvaccinated controls. Vaccination resulted in reduced placental infection, probably related to the reduction in maternal DNAemia. Although the pp65 homolog in GPCMV, GP83, is a known target of protective T cell immune responses, it is nevertheless dispensable for effective vaccination against maternal and fetal CMV disease in this model.
Keywords: Cytomegalovirus, Guinea pig cytomegalovirus, Cytomegalovirus vaccine, Live, attenuated CMV vaccine, CMV UL83, CMV pp65, GPCMV GP83, Vaccine efficacy, Guinea pig challenge model, cytomegalovirus immune evasion
Introduction
Maternal infection with human cytomegalovirus (HCMV) during pregnancy can lead to severe disease in newborns associated with long-term neurodevelopmental sequelae, particularly sensorineural deafness [1–3]. A vaccine capable of protecting newborns from the sequelae of congenital HCMV infection has accordingly been designated as a major public health priority by the Institute of Medicine [4]. It is not clear what would constitute an optimal vaccine. Subunit vaccines that target the major envelope glycoprotein gB (gpUL55) have been evaluated for immunogenicity and safety in clinical trials, and have demonstrated both excellent immunogenicity and varying degrees of efficacy against CMV infection and/or disease in high-risk populations, including young women [5–7]. However, it remains uncertain if a vaccine-induced antibody response to a single viral glycoprotein target would be sufficient for a vaccine designed to prevention congenital infection. Live, attenuated HCMV vaccines theoretically capable of inducing both antibody responses as well as broad-based cellular responses, including cytotoxic CD8+ T-cell responses, are also in clinical trials [8, 9], in addition to subunit vaccines, although safety considerations regarding such vaccines have dampened enthusiasm for this approach [10].
Given the species-specificity of CMVs, it is problematic to perform preclinical studies of HCMV vaccines in animal models. However, efficacy evaluations of vaccines can be performed using the guinea pig cytomegalovirus (GPCMV) model [11, 12]. GPCMV has some unique advantages for the study of vaccines against congenital infection, compared to other animal models. In contrast to the CMVs of most small animals, GPCMV causes maternal viremia and disease, can cross the placenta, and can infect fetuses resulting in stillborn offspring. Furthermore, the anatomy of the guinea pig placenta more closely resembles the human placenta than in other small laboratory animals. Preconception immunization of guinea pigs with recombinant GPCMV gB, administered either as a DNA vaccine or purified baculovirus-expressed protein vaccine, has been shown to protect against GPCMV challenge made during pregnancy, as measured by the reduction of pup mortality and infection, and by the reduction of maternal and fetal viral load [13, 14]. These observations support the ongoing efforts to optimize gB immunogenicity for HCMV vaccine clinical trials.
In addition to gB, most HCMV subunit based vaccines in clinical trials also contain various recombinant formulations or expression strategies for the major cell-mediated immune (CMI) target of HCMV infection, the tegument protein pp65 (pUL83), based on the presumption that inclusion of this CMI target will augment or synergize with the protection conferred by neutralizing antibodies engendered against gB [8]. Support for the role of pp65 in protection against HCMV disease comes from adoptive transfer studies in which passive administration of pp65-specific CD8+ cells was found to reduce the risk of HCMV disease in hematopoietic stem cell transplant patients [15]. The GPCMV homolog of pp65, GP83, has been shown to provide some protection against maternal and fetal GPCMV disease when administered as a preconception vaccine [16]. However, pp65 is itself an immunomodulatory protein, an immunomodulatory protein, and the impact of these immune modulation effects on the host response to vaccination and/or infection is incompletely understood. The pp65 protein has been demonstrated to block interferon activity [17] and the down-regulation of interferon-responsive genes [18]. Thus, in spite of its role in eliciting CD8+ T-cell responses involved in protection against HCMV disease, pp65 may also play an immunomodulatory role that could be relevant to vaccine design.
To examine the relative importance of the GPCMV homolog of pp65, GP83, in a preconception vaccinate, these studies were undertaken to assess the immune responses and protective capacity of an attenuated GPCMV mutant, vAM409, with a targeted deletion of the GP83 gene [19, 20]. Previous evaluation of this virus demonstrated that, although this mutation conferred only a minimum growth defect in cell culture, the mutant was highly attenuated for in vivo dissemination, with reduced recovery of recombinant virus noted in liver, spleen, lung, and salivary gland in experimentally inoculated non-pregnant animals [20]. We examined whether vaccination with the GP83 deletion virus would provide protection against maternal and fetal GPMCV infection and disease, of particular interest in light of the knowledge that this tegument phosphoprotein induces protective T cell responses in both humans [21] and guinea pigs [16]. In addition, we examined whether immunization results in reduced presence of virus in the placenta of immunized compared to control dams using an in situ hybridization assay.
Materials and methods
Animal studies
This study was performed at the University of Minnesota (Minneapolis, MN, USA) with full approval of the Institutional Animal Use and Care Committee (IACUC). Inbred adult strain-2 guinea pigs were used for preparation of salivary gland passaged-GPCMV stocks. Age-matched young female and breeder male Hartley guinea pigs were obtained from Elm Hill Laboratories (Chelmsford, MA, USA). All animals were confirmed to be GPCMV-seronegative by ELISA [14]. Animals were housed under conditions approved by the American Association of Accreditation of Laboratory Animal Care, in accordance with institutional animal use committee policies at the University of Minnesota.
