Abstract
Significance: Radiation exposure as a result of radiation treatment, accident, or terrorism may cause serious problems such as deficiency due to necrosis or loss of function, fibrosis, or intractable ulcers in the tissues and organs. When the skin, bone, oral mucous membrane, guts, or salivary glands are damaged by ionizing radiation, the management and treatment are very lengthy and difficult.
Critical Issues: In severe and irreversible injuries, surgery remains the mainstay of treatment. Several surgical procedures, such as debridement, skin grafting, and local and free-vascularized flaps, are widely used.
Recent Advances: In specific cases of major morbidity or in high-risk patients, a newly developed therapy using a patient's own stem cells is safe and effective. Adipose tissue, normally a rich source of mesenchymal stem cells, which are similar to those from the bone marrow, can be harvested, since the procedure is easy, and abundant tissue can be obtained with minimal invasiveness.
Future Directions: Based on the molecular basis of radiation injuries, several prospective treatments are under development. Single-nucleotide polymorphisms focus on an individual's sensitivity to radiation in radiogenomics, and the pathology of radiation fibrosis or the effect of radiation on wound healing is being studied and will lead to new insight into the treatment of radiation injuries. Protectors and mitigators are being actively investigated in terms of the timing of administration or dose.

Sadanori Akita, MD, PhD
Scope and Significance
Damage to normal tissues remains the most important limiting factor in the treatment of cancer by radiotherapy. Radiation exposure by accident or terrorism may also cause serious problems such as deficiency or loss of function, fibrosis, and intractable ulcers in the tissues and organs. When the skin, bone, oral mucous membrane, digestive tract, or salivary glands are damaged by ionizing radiation, properly managing and treating these injuries can be a long and difficult process. Ionizing radiation causes damage to molecules through energy transmission. This energy generates highly reactive chemical products such as free ion radicals that can ultimately combine with normal body chemicals and react with cellular components, resulting in intracellular and molecular changes and damage. If lipids and proteins are extensively destroyed, the cellular DNA becomes a primary candidate for the biological and ultimately lethal effects of free radicals by ionizing radicals. Most ionizing radiation-induced damage can be repaired, and enzymatic pathways continue, but radiobiological death may occur when the cells lose their ability to divide and proliferate. Endogenous free radicals, which are collectively named reactive oxygen species (ROS), arise from mitochondrial oxidative metabolism and other reactions in cells.1 The estimated average generation rate is around 109 ROS per cell per day,2 which results in 106 oxidative DNA damage, 105 DNA single-strand breaks, and 0.1 DNA double-strand breaks per cell per day.1 Additionally, cellular and molecular targets leading to broader issues of signaling and concerns regarding systemic responses to radiation injuries have been investigated, as well as three temporal categories of acute, subacute, and late (chronic), and these are uninterrupted.3 Decreased proliferation, adhesion, and an increased apoptotic cell ratio of fibroblasts occur after whole-body irradiation.4 Early signs of epithelial damage include erythema—warmth and tenderness because of transient dilatation of vessels caused by vasoactive amines. The next phase of erythema is caused by vessel damage observed 2–3 weeks after irradiation. The last phase of erythema occurs 10–16 weeks after initial injury and is related to epidermal basal cell death. Appendages such as sweat glands are radioresistant. Erythema may be accompanied by dry desquamation or discoloration. Chronic effects are seen 4–6 months after irradiation. Skin becomes fibrotic with a thin epidermis, which can be easily damaged. Radiation-injured wounds result from the collective effects of fibrosis, necrosis, ulceration, and fistula. Hyper- and hypopigmentation of the skin arise from enhanced activity or damage to melanocytes or the melanocyte–melanosome complex.5
Clinical Relevance
Radiation injuries solely to skin in interventional procedures have been increasingly reported since the 1990s. The use of fluoroscopic techniques in complicated procedures with a long fluoroscopy time, without proper awareness or dose monitoring, results in a high incidence of these injuries. A rough estimate of the total number of X-ray procedures that have ever been performed is 3.6 billion procedures, and typically 1% of X-ray procedures are considered angiography and interventional radiology. Thus, some 36 million of these procedures have taken place, and the frequency of major radiation injury is estimated to be between 1:10,000 and 1:100,000 procedures, although the true risk is still unknown.6 Thus, 360 to 3,600 cases of major radiation injuries are estimated. In a detailed clinical study of radiation, skin effects occurred from percutaneous coronary intervention (PCI) of the right coronary artery in six chronic total occlusion patients (mild erythema; occurrence rate 1.5%). In most cases, erythema was seen vividly 4 weeks after PCI. A few days after PCI, early erythema can be detected through careful observation. At 7–10 days after PCI, most erythematous pigmentation can be detected. Four weeks after PCI, most skin erythema appears clearly; however, some cases of skin erythema occur without back pain. Subsequently, follow-up every 6 months is needed to detect the reappearance of erythema.7 In other infrequent, but socially and psychologically significant, causes of radiation injuries such as direct contact with radioactive materials, accidents at nuclear power plants, and possible terrorism, a greater impact of radiation injury can be observed and the quality of life of patients is severely impaired.
Translational Relevance
Ionizing radiation is often used for cancer treatment or diagnosis and may be harmful if the dose and duration are not appropriate. Normal tissue toxicity and sensitivity to irradiation are also factors to consider in the development of radiation injuries. As is often seen, local radiation injury occurs due to direct manipulation of high-intensity radioactive sources. Other causes of radiation injuries include terrorist attack and nuclear power plant accidents. Although the clinical course, severity, and prognosis of local radiation injuries are dependent on the type, source, dose, rate, duration, distribution, location, and size, it is useful to understand the pathogenesis from the cellular to molecular levels for current treatment and future potential.