CMV stocks
GPCMV (strain no. 22122, ATCC VR682) was propagated in guinea pig fibroblast lung cell cultures (GPL; ATCC CCL 158) maintained in F-12 medium supplemented with 10% fetal calf serum (FCS, Fisher Scientific), 10,000 IU/l penicillin, 10 mg/l streptomycin (Gibco-BRL) and 7.5% NaHCO3 (Gibco-BRL). The vAM409 deletion mutant strain was similarly cultured and maintained in GPL cells as described previously [22]. Briefly, this recombinant virus was generated by gpt mutagenesis. A 250-bp out-of-frame NH-terminal deletion of coding sequences of GP83 was engineered into a plasmid, followed by insertion of a cassette containing the gpt/eGFP genes within the carboxy-terminal coding sequence of GP83. This plasmid was used in the generation of recombinant gpt/eGFP+ virus under metabolic selection with MPA and xanthine as previously described [22]. Salivary gland-passaged GPCMV stocks (SG virus) used for animal challenge studies were prepared by sequential passage in strain-2 guinea pigs.
Experimental design
Hartley strain guinea pigs were obtained from Elm Hill laboratories (Chelmsford, MA). All animals were determined to be GPCMV seronegative prior to vaccination by ELISA. Animals were immunized twice with an interval of 3 weeks between doses, with 5×104 pfu of vAM409 vaccine (n=8), by subcutaneous route in a total volume of 1 ml. Control animals (n=13) received an identical volume of phosphate-buffered saline. Small-volume bleeds were performed by toenail clip once a week for 6 weeks on all guinea pigs following vaccination for both quantitative PCR analysis of viral load and for serum analysis. After 6 weeks, bleeds were performed every other week until the conclusion of the study.
Three weeks following the second immunization, animals were mated with seronegative young male Hartley guinea pigs. Mating persisted until abdominal palpitation confirmed pregnancy. All 13 animals in the control group, and 7/8 animals in the vAM409 group, became pregnant. Approximately 4 weeks before the anticipated time of delivery, pregnant dams were challenged with a subcutaneous injection of 1×106 pfu/ml of salivary gland derived GPCMV. Bleeds were performed of infected dams day 5 post-challenge for quantitative PCR analysis of viral load and for plasma analysis. Upon delivery, pups were weighed and live-born pups were sacrificed within 96 hours of birth. Blood was obtained from pups for quantitative PCR analysis of viral load. Lung, liver, spleen when available, were also extracted for evaluation of organ pathology and quantitative PCR analysis of viral load. Retrievable placentas were also obtained and stored at −80 °C for subsequent in situ hybridization studies. The overall study outline is schematically presented in Fig. 1.
Figure 1.
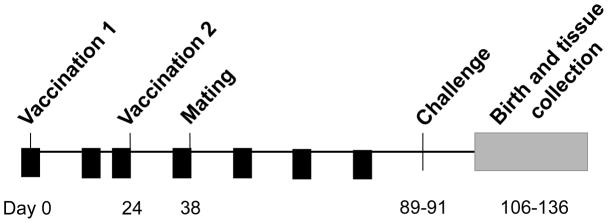
Schematic overview and time course of vaccine study. GPCMV seronegative outbred Hartley guinea pigs were immunized twice (interval of 3 weeks between doses), with vAM409 vaccine, or placebo. Small-volume bleeds were performed by toenail clip were obtained (arrowheads) on all guinea pigs following vaccination at weeks 2, 3, 5, 7, 9 and 11, for both quantitative PCR analysis of viral load and for analysis of antibody response. Challenge with SG-passaged GPCMV was administered on days 89–91 of the experiment, approximately 7–8 weeks after initiation of breeding.
ELISA and western blot assay
ELISA analysis (Fig. 2a) was performed as previously described [14], with titer determined by limiting dilution assay (initial dilution, 1:80). A positive result was determined by calculating the reciprocal of the highest dilution that produced an absorbance of at least 0.1 and twice the absorbance observed using a negative control antigen. Plates were read at a wavelength of 450 nm using the SpectraMax M2 Spectrophotometer (Molecular Devices) and the ScanMax Pro program. ELISA was performed to compare antibody titers in vaccinated animals to those found in polyclonal serum obtained from guinea pig inoculated with wild-type GCMV. Western blot analysis was performed on serum collected from a subset of guinea pigs from the vaccine and control groups (Fig. 2b). Serum was collected before vaccination from each experimental group (preimmune) and at time points 6 days post vaccination (dpv), 20 dpv and 48 dpv. Virus particles were subjected to SDS-PAGE and were then transferred onto nitrocellulose membrane by electroblotting. Membranes were blocked using membrane blocking agent (GE Healthcare) resuspended in Tris-buffered saline plus 0.5% Tween (TBST) and then incubated for 3.5 hours with the above mentioned collected sera (1:800). Along with serum samples obtained from the above time points, a gB-specific polyclonal rabbit antiserum (1:500) and a guinea pig polyclonal antiserum (1:10,000) from an experimentally infected animal were used as controls [23]. After washing in TBST, blots were incubated with either a horseradish peroxidase-conjugated rabbit anti-guinea pig antiserum (1:10,000; Santa Cruz Biotechnology) or a horseradish peroxidase-conjugated donkey anti-rabbit antiserum (1:10,000; Santa Cruz Biotechnology) for 2 hours at 27°C. Antibody binding was then detected using the ECL western Blot Detection Kit (GE Healthcare), followed by autoradiography (Hyblot CL; Denville Scientific, Inc.).
Figure 2.
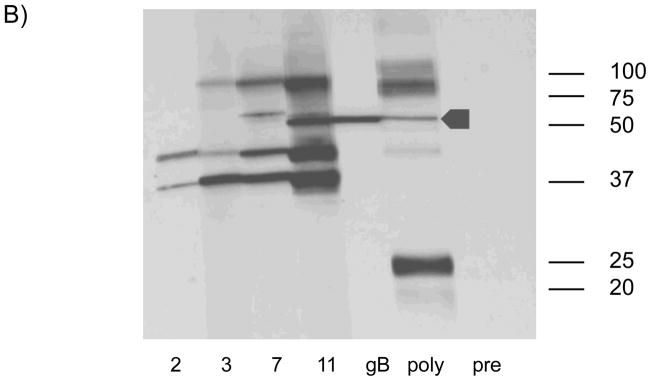
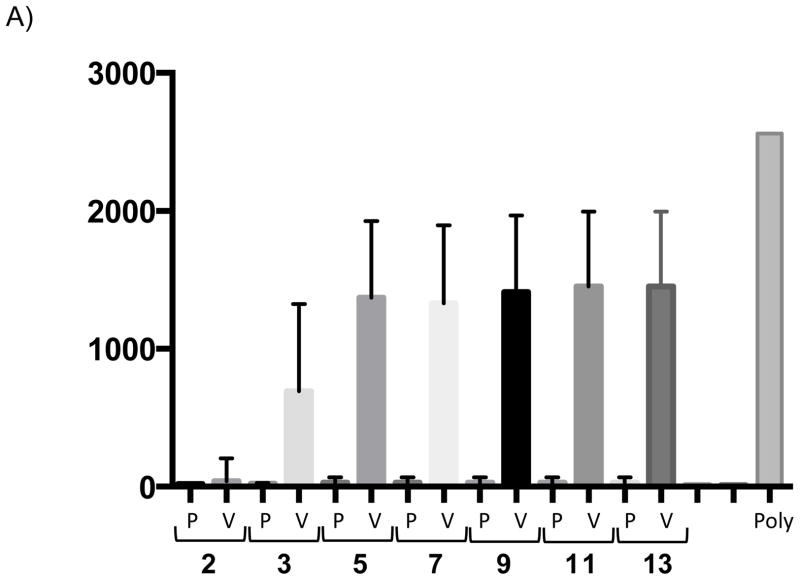
ELISA response following vAM409 vaccination. A) Antibody titers in response to vaccine (V) or placebo (P) were compared at multiple time points post-immunization. Antibodies titers (mean ± SE) were measured by ELISA using sonicated, clarified guinea pig lung fibroblast lysate from GPCMV-infected cells as previously described [13]. A control ELISA titer obtained using a polyclonal anti-GPCMV antisera from an animal inoculated with wild-type GPCMV is shown for comparison. B) Western blot demonstrating evolution of antibody response following vAM409 immunization. Immunized animals evolve antibody responses that include gB (GP58) as evidenced by comparison with a monospecific anti-gB polyclonal antibody (arrowhead).
In situ hybridization
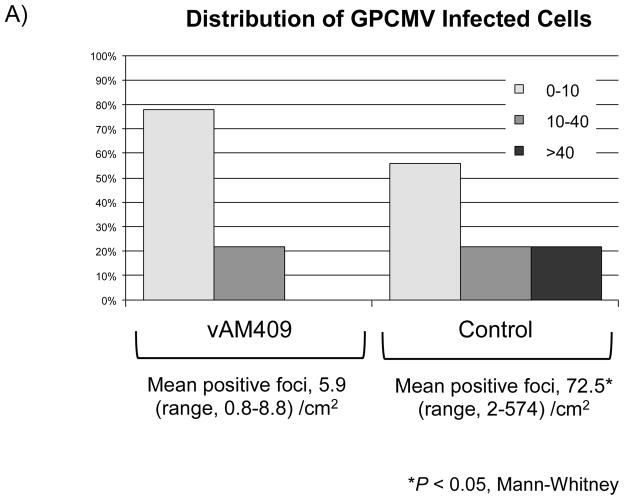
For in situ hybridization (ISH) studies, a digoxinogen-labeled probe was generated, using the Roche PCR Dig Probe synthesis kit (Roche Applied Sciences, Madison, WI) according to the manufacturer’s specification. Briefly, a 148 bp digoxinogen-labeled probe was generated by PCR corresponding to amino-terminal coding sequences in the GPCMV gB (GP55) ORF (nt 96,562-96,709; Accession NC_020231.1). The primer sequences used for amplification of probe were: 5′ – ATCATTCGGACGCATCGC – 3′ and 5′ – CGGATTGAAGGGTACGCACT – 3′. A digoxinogen-labeled tPA probe was used as a control. For detection of hybridized probe, a mouse anti-digoxinogenic monoclonal antibody was used (Roche Applied Sciences). Tissue preparation and processing for in situ visualization employed techniques from previously published protocols [24, 25] with a number of modifications. Frozen sections were cut at 10 μ thickness and slides were pretreated for 10 m at 4 °C in 4% paraformaldehyde prepared in phosphate-buffered saline (PBS), pH 7.0, washed in PBS, then incubated for 10 m in 0.5% saponin and triton-X 100 in PBS. Slides were next washed with PBS, incubated for 30 m in 200 mM HCl, washed with PBS, then incubated 15 m at 37 °C in 0.1 μg/ml proteinase K in 10 mM Tris, pH 7.0, prior to a final wash with PBS. Hybridization was performed for 30 m at 37 °C in hybridization buffer (50% deionized formamide, 2X SSC, 1 mM EDTA, 0.2 mg/ml salmon sperm DNA, 5% dextran sulfate, 0.2 mg/ml yeast tRNA, and 1X Denhardt’s Solution) containing probe diluted per the manufacturer’s specifications. Slides were incubated for 5 m at 77 °C then incubated overnight at room temperature. Stringent washes consisted of two 15 m washes at 60 °C in 2X SSC and one 20 m wash at 45 °C in 0.1X SSC. Slides were washed in PBS, then detection was performed by first pre-blocking for 30 m in 2% non-fat dry milk, and next treating for a total of 60 m with anti-digoxinogen secondary antibody diluted 1:500 in PBS, 1% non-fat dry milk, 0.3% triton-X 100. Next, slides were washed with PBS, incubated 5 m in 100 mM Tris-HCl, pH 9.5, then incubated with 75 ml of BCIP/NBT overnight, per the manufacturer’s specifications. Slides were sequentially washed with 100 mM Tris-HCl pH 9.5, and H20, and then mounted with Vectamount AQ (Vector Laboratories, Burlingame, CA, USA) prior to examination. Slides were quantified by microscope as whole sections. A minimum of three, non-contiguous sections were processed and counted for each available placenta. The investigator was blinded to as to whether slides were from pregnancy outcomes from dams immunized with vAM409, or placebo. The number of positive cells per slide were counted and quantified as number of positive cells/cm2 (Fig. 3).