Discussion
Safety of radiation for the body
Many calculations and studies of the effects of radiation on the human body have been performed in an attempt to maintain patients' safety. Many safety aspects are mentioned in radiation therapy for both external beam radiation therapy and radionuclide therapy. In both procedures, the goal is to deliver a tumoricidal radiation dose to a known tumor while sparing the normal tissue as much as possible. The concept of a minimal and maximal tissue tolerance dose (TD) was introduced and applied to the whole or partial organ volume that received a uniform dose of external beam radiation at as high as >1 Gy/min, with daily fractions of 1.8–2 Gy.8 Data were collected of the predicted TD, which was related to a 5% rate of complications within 5 years (TD5/5) of the maximum TD, TD50/5, which caused a 50% complication rate by 5 years. Depending on the tissue, a 20-Gy single-fraction dose could be biologically comparable to 50 Gy, which is typical for tumor cells, and 10 Gy, which includes some normal tissue. With radionuclide therapy, the tolerance of normal tissues seems greater, but is more variable. Variability is based on differences in dosimetry methodology and in heterogeneous distribution of the radionuclides, which may lead to complications of potential organ toxicity. Measurement and quantification of the heterogeneous radionuclide dose are considered less precise than those of external beam radiation. Heterogeneity of the dose deposition at the cellular level of radionuclides remains a concern.9
Effect of radiation on cells, signaling, and the genome
Radiogenomics in prediction of normal tissue toxicity
Radiogenomics aims to develop an assay capable of predicting which radiation-treated patients are most likely to progress after radiotherapy and may personalize and optimize radiation therapy. Single-nucleotide polymorphisms (SNPs) focus on an individual's sensitivity to radiation. An SNP is a DNA sequence alteration targeting a single nucleotide, a point mutation. Considering the polymorphism, the variant form, a minor allele, has to be present in at least 1% of the population. Even though the frequency of this variant may differ among ethnic groups or geographic regions, the genetic ancestral foundation is largely maintained, or small changes have occurred by natural selection. As human paired homologous chromosomes and each pair of chromosomes carry an allele of the polymorphism, each individual is homozygous for the common allele, and is heterozygous (two of each allele) or minor homozygous for the minor allele. It is estimated that there are 1 million SNPs among 3 billion nucleotide base pairs that cover the human genome. Since haplotype blocks are identical by descent and separated via short recombination hot spots, a genome-wide association study (GWAS), which is necessary for understanding human developmental history, can genotype just a few SNPs (tag SNPs) in a block to estimate nearly all the other SNPs in the same block. In the first GWAS to identify SNPs allied to adverse reactions in patients treated with radiotherapy, SNPs causing erectile dysfunction were found to be most significantly associated, and rs2268363 was located in the FSHR gene, the encoded product of which plays a role in male gonad development and function.10 Since this gene is not related to the radiation response, it might be impossible to detect this SNP with a candidate gene approach. In addition, 4 SNPs found to be related to erectile dysfunction have African ancestry and may not be discovered if searching and investigating only the European ancestry. In GWAS screening of lymphoblastoid cell lines, BORA, MAD2L1, PLK4, TPD52, and DEPDC1B SNPs were associated with radiation cytotoxicity.11 Even in SNP profiling to estimate the normal tissue response, it is clearly true that other traits and diseases that are likely to increase understanding of genetic variation are associated with radiosensitivity and novel pathways. Whole-genome sequencing and analysis of SNPs associated with the risk of disease have been demonstrated to produce useful and clinically relevant information for individual patients.12
Pathology of radiation fibrosis
Deregulated processes after radiation therapy have much in common with the processes of associated diseases that affect the heart, lung, skin, kidney, gastrointestinal tract, and liver. Of the secretory factors driving fibrosis, transforming growth factor beta-1 (TGFβ1), produced by wide-ranging inflammatory, mesenchymal, and epithelial cells, induced fibroblasts and other cells to transform into matrix-producing myofibroblasts. After myofibroblast activation, collagen production can continue independent of TGFβ1 by autocrine induction of connective tissue growth factor (CTGF). Oxidative stress, hypoxia, and microvascular insults are considered in terms of radiotherapy. Immediate oxidative damage to DNA, protein, and lipid is the primary chemical event. Cell death is a relevant biological response to these early chemical events; however, responses of sublethal damage to cells give rise of fibrogenesis. The direct action of ROS on the extracellular matrix (ECM) is related to this event, because ROS are able to release activated cytokines from extracellular reservoirs.
Microscopically, fibrosis is often focal in distribution and develops an interstitial fibrin network for a long time after radiation exposure. Microvascular injury is also observed. Intimal and media fibrosis causes arterial constriction and contributes to organ fibrosis with atrophy through ischemic hypoxia. Microvascular diminution leads to tissue ischemia, and tissue ischemia develops to fibrosis. Subintimal accumulation of lipid-burdened macrophages is observed in chronic radiation vasculitis, similar to atherosclerosis, where macrophages are a major source of ROS and fibrogenic cytokines. In the radiated human ileum and colon, marked fibrosis of the submucosa, muscularis propria, and subserosa related to the increase of both CTGF mRNA and protein after pelvic radiotherapy has been observed.13 Deregulated ECM metabolism after radiotherapy is accompanied by the increase of N- and C-terminal collagen peptides assayed in interstitial fluid from patients postradiotherapy.14
A study using breast skin 1–5 years after 50 Gy in 25 fractions demonstrated a twofold increase of collagen I and III in the early years and increased collagen degradation.15 Increased ECM degradation and synthesis are specific characteristics of chronic fibrosis. Increased TGFβ1 immunostaining and phosphorylated Smad 2/3 expression are seen in irradiated vascular smooth muscle cells (VSMCs), but not in nonirradiated cells. When human endothelial cells and VSMCs are irradiated, VSMCs stimulate their transition to myofibroblasts.16 Radiation fibrosis in humans is related to increased collagen synthesis, deteriorated remodeling, and continual activation of fibrogenic growth factors such as TGFβ1 and CTGF. It is difficult to establish an irradiated skin fibroblast cell culture, because irradiated skin in culture has residual DNA damage and radiation-induced differentiation and demonstrates long-term cell cycle arrest. Both postmitotic and mitotic fibroblasts obtained from irradiated skin have contractile and secretory features due to the induction of TGFβ1, upregulating collagen synthesis and alpha–smooth muscle actin expression.17 In dermal fibroblasts obtained from eight patients postmastectomy radiotherapy, the differentiation state was significantly different from the preirradiation cells according to the severity of clinical fibrosis.18 In contrast to radiated skin, primary cell cultures from human intestines demonstrate increased CTGF mRNA expression and collagen I protein.13,19 Although microvascular injury provides early stimulus of fibrosis and induction of CTGF is dependent on TGFβ1/Smad signaling in normal skin fibroblasts, the active TGFβ1 level in radiation enteropathy remains very low.20 Rho/ROCK (rho kinase) activation may be reversed in the cellular phenotype with inhibition of fibrosis in an experimental rat radiation fibrosis model using a rho/ROCK inhibitor statin, and this is very relevant to clinical phenomena.21 As TGFβ1 is one of the most characterized fibrogenic cytokines and can activate myofibroblast progenitors and ECM may be a reservoir of inactive TGFβ1, oxidative leverage is required to release activated TGFβ1. ROS are created by radiation exposure, and TGFβ1 is activated by radiation exposure and upregulated collagen synthesis, which is enhanced by autocrine induction of CTGF. TGFβ1 is also triggered by radiation through a transcription factor, AP-1 family, and also activated by the stress response to DNA damage in skin fibroblasts. A threshold of TGFβ1 activation may exist, and a very early TGFβ1 cascade may be a target of ameliorating radiation-induced late effects.22 High-dose ionizing radiation in rats causes ROS in radiation fibrosis, and ROS release activated TGFβ1 from the ECM and contributes to intracellular signaling molecules in the TGFβ1-dependent pathway.23 Antioxidant therapy employed after high and single dose of radiation decreases subsequent fibrosis in animals, and the problem remains if these data are related to humans and fractionated radiotherapy.