Figure 3.
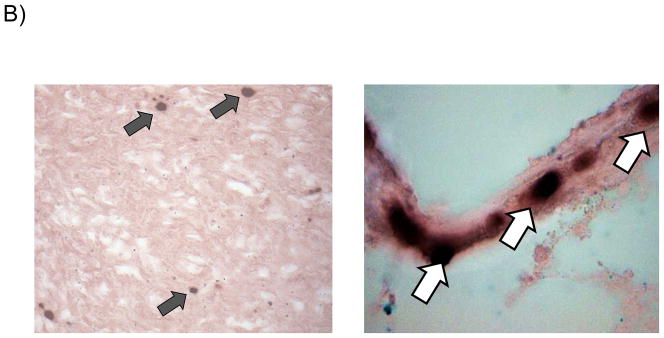
Reduced placental infection in vAM409-immunized dams compared to controls. (A) Distribution of in situ positive foci in vAM409-vaccinated (left panel) and control (right panel) animals. An average of 72.5 (range, 2–574) positive foci/cm2 were noted in control placentas. In contrast, in placentas of vAM409-vaccinated animals, an average of 5.9 positive foci (range, 0.8–8.8)/cm2 were noted (P < 0.05). (B) Demonstration of positive signal in control placenta. Left panel, 20× magnification of in situ labeled control dam demonstrating multiple positive foci (arrows) of GPCMV infection. Right panel, 80× magnification demonstrating positive cells (arrows) in trophoblasts of infected placenta.
For analysis of ISH data, both qualitative and semi-quantitative comparisons were made. Placentas from experimental and control animals were examined in blinded fashion by microscopy. Sections were examined in all fields and scored as positive if any ISH signal was detected. The number of GPCMV+ cells by ISH in each slide was also enumerated and expressed as positive cells/cm2. Positive slides were designated as low positive (1–10 GPCMV+ cells/cm2), intermediate positive (10–40 GPCMV+ cells/cm2) or high positive (>40 GPCMV+ cells/cm2).
Real-time PCR
Viremia was defined as the detection of GPCMV genomic DNA (DNAemia) and viral load was defined as the concentration of GPCMV genomic DNA in maternal blood, measured 5 days post-challenge, using GPCMV gB specific primers, probes and control plasmid as described elsewhere [26]. Maternal-fetal transmission was confirmed by detection of GPCMV genomic DNA in the organs of necropsied pups using this same quantitative assay. The following primers were used: forward primer, 5′-CTTCGTGGTTGAACGGG-3′; reverse primer, 5′-GTAGTCGAAAGGACGTTGC-3′; probe 1, 5′-TGGTGACCTTCGTTACCAATCCGTTTGGA-fluorescein; probe 2, 5′-LC Red 640-CTTCGTGGTGTTCCTGTTCTGCGT-Phosphate. DNA was extracted from whole blood or (neonatal) liver/spleen homogenates using Roche MagNa Pure LC Total Nucleic Acid Isolation Kit. PCR reaction mixtures were prepared using Lightcycler® Fast Start Master hybridization probes (Roche Applied Sciences) supplemented with 2.5 mM MgCl2, 0.5 μM primers and 0.2 μM probes. PCR was performed in the Lightcycler® instrument.
Statistical analyses
Proportions of live-born pups were compared using Fisher’s exact test. Antibody titers were compared using the paired Student’s t test. Statistics were analyzed using the Prism 5.0 software package (GraphPad Software, San Diego).
Results
Immune response to vAM409 vaccine
All animals were confirmed to be GPCMV-seronegative by ELISA at the beginning of the study. A total of 8 young Hartley female guinea pigs were immunized with a two-dose series of vAM409 and compared to age-matched mock-immunized controls as described in materials and methods. By 11 weeks post-vaccination, vAM409-immunized animals had an ELISA titer of 3.0 log10, compared to 1.3 log10 in sham-immunized controls (P< 0.0001). GPCMV-specific bands were first noted 2 weeks post-immunization by western blot (Fig. 2B) and additional bands were discernable at later time points. Immunized animals were confirmed by western to have generated antibody responses to gB, based on the comparison of the Mr of bands in immunized animals to those observed with a gB-specific antibody (Fig. 2B, lane 5).