Effect of radiation on wound healing
Tissue damage by radiation may lead to dermatitis, skin necrosis, and ulcer. In breast cancer patients, an estimated half of the patients underwent radiotherapy. In the course of radiotherapy, over 70% patients experience radiodermatitis, ranging from faint erythema, dry desquamation, moist desquamation, and necrosis. After radiotherapy, patients with radiodermatitis may experience skin fibrosis, which is demonstrated as a change of skin texture, retraction, discomfort, telangiectasia, pain, and itching.24 Grading criteria for radiation dermatitis were introduced by the National Cancer Institute (NCI) in 2003 and comprise grade 1: faint erythema or dry desquamation; grade 2: moderate to brisk erythema, patchy moist desquamation, mostly confined to skin folds and creases, and moderate edema; grade 3: moist desquamation other than skin folds and creases and bleeding induced by minor trauma or abrasion; grade 4: skin necrosis or ulceration of the full-thickness dermis, spontaneous bleeding from involved sites; and grade 5: death.25 In a randomized phase-III Radiation Therapy Oncology Group (RTOG) study (RTOG 9003), the incidence of grade 3 or worse acute side effects was 35% for conventional fractionation and 54.5%, 50.4%, and 58.8% for hyperfractionation, which divides the frequency of radiation more than usual, split-course-accelerated fractionation, and accelerated fractionation with a concomitant boost, respectively.26 In most patients, radiation dermatitis is mild to moderate (grade 1 and 2), but 20 to 25% of patients experience severe reactions.27 The incidence of severe reactions is dependent on the total radiation dose, dose per fraction, overall treatment time, beam type, energy, and surface area of the skin. In the RTOG 9003 study, the rates of grade 3 and 4 skin reactions were higher in hyperfractionation (11%) and accelerated fractionations with a concomitant boost (11%) than in standardized fractionation (7%).26 In a more recent phase III study, most patients who received over 60 Gy with a concomitant boost regimen, with 53% also receiving chemotherapy, demonstrated mean rates of grades 2, 3, and 4 radiation dermatitis in 54%, 20%, and 4%, respectively.28 In the RTOG 9003, these rates were 49%, 8%, and 0%, respectively.26
Other than skin, radiation injuries are often observed in the oral cavity and intestines. Radiation-induced xerostomia is a common complication of radiotherapy after head and neck cancer. Salivary flow reduces to 50% to 70% of the baseline after 10–16-Gy radiation and is undetectable after 40–42 Gy,29 and radiation-induced salivary gland damage has been investigated. The serum amylase levels rose immediately after radiotherapy, peaked at 48 h, returned to the normal level by day 7, and were undetectable by day 10. Radiation is thought to induce apoptosis of serous cells. Radiation-induced damage to the salivary glands changes the volume and pH consistency of secreted saliva, and secretions become thick and persistent with increased acidity after radiotherapy. In intensity-modulated radiotherapy with partial-volume irradiation, long-term recovery of salivary gland function is reported up to 2 years after completion of radiotherapy.30
Radiation enteritis can be caused by direct and indirect targeting of cellular DNA, leading to impaired cell division or immediate cell death.31 The most vulnerable cells to radiation are rapidly replicating cells during the G2 and M phases of mitosis, predisposing to radiation-induced damage, the continually replicating intestinal mucosa, especially stem cells residing in the intestinal crypts. Damage and death of these replicating and differentiating cells may lead to a decrease of differentiated intestinal epithelial cells and loss of mucosal integrity.31
Protectors against and mitigators of radiation injury
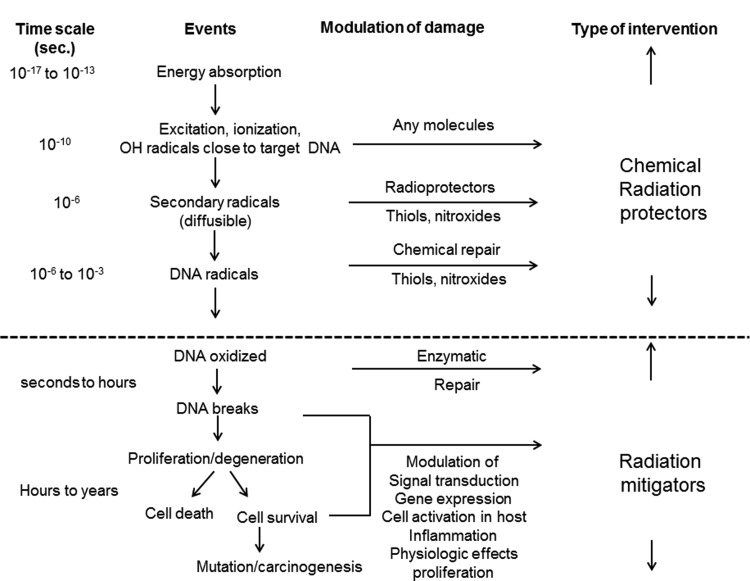
Agents delivered before or at the time of irradiation with the intent of preventing or reducing damage to normal tissues are known as radioprotectors, while agents supplied at the time of or after irradiation is completed, but before the manifestation of normal tissue toxicity, are called radiomitigators (Fig. 1).