Effect of vaccination on pregnancy outcomes
The impact of pre-conception vaccination was assessed by comparing pup mortality and weights in the vAM409 and control groups (Table 1). Results from this control group have been previously reported in another live attenuated vaccine study (with slightly different set of study endpoints for the vaccine group) performed in parallel with this study [27]. The effect of the vAM409 attenuated virus vaccine on pup mortality due to disseminated GPCMV in Hartley guinea pigs (two doses of vaccine) was analyzed. A total of 7 dams in the vAM409 vaccine group completed pregnancy. In the control group, 13 animals became pregnant, and had evaluable pregnancy outcomes. In the control group, pup mortality was 35/50 (70%). In contrast, pup mortality in litters born to vAM409-vaccinated dams was 3/29 (10%; p<0.0001 compared to vaccine group). Mean birth weights of the pups delivered in each group were also compared. Among pups born in the control group, live-born pups (n=15) had a mean weight of 87.1 +/− 16.5 grams, while dead pups (n=35) had a mean weight of 62.2 grams +/−29.5 grams. In contrast, in the vAM409 vaccine group, live-born pups (n=26) had an average weight of 104.3 +/− 11 grams, while stillborn pups had a mean weight of 75.4 +/− 21 grams. Overall, mean pup weight was 71.2 +/− 28 grams in the control group, and 101.3 +/− 15 grams in the vaccine group (p<0.0001, unpaired t-test). Of 7 litters born to vaccinated dams, 3 (43%) had at least one dead pup, while 12/13 litters born to dams in the control group had at least one dead pup (p<0.001, Fisher’s exact test).
Table 1.
Pregnancy outcomes (pup mortality and weights [grams]) after GPCMV challenge of vAM409- and control-immunized dams.
| Pup Mortality | Pup Weights | ||||
|---|---|---|---|---|---|
| Vaccine | Litters, no | Dead/total (%) | Liveborn Pups | Dead Pups | All Pups |
| Control | 13 | 35/50 (70%) | 87.1 | 62.2 | 71.2 |
| vAM409 | 7 | 3/29 (10%) | 104.3 | 75.4 | 101.3 |
Impact of vaccination on GPCMV transmission and on maternal and fetal viral load
The vAM409 vaccine had an impact on the magnitude of maternal viremia and congenital GPCMV infection (Table 2). All maternal blood samples taken before SG virus challenge were negative by qPCR. This confirmed that the vaccine itself did not result in DNAemia. Following SG virus challenge, in control dams, 12/13 demonstrated DNAemia at day 5 post-infection, with a mean viral load of 4.4 +/−0.4 log10 genomes/ml. In contrast, although 5 of 7 dams immunized with vAM409 vaccine demonstrated DNAemia day 5 post-salivary gland virus challenge, the viral load concentration was significantly lower than that of the control, with a level of 3.8 +/− 0.37 log10 genomes/ml (P=0.01). PCR of DNA purified from pup organs (liver, spleen and lung) was compared between groups to evaluate for congenital GPCMV transmission rates in vaccinated and control animals. Overall, 35/50 pups (10/15 among live-born pups, and 25/35 among dead pups) in the control group had congenital infection, as evidence by at least one positive tissue PCR (70% overall congenital infection rate). In the vAM409 vaccine group, PCR of DNA purified from pup organs demonstrated congenital GPCMV transmission in 5/29 pups (4/26 live-born pups and 1/3 dead pups) for an overall 17% congenital infection rate (p<0.0001 compared to control).
Table 2.
Maternal viral load and congenital viral transmission.
| Vaccine | Maternal DNAemia | Viral Load (genomes/ml) | Congenital GPCMV Transmission |
|---|---|---|---|
| Control | 12/13 (92%) | 4.4 +/−0.4 log10 | 35/50 (70%) |
| vAM409 | 5/7 (71%) | 3.8 +/− 0.4 log10* | 5/29 (17%)** |
P = 0.01, Mann-Whitney Test
P < 0.0001, Fisher Exact Test
Placental studies in vaccinated and control dams
Term placentas were retrieved when available and evaluated for the presence of GPCMV using in situ hybridization studies as described in materials and methods. A total of 17 placentas were available from the vAM409 group, corresponding to 5 litters. Of these, 9 (53%) were positive by in situ hybridization, with an average of 5.9 positive foci (range, 0.8–8.8)/cm2 enumerated. A total of 21 control group placentas, corresponding to 10 litters, were examined. There were 18 in situ positive placentas in this group (86%). An average of 72.5 (range, 2–574) positive foci/cm2 were noted in control placentas (P<0.05, Mann-Whitney test).
Discussion
In this study, a live, attenuated CMV vaccine was generated, based on deletion of the GPCMV pp65 homolog, GP83, from the GPCMV genome. This recombinant virus, generated using a gpt-based mutagenesis approach, was previously demonstrated to be highly attenuated for replication in guinea pigs [20]. In spite of this attenuation, the vaccine, when administered to non-pregnant guinea pigs, was capable of eliciting high-titer ELISA antibody responses comparable to those observed in natural infection (Fig. 2). Vaccinated animals, following establishment of pregnancy, were protected against DNAemia after virulent salivary-gland virus challenge, and their pups were protected both against GPCMV-associated mortality and GPCMV infection. Similar observations were made in a study by Bia and colleagues, examining the efficacy of pre-conception vaccination with a tissue culture-passaged live, attenuated GPCMV in the guinea pig model. This study found that pregnant animals previously inoculated with live CMV vaccine had lower incidences of viremia and generalized maternal and fetal infection [28].
The concept of using molecular genetic approaches to engineer recombinant CMVs, toward the goal of creating less pathogenic and/or more immunogenic live, attenuated vaccine candidates, has been previously described for both GPCMV and MCMV [29–31]. The targeting of immune modulation genes in vaccine design is of particular appeal given that there are substantial regulatory concerns about the potential long-term risks of live, attenuated HCMV vaccines, which theoretically include latency, oncogenesis, autoimmune disease, and atherosclerosis [32]. The HCMV pp65 (pUL83) is an important target of CD8+ cytotoxic T-cells in the course of the immune response to HCMV [21] infection. The GP83 (pp65 homolog) is similarly a highly abundant virion protein in GPCMV [19]. Previous studies have shown that GP83-specific immune responses induced by vectored vaccination, using a Venezuelan Equine Encephalitis virus replicon system, are capable of modifying congenital GPCMV infection and disease [33]. However, although HCMV pp65 is frequently included in subunit vaccines [8], this protein elicits multiple immunomodulatory functions that might impact primary and persistent infection, and may not be desirable in a subunit vaccine. These immune modulation functions include the blocking of the induction of interferon-response genes [34], primarily through modulation of the interferon response factor 3, IRF-3 [35]. Viral subversion of the IRF-3 response by pp65 raises the question of whether a live, attenuated vaccine deleted of pp65 might not only be highly attenuated but also provide improved immune protection as an HCMV vaccine, the loss of the anti-pp65 T cell response notwithstanding. For this reason, it was of interest to examine whether a vaccine with a deletion in the pp65 homolog could nevertheless induce protective immunity. Although a formal role for GP83 in immune evasion of GPCMV infection in guinea pigs remains to be formally demonstrated, these studies nonetheless demonstrate that a highly protective vaccine can be engineered without this T-cell target, and that this vaccine virus is highly attenuated relative to viruses retaining the GP83 locus.
These studies examined placentas from both vAM409-immunized dams as well as sham-immunized dams to test whether vaccine-mediated protection was associated with decreased evidence of placental GPCMV infection. Griffith and colleagues examined the role of the placenta as a site of GPCMV infection in Hartley guinea pigs inoculated at mid-gestation [36]. These studies demonstrated a hematogenous spread of GPCMV from the mother to the placenta, and virus was noted to remain in placental tissues long after clearance from maternal blood. GPCMV-induced histopathological lesions bearing CMV antigen, identified by immunoperoxidase staining using anti-GPCMV polyclonal antibody, were observed at the transitional zone between the capillarized labyrinth and the noncapillarized interlobium. Although GPCMV was invariantly isolated from the placenta for an infected fetus, only 27% of fetuses were infected when the accompanying placenta was infected. We chose to use an in situ hybridization assay for the evaluation of GPCMV-specific placental involvement, similar to the approach described by other investigators [24, 37], with the exception that the experiments performed in our study used a much smaller GPCMV-specific probe. Unfortunately, we were unable for the most part to link placentas with pups, since placentas were retrieved typically several hours after birth. In addition, some placentas were eaten by the dams prior to recovery. These limitations notwithstanding, blinded analysis of the in situ studies did demonstrate a clear effect of vAM409 vaccination on both the frequency and magnitude of viral antigen expression in retrievable placentas (Fig. 3). A recent report of an adenovirus-vectored GPCMV gB vaccine similarly noted reduced placental infection in vaccinated dams challenged with GPCMV, compared to unimmunized controls [38].
One limitation of the analysis of the GPCMV GP83 knockout virus analyzed in these studies is the viral mutant was generated using a highly tissue culture-derived (ATCC) viral stock as the parental virus. Subsequent to the initiation of these studies, it was demonstrated by Inoue and colleagues that the predominant variant of GPCMV that is selected for in tissue culture, following cultivation of ATCC virus in fibroblasts, is a virus that contains a 1.6 kb deletion that attenuates replication and results in reduced disease in vivo [39]. This region of the genome encodes potential homologs to HCMV UL128, UL130, and UL131 proteins, respectively, proteins which play a critical role in the tropism of HCMV for endothelial and epithelial cells [40, 41]. The GPCMV homologs of these proteins, (GP129, 131, and 133) form a pentameric complex with the gH and gL homologs [41]. Viruses lacking GP129–133 are impaired for replication in vivo following sc inoculation relative to viruses that retain these gene products [41]. Since the vAM409 mutant was generated against an ATCC background lacking this 1.6 kb region, the deletion of GP83 in this virus is superimposed upon a virus already lacking in genes that play an important role in the pathogenesis of infection. On the other hand, the attenuation of vAM409 pathogenicity reported in this and other studies [20] is striking compared to another GPCMV recombinant, vAM403, which also lacks this 1.6 kb virulence-conferring locus. Therefore, there is a clear additional attenuation conferred by deletion of GP83 gene in the vAM409 virus, compared to vAM403, in which the GP83 gene is intact. Additional studies of recombinant GPCMVs deleted of key immune evasion and/or pathogenesis genes may shed light on attractive strategies for future clinical development for human trials.
Conclusions
Compared to placebo-immunized controls, vaccination with a live, attenuated GPCMV vaccine with a targeted deletion in the GP83 gene (pUL83 homolog of HCMV) resulted in significantly reduced maternal DNAemia following SG virus challenge, and there was significantly decreased pup mortality in litters born to vaccinated compared to control dams. By in situ hybridization study, recovered placentas in the vAM409 vaccine group demonstrated reduced infection and fewer infectious foci compared to the control group. These results suggest that reduction in maternal DNAemia results in reduced placental interface, which in turn improves pregnancy outcomes in this uniquely useful small animal model of congenital CMV infection. Moreover, these data suggest that the major tegument protein, pp65, although an important T-cell immunogen for both HCMV and GPCMV, may not be required for an effective attenuated CMV vaccine.
Summary Points.
Congenital CMV infection is a major public health problem.
CMV vaccines are a major priority, but must be tested in preclinical models of congenital infection.
Subunit vaccines based on envelope glycoproteins have, to date, been disappointing in clinical trials.
Live attenuated vaccines can be generated using mutagenesis techniques to delete putative immune modulation genes for improved safety.
Although pp65 is a major target antigen for the cellular immune response, it is also an immune modulation protein.
Deletion of the pp65 homolog in the guinea pig cytomegalovirus (GPCMV) attenuates the virus substantially, yet the virus remains immunogenic and protective as a vaccine against congenital CMV in the guinea pig model.
Further study of attenuated CMV vaccines are warranted, both in preclinical models and in clinical trials.