Figure 1.
Radioprotectors and radiomitigators.
After DNA damage has occurred, many processes take place in the damaged cells, tissue, and organs, including DNA repair activation, signal transduction, expression of radiation-response genes, enhancement of proliferation, and regulation of inflammation. These pathways are important for cell or tissue recovery after radiation exposure, but may also play a pivotal role in the development of tissue toxicity.
Radioprotectors
Free radicals are responsible for continuing a large amount of damage triggered by ionizing radiation. Thus, agents to protect cells from primary free radical damage need to be present at the time of radiation and in sufficient enough concentrations to compete with free radicals produced through radical-scavenging mechanisms. Plenty of radical scavengers and antioxidants exist, which may limit the oxidative stress induced by free radicals. Superoxide dismutase (SOD), catalase, glutathione peroxidase, and glutathione reductase are examples of naturally occurring antioxidants that defend against free radical–mediated damage; in contrast, the substrates are specific to each enzyme. Generally, the antioxidants used have a low molecular weight, such as hydrogen atom–donating reducing agents, for example ascorbic acid, tocopherols, polyphenols, and thiols such as glutathione. Oxidants are neutralized by hydrogen atom donation, which results in a less- or nonreactive product compared to the original oxidant and a radical product formed by the antioxidant that is no longer detrimental. Scavenging hydroxyl radicals, which are formed by radiation-induced damage, can be neutralized by almost all unsaturated organic molecules or molecules that can donate hydrogen atoms. Although hydroxyl radicals can be scavenged with equal efficacy by both radioprotectors and antioxidants, cellular and in vivo radioprotection is noted only in radioprotectors. Thus, secondary species generated by hydroxyl radicals are responsible for DNA damage. Thiols such as amifostine and newly developing nitroxides have abundant reactivity to sufficiently scavenge secondary radicals. In contrast, antioxidants such as vitamin C and tocopherol (vitamin E) do not serve as radioprotectors.
Amifostine: a clinically used radioprotector
Sulfhydryl compounds such as cysteine and cysteamine have been known to serve as radioprotectors through free radical scavenging and the donation of hydrogen atoms. Since the first depiction of sulfhydryl/thiol compounds as radioprotectors, more-efficient and less-toxic agents have been developed, such as amifostine. Amifostine is a phosphorothioate that does not enter cells until it is dephosphorylated by alkaline phosphatase.32 When dephosphorylated, the agents freely diffuse into cells and work as free radical scavengers. Amifostine has been proven to disseminate more rapidly in normal tissues than in tumor tissues.33 This may be explained by several factors, including tumor blood flow, acidosis of tumors, and lower expression of alkaline phosphatase. Induction of hypoxia via escalated oxygen consumption and DNA concentration may be another mechanism.34,35 In many clinical trials, including phase III trials, amifostine has been investigated for the prevention and reduction of acute and late xerostomia, mucositis, dysphagia, dermatitis, pneumonitis, proctitis, and cystitis. Recently, amifostine was tested in randomized trials led by the American Society of Clinical Oncology (ASCO) for the purpose of preventing xerostomia during fractionated radiotherapy, and proved to reduce radiation-induced toxicity.36 In the 2008 ASCO guidelines, amifostine is not supported as a chemotherapy agent targeting advanced head and neck malignancies. Amifostine is recommended for the prevention of mucositis with radiotherapy associated with head and neck malignancies or esophagitis associated with non-small-cell lung cancer. Amifostine toxicity and tumor protection are controversial and have been discussed.37
Nitroxides in clinical development
In addition to amifostine, which is the only clinically available radioprotector, nitroxides are the most promising agents as radiation protectors. Both preclinical in vivo and in vitro studies have demonstrated the oxidized form of a nitroxide as a radioprotector.38,39 The leading compound in this class for radioprotection is tempol (4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl). A phase I clinical trial in patients receiving whole-bran radiotherapy demonstrated that tempol is effective in preventing radiation-induced alopecia to mild (grade 1 and 2) toxicity, and the systemic level of tempol is negligible after topical application.40
Other antioxidants
Antioxidants are radioprotectors, and SOD is used to prevent radiation-induced toxicity. SOD is an enzyme present in human cells which catalyzes the conversion of superoxide to oxygen and hydrogen peroxide and works as an antioxidant after radiation. As SOD is too large to enter cells freely, gene therapy has been used to increase SOD levels in tissues and to prevent or attenuate radiation-induced mucositis in mice41 or fibrosis in pigs.42
Radioprotectors other than antioxidants
A hormone, melatonin, is considered to act as an antioxidant and increase the expression of antioxidant enzymes such as SOD and glutathione peroxidase. Clinically, melatonin was tested as a radiosensitizer against tumor cells and as a radioprotector for normal cells. In this study, there was no evidence of a longer survival time or better neurologic function than in a historically compared control group.43
Targeting signal transduction pathways have been explored as a mechanism to protect organisms and cells and tissues from ionizing radiation. A polypeptide, CBLB502, delivered before or shortly after radiation protected mice and monkeys from lethal total-body irradiation with less damage to the intestine and bone marrow.44 This type of protector is suitable for therapeutic radiation as well as accidental exposure when large areas of the intestine or bone marrow are affected.
Mitigators of radiation
Late-normal tissue toxicity induced by radiation progresses in tissue before the appearance of toxicity. This includes progressive mitotic cell death and eternally active cytokine cascades, which lead to vascular impairment, local tissue hypoxia, and excessive ECM deposition.45 The primary aim of radiation mitigators is to stop these cascades or to prevent the continuation of damage, thus reducing the expression of toxicity. In reverse, radiation mitigators can be delivered shortly after exposure to reorganize a critical cell compartment of mucous membranes or bone marrow. In this situation, mitigators are agents used to avoid acute toxicity. In radiological terrorism, nuclear accidents, and space research, mitigators are a focus in the development of chemopreventatives to decrease carcinogenesis of total-body exposure.