References
- 1.Whitley RJ. Congenital cytomegalovirus infection: epidemiology and treatment. Adv Exp Med Biol. 2004;549:155–160. doi: 10.1007/978-1-4419-8993-2_21. [DOI] [PubMed] [Google Scholar]
- 2.Williamson WD, Demmler GJ, Percy AK, Catlin FI. Progressive hearing loss in infants with asymptomatic congenital cytomegalovirus infection. Pediatrics. 1992;90(6):862–866. [PubMed] [Google Scholar]
- 3.Cheeran MC, Lokensgard JR, Schleiss MR. Neuropathogenesis of congenital cytomegalovirus infection: disease mechanisms and prospects for intervention. Clin Microbiol Rev. 2009;22(1):99–126. doi: 10.1128/CMR.00023-08. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arvin AM, Fast P, Myers M, Plotkin S, Rabinovich R. Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2004;39(2):233–239. doi: 10.1086/421999. [DOI] [PubMed] [Google Scholar]
- 5.Pass RF, Zhang C, Evans A, et al. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med. 2009;360(12):1191–1199. doi: 10.1056/NEJMoa0804749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffiths PD, Stanton A, Mccarrell E, et al. Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: a phase 2 randomised placebo-controlled trial. Lancet. 2011;377(9773):1256–1263. doi: 10.1016/S0140-6736(11)60136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabbaj S, Pass RF, Goepfert PA, Pichon S. Glycoprotein B vaccine is capable of boosting both antibody and CD4 T-cell responses to cytomegalovirus in chronically infected women. The Journal of Infectious Diseases. 2011;203(11):1534–1541. doi: 10.1093/infdis/jir138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sung H, Schleiss MR. Update on the current status of cytomegalovirus vaccines. Expert Rev Vaccines. 2010;9(11):1303–1314. doi: 10.1586/erv.10.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heineman TC, Schleiss M, Bernstein DI, et al. A phase 1 study of 4 live, recombinant human cytomegalovirus Towne/Toledo chimeric vaccines. The Journal of infectious diseases. 2006;193(10):1350–1360. doi: 10.1086/503365. [DOI] [PubMed] [Google Scholar]
- 10.Krause PR, Bialek SR, Boppana SB, et al. Priorities for CMV vaccine development. Vaccine. 2013 doi: 10.1016/j.vaccine.2013.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bia FJ, Miller SA, Davidson KH. The guinea pig cytomegalovirus model of congenital human cytomegalovirus infection. Birth Defects Orig Artic Ser. 1984;20(1):233–241. [PubMed] [Google Scholar]
- 12.Schleiss MR. Nonprimate models of congenital cytomegalovirus (CMV) infection: gaining insight into pathogenesis and prevention of disease in newborns. ILAR J. 2006;47(1):65–72. doi: 10.1093/ilar.47.1.65. [DOI] [PubMed] [Google Scholar]
- 13.Schleiss MR, Bourne N, Bernstein DI. Preconception vaccination with a glycoprotein B (gB) DNA vaccine protects against cytomegalovirus (CMV) transmission in the guinea pig model of congenital CMV infection. J Infect Dis. 2003;188(12):1868–1874. doi: 10.1086/379839. [DOI] [PubMed] [Google Scholar]
- 14.Schleiss MR, Bourne N, Stroup G, Bravo FJ, Jensen NJ, Bernstein DI. Protection against congenital cytomegalovirus infection and disease in guinea pigs, conferred by a purified recombinant glycoprotein B vaccine. J Infect Dis. 2004;189(8):1374–1381. doi: 10.1086/382751. [DOI] [PubMed] [Google Scholar]
- 15.Walter EA, Greenberg PD, Gilbert MJ, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333(16):1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 16.Schleiss MR, Lacayo JC, Belkaid Y, et al. Preconceptual administration of an alphavirus replicon UL83 (pp65 homolog) vaccine induces humoral and cellular immunity and improves pregnancy outcome in the guinea pig model of congenital cytomegalovirus infection. J Infect Dis. 2007;195(6):789–798. doi: 10.1086/511982. [DOI] [PubMed] [Google Scholar]
- 17.Browne EP, Wing B, Coleman D, Shenk T. Altered cellular mRNA levels in human cytomegalovirus-infected fibroblasts: viral block to the accumulation of antiviral mRNAs. Journal of virology. 2001;75(24):12319–12330. doi: 10.1128/JVI.75.24.12319-12330.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abate DA, Watanabe S, Mocarski ES. Major human cytomegalovirus structural protein pp65 (ppUL83) prevents interferon response factor 3 activation in the interferon response. J Virol. 2004;78(20):10995–11006. doi: 10.1128/JVI.78.20.10995-11006.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schleiss MR, Mcgregor A, Jensen NJ, Erdem G, Aktan L. Molecular characterization of the guinea pig cytomegalovirus UL83 (pp65) protein homolog. Virus Genes. 1999;19(3):205–221. doi: 10.1023/a:1008136714136. [DOI] [PubMed] [Google Scholar]
- 20.Mcgregor A, Liu F, Schleiss MR. Molecular, biological, and in vivo characterization of the guinea pig cytomegalovirus (CMV) homologs of the human CMV matrix proteins pp71 (UL82) and pp65 (UL83) Journal of virology. 2004;78(18):9872–9889. doi: 10.1128/JVI.78.18.9872-9889.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wills MR, Carmichael AJ, Mynard K, et al. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. Journal of virology. 1996;70(11):7569–7579. doi: 10.1128/jvi.70.11.7569-7579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mcgregor A, Liu F, Schleiss MR. Molecular, biological, and in vivo characterization of the guinea pig cytomegalovirus (CMV) homologs of the human CMV matrix proteins pp71 (UL82) and pp65 (UL83) J Virol. 2004;78(18):9872–9889. doi: 10.1128/JVI.78.18.9872-9889.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schleiss MR. Cloning and characterization of the guinea pig cytomegalovirus glycoprotein B gene. Virology. 1994;202(1):173–185. doi: 10.1006/viro.1994.1333. [DOI] [PubMed] [Google Scholar]