Cytokines as mitigators
Many cytokines and growth factors are actually radiation mitigators if used at or near the time of radiation. They stimulate the differentiation of stem cells in the bone marrow, intestine, or mucosa and inhibit bone marrow failure, gastrointestinal syndrome, or mucositis or xerostomia after total-body exposure. Granulocyte colony-stimulating factor is effectively able to reduce the lethality of total-body radiation exposure by assisting in bone marrow recovery.46 Another growth factor, keratinocyte growth factor (KGF), fibroblast growth factor 7 (FGF-7), stimulates many cellular processes, including differentiation, proliferation, DNA repair, and detoxification of ROS.47 The characteristics of KGF are associated with the recovery of mucosa after ionizing radiation and prevent radiation-induced xerostomia48 and mucositis.49 The use of palifermin, a recombinant human KGF, in patients with mucositis after cytotoxic therapy50 led to evaluating its use in patients with head and neck cancer who were receiving chemoradiotherapy and experiencing severe and extensive mucositis. Patients receiving hyperfractionated radiotherapy in a phase II study demonstrated a lower incidence of mucositis and shorter duration.51 Another FGF family cytokine, basic FGF (bFGF) or FGF-2, is primarily found as a potent angiogenic growth factor because of its high capacity for inducing endothelial cell proliferation and migration as well as smooth cell proliferation,52 and also accelerates second-degree burn wound healing and improves scar quality.53 In burns, resurfacing with a dermal component is required, and bFGF stimulates wound healing and enhances human skin-derived mesenchymal stem cells under serum-free conditions in a dose-dependent manner.54 In a mini-pig receiving 10-Gy external X-ray radiation to the skin and subcutaneous tissue with a tissue expander underneath the subcutaneous tissue, immediate use of bFGF after radiation successfully protected the tissue from subsequent damage by increasing epithelial proliferation, suppressing the induction of apoptotic cells, and enhancing angiogenic cells.55
Radiation fibrosis is considered late radiation damage, and TGFβ1 plays a pivotal role in the establishment of radiation fibrosis. For the purpose of neutralizing TGF-β antibodies to prevent lung fibrosis in rats56 or using a small molecule to inhibit TGFβ signaling, halofuginone was tested in mice.57
Surgical treatment for radiation injury
When conservative therapy such as protectors and mitigators fail to function for late effects, surgery is attempted to remodel and maintain the structures, since irreversible changes and voluntary recovery are not expected. Plastic and reconstructive surgery consists of necrotic tissue removal, i.e., debridement, skin grafting, and local and free-vascularized flaps; in essence, surgical reconstruction, thorough debridement, and anatomically appropriate reconstruction with minor morbidity. This may require multistep surgeries, since the extent and depth of the damaged tissue are obscure.58 Radiation wounds may persist for years after radiation with severe pain and skin lesions, limiting range of motion and activities of daily living.59 Recent advances in the knowledge and techniques for using somatic progenitor cells or stem cells has promoted less-invasive and more-effective therapy. A mini-pig chronic radiation syndrome model with 50- and 60-Gy cobalt irradiation was similar to humans after a latency period of several weeks and developed skin necrosis over months. Autologous cultured bone marrow–derived stem cells (BMMSCs) applied multiple times enhanced lymphocyte accumulation at the border between the dermis and subdermis and improved vascularization.60 In humans, the application of multiple cultured autologous mesenchymal stem cells with skin grafting modulated the radiation inflammatory process by decreasing C-reactive protein.58 Two-dimensional gel electrophoretic gel proteomic analysis of the intracellular protein of BMMSCs and adipose-derived stem cells (ADSCs) revealed that the proteins were similar, which suggests that ADSCs can replace BMMSCs in cell therapy.61 Using noncultured ADSCs, which are 100 times more abundant than BMMSCs per tissue, chronic radiation injuries, including those adjacent to the major organs such as the carotid artery, were effectively treated and showed no recurrence in patients with autologous noncultured adipose-derived regenerative cells, which contain many adipose-derived regenerative cells.62 By subcutaneously harvesting autologous adipose-derived cells with a minimal cell-harvesting port and morbidity in patients suffering from chronic radiation wounds and fibrosis for many years after high radiation exposing the bone, tendon, ligament, or cartilage, and demonstrating either necrosis or infection, with meticulous surgical debridement but no additional skin grafting or flap, angiogenic factors such as bFGF and artificial dermis successfully resulted in complete regeneration and its maintenance for months.59,62 As seen in Fig. 2, a 78-year old female patient who underwent a standardized mastectomy and 60-Gy fractionated radiation developed exposed costal rib cartilage and bone in her right chest (Fig. 2). Wound contracture due to fibrosis also occurred (Fig. 2, red arrows). Extensive debridement with autologous adipose-derived regenerative cell therapy with artificial dermis (Fig. 3A, B) healed within 5 weeks, and the scar is more matured and fibrosis is improved (Fig. 3C, black arrows). Ex vivo cell culture of adipose-derived cells to investigate their in vitro properties demonstrated cell proliferation and differentiation. An ADSC-like appearance emerged in a regular cell culture medium at day 3 (Fig. 4A). Cell growth was uneventful by day 8 (Fig. 4B). These cells are shown in an ES cell culture medium at day 15 at confluence (Fig. 4C), and in the ES cell culture medium at day 15 by differentiation induction (Fig. 4D). In these cells, in ES cell culture medium at day 15, lipid is marked in red by Oil-Red-O staining (Fig. 4E). In this ex vivo culture, these cells may be a characteristic of stem cells with the ability to proliferate and differentiate. Therapy with the patient's own abundant adipose-derived regenerative and stem cells with no need of an extensive cell culture facility at a cell processing center can be applied to other surgeries such as for liver disease, type 1 diabetes mellitus, and Crohn's disease,63 and further clinical use may be explored in these organs with radiation injuries.
Figure 2.

Breast cancer, standardized mastectomy (Halsted), and 60-Gy irradiation. Ulcer and scar contracture continued for 35 years. Arrows indicate the scar contractures. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Figure 3.
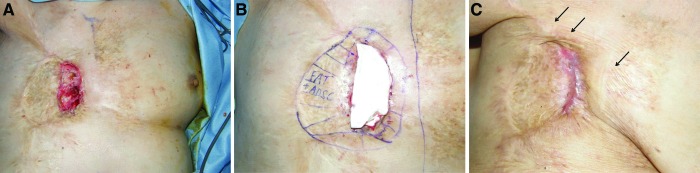
(A) Meticulous debridement, including necrotic cartilage and bone. (B, C) After debridement, adipose-derived regenerative cells were injected in the edges and bottom of the wound and covered with artificial dermis. The arrows in (C) indicate the loosened tissue fibroses in the axilla and in the parasternal and the peri-wounded areas. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Figure 4.
Ex vivo cultured adipose-derived stem cells, days 3 (A), 8 (B), and 15 (C–E). (D) Induction medium; (E) Oil Red-O. Magnification, ×100. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Take-Home Messages.
• Development of radiation injuries can be a long-term process.
• The study of radiogenomics for the prediction of normal tissue toxicity shows single-nucleotide polymorphisms (SNPs) in 1% of the population regardless of racial/ethnic background.
• Radioprotectors and radiomitigators can be used before or after radiation exposure to reduce injury.
• Surgery remains the cornerstone of treatment for chronic ulcers and fibrosis.
• Novel therapy using noncultured adipose-derived regenerative cells (including stem cells) is less invasive and has proven effective in elderly patients with meticulous surgical debridement.
Abbreviations and Acronyms
- ADSC
adipose-derived stem cell
- ASCO
American Society of Clinical Oncology
- BMMSC
bone marrow-derived stem cell
- CTGF
connective tissue growth factor
- ECM
extracellular matrix
- FGF
fibroblast growth factor
- GWAS
genome-wide association study
- KGF
keratinocyte growth factor
- PCI
percutaneous coronary intervention
- ROS
reactive oxygen species
- SNP
single-nucleotide polymorphism
- SOD
superoxide dismutase
- TD
tolerance dose
- TGFβ1
transforming growth factor beta-1
- VSMC
vascular smooth muscle cells
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the author listed.No ghostwriterswere used towrite this article.
About the Authors
Sadanori Akita, MD, PhD, is an Associate Professor at the Department of Plastic and Reconstructive Surgery of Nagasaki University Hospital. He is also a visiting professor of St. Petersburg Medical Academy of Postgraduate Education, St. Petersburg, Russia.
References
- 1.Pollycove M. and Feinendegen LE: Radiation-induced versus endogenous DNA damage: possible effect of inducible protective response in mitigating endogenous damage. Hum Exp Toxicol 2003; 22:290. [DOI] [PubMed] [Google Scholar]
- 2.Beckman KD. and Ames BN: The free radical theory of aging matures. Physiol Rev 1998; 78:547. [DOI] [PubMed] [Google Scholar]
- 3.McBride WH: Cytokine cascades in late normal tissue radiation responses. Int J Radiat Oncol Biol Phys 1995; 33:233. [DOI] [PubMed] [Google Scholar]
- 4.Qu J, Cheng T, Shi C, Lin Y, and Ran X: A study on the activity of fibroblast cells in connection with tissue recovery in the wounds of skin injury after whole-body irradiation. J Radiat Res 2004; 45:341. [DOI] [PubMed] [Google Scholar]
- 5.Dressler J, Busuttil A, Koch R, and Harrison DJ: Sequence of melanocyte migration into scar tissue Int J Legal Med 2001; 115:61. [DOI] [PubMed] [Google Scholar]
- 6.Miller DL, Balter S, Schueler BA, Wagner LK, Strauss KJ, Vano E: Clinical radiation management for fluoroscopically guided interventional procedures. Radiology 2010; 257:321. [DOI] [PubMed] [Google Scholar]
- 7.Kato M, Chida K, Sato T, Oosaka H, Tosa T, Munehisa M, and Kadowaki K: The necessary of follow-up for radiation skin injuries in patients after percutaneous coronary interventions: radiation skin injuries will often be overlooked clinically. Acta Radiol 2012; 53:1040. [DOI] [PubMed] [Google Scholar]
- 8.Rubin P. and Casarett GW: A direction for clinical radiation pathology: a tolerance dose. In: Frontiers of Radiation Oncology and Therapy, vol. 6, edited by Vaeth JM. Baltimore, MD: University Park Press, 1972, pp. 1–16 [Google Scholar]
- 9.Neti PV, and Howell RW: Log normal distribution of cellular uptake of radioactivity: implications for biologic responses to radiopharmaceuticals. J Nucl Med 2006; 47:1049. [PMC free article] [PubMed] [Google Scholar]
- 10.Kerns SL, Ostrer H, Stock R, Li W, Moore J, Pearlman A, Campbell C, Shao Y, Stone N, Nusnetz L, and Rosenstein BS: Genome-wide association study to identify single nucleotide polymorphisms (SNPs) associated with the development of erectile dysfunction in African-American men after radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 2010; 78:1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niu N, Qin Y, Firdley BL, Hou J, Kalari KR, Zhu M, Wu TY, Jenkins GD, Batzler A, and Wand L: Radiation pharmacogenomics: a genome-wide association approach to identify radiation response biomarkers using human lymphoblastoid cell lines. Genome Res 2010; 20:1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashley EA, Butte AJ, Wheeler MT, Chen R, Klein TE, Dewey FE, Dudley JT, Ormond KE, Pavlovic A, Morgan AA, Pushkarev D, Neff NF, Hudgins L, Gong L, Hodges LM, Berlin DS, Thom CF, Sanqkuhl K, Hebert JM, Woon M, Sagreiya H, Whaley R, Knowles JW, Chou MF, Thakuria JV, Rosenbaum AM, Zaranek AW, Church GM, Greely HT, Quake SR, and Altman RB: Clinical assessment incorporating a personal genome. Lancet 2010; 375:1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vozenin-Brotons MC, Milliat F, Sabourin JC, de Gouville AC, Francois A, Lasser P, Morice P, Haie-Meder C, Lusinchi A, Antoun S, Bourhis J, Mather D, Grinsky T, and Alqueperse J: Fibrogenic signals in patients with radiation enteritis are associated with increased connective tissue growth factor expression. Int J Radiot Oncol Bio Phy 2003; 56:561. [DOI] [PubMed] [Google Scholar]