- 24.Griffith BP, Chen M, Isom HC. Role of primary and secondary maternal viremia in transplacental guinea pig cytomegalovirus transfer. J Virol. 1990;64(5):1991–1997. doi: 10.1128/jvi.64.5.1991-1997.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffith BP, Aquino-De Jesus MJ. Guinea pig model of congenital cytomegalovirus infection. Transplant Proc. 23(3 Suppl 3):29–31. discussion 31 (1991) [PubMed] [Google Scholar]
- 26.Schleiss MR, Choi KY, Anderson J, et al. Glycoprotein B (gB) vaccines adjuvanted with AS01 or AS02 protect female guinea pigs against cytomegalovirus (CMV) viremia and offspring mortality in a CMV-challenge model. Vaccine. 2013 doi: 10.1016/j.vaccine.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leviton MP, Lacayo JC, Choi KY, Hernandez-Alvarado N, Wey A, Schleiss MR. An Attenuated Cytomegalovirus Vaccine with a Deletion of a Viral Chemokine Gene Is Protective against Congenital CMV Transmission in a Guinea Pig Model. Clin Dev Immunol. 2013;2013:906948. doi: 10.1155/2013/906948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bia FJ, Griffith BP, Tarsio M, Hsiung GD. Vaccination for the prevention of maternal and fetal infection with guinea pig cytomegalovirus. J Infect Dis. 1980;142(5):732–738. doi: 10.1093/infdis/142.5.732. [DOI] [PubMed] [Google Scholar]
- 29.Cicin-Sain L, Bubic I, Schnee M, et al. Targeted deletion of regions rich in immune-evasive genes from the cytomegalovirus genome as a novel vaccine strategy. Journal of virology. 2007;81(24):13825–13834. doi: 10.1128/JVI.01911-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohr CA, Cicin-Sain L, Wagner M, et al. Engineering of cytomegalovirus genomes for recombinant live herpesvirus vaccines. Int J Med Microbiol. 2008;298(1–2):115–125. doi: 10.1016/j.ijmm.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Crumpler MM, Choi KY, Mcvoy MA, Schleiss MR. A live guinea pig cytomegalovirus vaccine deleted of three putative immune evasion genes is highly attenuated but remains immunogenic in a vaccine/challenge model of congenital cytomegalovirus infection. Vaccine. 2009;27(31):4209–4218. doi: 10.1016/j.vaccine.2009.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soderberg-Naucler C. Does cytomegalovirus play a causative role in the development of various inflammatory diseases and cancer? J Intern Med. 2006;259(3):219–246. doi: 10.1111/j.1365-2796.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- 33.Schleiss MR, Lacayo JC, Belkaid Y, et al. Preconceptual administration of an alphavirus replicon UL83 (pp65 homolog) vaccine induces humoral and cellular immunity and improves pregnancy outcome in the guinea pig model of congenital cytomegalovirus infection. The Journal of infectious diseases. 2007;195(6):789–798. doi: 10.1086/511982. [DOI] [PubMed] [Google Scholar]
- 34.Browne EP, Shenk T. Human cytomegalovirus UL83-coded pp65 virion protein inhibits antiviral gene expression in infected cells. Proc Natl Acad Sci U S A. 2003;100(20):11439–11444. doi: 10.1073/pnas.1534570100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abate DA, Watanabe S, Mocarski ES. Major human cytomegalovirus structural protein pp65 (ppUL83) prevents interferon response factor 3 activation in the interferon response. Journal of virology. 2004;78(20):10995–11006. doi: 10.1128/JVI.78.20.10995-11006.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Griffith BP, Mccormick SR, Fong CK, Lavallee JT, Lucia HL, Goff E. The placenta as a site of cytomegalovirus infection in guinea pigs. Journal of virology. 1985;55(2):402–409. doi: 10.1128/jvi.55.2.402-409.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Griffith BP, Isom HC, Lavallee JT. Cellular localization of cytomegalovirus nucleic acids in guinea pig salivary glands by in situ hybridization. J Virol Methods. 1990;27(2):145–157. doi: 10.1016/0166-0934(90)90131-x. [DOI] [PubMed] [Google Scholar]
- 38.Hashimoto K, Yamada S, Katano H, et al. Effects of immunization of pregnant guinea pigs with guinea pig cytomegalovirus glycoprotein B on viral spread in the placenta. Vaccine. 2013 doi: 10.1016/j.vaccine.2013.04.078. [DOI] [PubMed] [Google Scholar]
- 39.Nozawa N, Yamamoto Y, Fukui Y, et al. Identification of a 1.6 kb genome locus of guinea pig cytomegalovirus required for efficient viral growth in animals but not in cell culture. Virology. 2008;379(1):45–54. doi: 10.1016/j.virol.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 40.Yamada S, Nozawa N, Katano H, et al. Characterization of the guinea pig cytomegalovirus genome locus that encodes homologs of human cytomegalovirus major immediate-early genes, UL128, and UL130. Virology. 2009;391(1):99–106. doi: 10.1016/j.virol.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 41.Auerbach M, Yan D, Fouts A, et al. Characterization of the guinea pig CMV gH/gL/GP129/GP131/GP133 complex in infection and spread. Virology. 2013 doi: 10.1016/j.virol.2013.03.008. [DOI] [PubMed] [Google Scholar]