- 14.Autio P, Saarto T, Tenhunen M, Elomaa I, Risrteli J, Lahtinen T.Demonstration of increased collagen synthesis in irradiated human skin in vivo. Br J Cancer 1998; 77:2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sassi M, Jukkola A, Riekki R, Hoyhtya M, Risteli L, Oikarinen A, and Risteli J: Type I collagen turnover and cross-linking are increased in irradiated skin of breast cancer patients. Radiother Oncol 2001; 58:317. [DOI] [PubMed] [Google Scholar]
- 16.Milliat F, Francois A, Isoir M, Deutsch E, Tamarat R, Tarlet G, Atfi A, Validire P, Bourhis J, Sabourin JC, and Benderitter M: Influence of endothelial cells on vascular smooth muscle cells phenotype after irradiation: implication in radiation-induced vascular damage. Am J Pathol 2006; 169:1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeSimone JN, Dolezalova H, Redpath JL, and Stanbridge EJ: Prolonged cell cycle arrest in irradiated human diploid skin fibroblasts: the role of nutrient deprivation. Radiat Res 2000; 153:131. [DOI] [PubMed] [Google Scholar]
- 18.Herskin C, Bentzen SM, Overgaard J, Overgaard M, Bamberg M, and Rodemann HP: Differentiation state of subcutaneous fibrosis after radiotherapy. Radiother Oncol 1998; 47:263. [DOI] [PubMed] [Google Scholar]
- 19.Bourgier C, Haydont V, Millat F, Francois a, Holler V, Lasser P, Bourhis J, Mathe D, and Vozenin-Brotons MC: Inhibition of Rho kinase modulates radiation induced fibrogenic phenotypes in intestinal smooth muscle cell through alteration of the cytoskeleton and connective tissue growth factor expression. Gut 2005; 54:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haydont V, Riser BL, Aigueperse J, and Vozenin-Brotons MC: Specific signals involved in the long-term maintenance of radiation-induced fibrogenic differentiation: a role for CCN2 and low concentration of TGF-beta1. Am J Physiol Cell Physiol 2008; 294:C1332. [DOI] [PubMed] [Google Scholar]
- 21.Holler V, Buard V, Gaugler MH, Guipaud O, Baudelin C, Sache A, Perez Mdel R, Squiban C, Tamarat R, Milliat F, and Benderitter M: Pravastatin limits radiation-induced vascular dysfunction in the skin. J Invest Dermatol 2009; 129:1280. [DOI] [PubMed] [Google Scholar]
- 22.Anscher MS, Thrasher B, Zqonianin L, Rabbani ZN, Corbley MJ, Fu K, Sun L, Lee WC, Ling LE, and Vujaskovic Z: Small molecular inhibitor of transforming growth factor-beta protects against development of radiation-induced lung injury. Int J Radiat Oncol Biol Phys 2008; 71:829. [DOI] [PubMed] [Google Scholar]
- 23.Jackson IL, Chen L, Batinic-Haberle I, and Vujaskovic Z: Superoxide dismutase mimetic reduces hypoxia-induced O2*-, TGF-beta, and VEGF production by macrophages. Free Radic Res 2007; 41:8. [DOI] [PubMed] [Google Scholar]
- 24.Cox JD, Stetz J, and Pajak TF: Toxicity criteria of the radiation therapy oncology group (RTOG) and the European organization for research and treatment of cancer (EORTC). Int J Radiat Oncol Biol Phys 1995; 31:1341. [DOI] [PubMed] [Google Scholar]
- 25.Trotti A and Bentzen SM: The need for adverse effects reporting standards in oncology clinical trials. J Clin Oncol 2004; 22:19. [DOI] [PubMed] [Google Scholar]
- 26.Fu KK.Pajak TF, Trotti A, Jones CU, Spencer SA, Phillips TL, Garden AS, Ridge JA, Cooper JS, and Ang KK: A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys 2000; 48:7. [DOI] [PubMed] [Google Scholar]
- 27.Bonner JA, Harari PM, Giralt J, Azamia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, Ove R, Kies MS, Baselga J, Youssoufian H, Amellal N, Rowinsky EK, and Ang KK: Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl Med 2006; 354:567. [DOI] [PubMed] [Google Scholar]
- 28.Elliott EA, Wright JR, Swann RS, Nguyen-Tan F, Takita C, Bucci MK, Garden AS, Kim H, Hug EB, Eyu J, Greenberg M, Saxton JP, Ang K, Berk L; Radiation Therapy Oncology Group Trial 99–13: Phase III trial of an emulsion containing trolamine for the prevention of radiation dermatitis in patients with advanced squamous cell carcinoma of the head and neck: results of Radiation Therapy Oncology Group Trial 99–13. J Clin Oncol 2006; 24:2092. [DOI] [PubMed] [Google Scholar]
- 29.Chao KS, Deasy JO, Markman J, Haynie J, Perez CA, Purdy JA, and Low DA: A prospective study of salivary function sparing in patients with head-and-neck cancers receiving intensity-modulated or three-dimensional radiation therapy: initial results. Int J Radiat Oncol Bio Phys 2001; 49:907. [DOI] [PubMed] [Google Scholar]
- 30.Eisbruch A, Ten Haken RK, Kim HM, Marsh LH, and Ship JA: Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int Radiat Oncol Bio Phys 1999; 45:577. [DOI] [PubMed] [Google Scholar]
- 31.Bismar MM, and Sinicrope FA: Radiation enteritis. Curr Gastroentero Rep 2002; 4:361. [DOI] [PubMed] [Google Scholar]
- 32.Calabro-Jones PM, Fahey RC, Smoluk GD, and Ward JF: Alkaline phosphatase promotes radioprotection and accumulation of WR-1065 in V79-171 cells incubated in medium containing WR-2721. Int J Radiat Biol Relat Stud Phys Chem Med 1985; 47:23. [DOI] [PubMed] [Google Scholar]
- 33.Yuhas JM. Active versus passive absorption kinetics as the basis for selective protection of normal tissues by S-2-(3-aminopropylamono)-ethylphosphorothioic acid. Cancer Res 1980;40:1519. [PubMed] [Google Scholar]
- 34.Glover D, Negendank W, Delivoria-Papadopoulos M, and Glick JH: Alterations in oxygen transport following WR-2721. Int J Radiat Oncol Biol Phys 1984; 10:1565. [DOI] [PubMed] [Google Scholar]
- 35.Savoye C, Swenberg C, Hugot S, Sy D, Sabattier R, Charlier M, and Spotheim-Maurizot M: Thiol WR-1065 and disulphide WR-33278, two metabolites of the drug ethyol (WR-2721), protect DNA against fast neutron-induced strand breakage. Int J Radiat Biol 1997; 71:193. [DOI] [PubMed] [Google Scholar]
- 36.Hensley ML, Hagerty KL, Kewalramani T, Green DM, Meropol NJ, Wasserman TH, Cohen GI, Emami B, Gradishar WJ, Mitchell RB, Thigpen JT, Trotti A, 3rd, von Hoff D, and Schuchter LM: American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants. J Clin Oncol 2009; 27:127. [DOI] [PubMed] [Google Scholar]
- 37.Brizel DM. and Overgaard J: Does amifostine have a role in chemoradiation teatmentt? Lancet Oncol 2003; 4:378. [DOI] [PubMed] [Google Scholar]
- 38.Hahn SM, Wilson L, Krishna CM, Liebmann J, DeGraff W, Gamson J, Samuni A, Venzon D, and Mitchell JB: Identification of nitroxide radioprotectors. Radiat Res 1992; 132:87. [PubMed] [Google Scholar]
- 39.Hahn SM, Krishna MC, DeLuca Am, Coffin D, and Mitchell JB: Evaluation of the hydroxylamine Tempo-H as an in vivo radioprotector. Free Radic Biol Med 2000; 28:953. [DOI] [PubMed] [Google Scholar]
- 40.Metz JM, Smith D, Mick R, Lustig R, Mitchell J, Cherakuri M, Glatstein E, and Hahn SM: A phase I study of topical Tempol for the prevention of alopecia induced by whole brain radiotherapy. Clin Cancer Res 2004; 10:6411. [DOI] [PubMed] [Google Scholar]
- 41.Guo H, Seixas-Silva JA, Jr, Epperly MW, Gretton JE, Shin DM, Bar-Sagi D, Archer H, and Geenberger JS: Prevention of radiation-induced oral cavity mucositis by plasmid/liposome delivery of the human manganese superoxide dismutase (SOD2) transgene. Radiat Res 2003; 159:361. [DOI] [PubMed] [Google Scholar]
- 42.Lefaix JL, Delanian S, Leplat JJ, Tricaud Y, Martin M, Nimrod A, Baillet F, and Daburon F: Successful treatment of radiation-induced fibrosis using Cu/Zn-SOD and Mn-SOD: an experimental study. Int J Radiat Oncol Biol Phys 1996; 35:305. [DOI] [PubMed] [Google Scholar]
- 43.Berk L, Berkey B, Rich T, Hrushesky W, Blask D, Gallagaher M, Kudrimonti M, McGarry RC, Suh J, and Mehta M: Randomized phase II trial of high-dose melatonin and radiation therapy for RPA class 2 patients with brain metastases (RTOG 0119). Int J Radiat Oncol Biol Phys 2007; 68:852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burdelya LG, Krivokrysenko VI, Tallant TC, Strom E, Gleiberman AS, Gupta D, Kurnasov OV, Fort FL, Osterman AL, Didonato JA, Feinstein E, and Gudkov AV: An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science 2008; 320:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bentzen SM: Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rec Cancer 2006; 6:702. [DOI] [PubMed] [Google Scholar]
- 46.Bertho JM, Frick J, Prat M, Demarquay C, Dudoignon N, Trompier F, Gorin NC, Thierry D, and Gourmelon P: Comparison of autologous cell therapy and granulocyte-colony stimulating factor (G-CSF) injection vs. G-CSF injection alone for the treatment of acute radiation syndrome in a non-human primate model. Int J Radiat Oncol Biol Phys 2005; 63:911. [DOI] [PubMed] [Google Scholar]
- 47.Finch PW. and Rubin JS: Keratinocyte growth factor/fibroblast growth factor 7, a homeostatic factor with therapeutic potential for epithelial protection and repair. Adv Cancer Res 2004; 91:69. [DOI] [PubMed] [Google Scholar]
- 48.Lombaert IM, Brunsting JF, Wierenga PK, Kampinga HH, de Haan G, and Coppes RP: Keratinocyte growth factor prevents radiation damage to salivary glands by expansion of the stem/progenitor pool. Stem Cells 2008; 26:2595. [DOI] [PubMed] [Google Scholar]
- 49.Farrell CL, Rex KL, Kaufman SA, Dipalma CR, Chen JN, Scully S, and Lacey DL: Effects of keratinocyte growth factor in the squamous epithelium of the upper aerodigestive tract of normal and irradiated mice. Int J Radiat Biol 1999; 75:609. [DOI] [PubMed] [Google Scholar]
- 50.Spielberger R, Stiff P, Bensinger W, Gentile T, Weisdorf D, Kewalramani T, Shea T, Yanovich S, Hansen K, Noga S, McCarty J, LeMaistre CF, Sung EC, Blazar BR, Elhardt D, Chen MG, and Emmanouilides C: Palifermin for oral mucositits after intensive therapy for hematologic cancers. N Engl J Med 2004; 351:2590. [DOI] [PubMed] [Google Scholar]
- 51.Bizel DM, Murphy BA, Rosenthal DI, Pandya KJ, Gluck S, Brizel HE, Meredith RF, Berger D, Chen MG, and Mendenhall W: Phase II study of paliferamin and concurrent chemoradiation in head and neck squamous cell carcinoma. J Clin Oncol 2008; 26:2489. [DOI] [PubMed] [Google Scholar]
- 52.Basilico C. and Moscatelli D: The FGF family of growth factors and oncogenes. Adv Cancer Res 1992; 59:115. [DOI] [PubMed] [Google Scholar]
- 53.Akita S, Akino K, Imaizumi T, and Hirano A: Basic fibroblast growth factor accelerates and improves second-degree burn wound healing. Wound Repair Regen 2008; 16:635. [DOI] [PubMed] [Google Scholar]
- 54.Riekstina U, Muceniece R, Cakstina I, Muiznieks I, and Ancans J: Characterization of human skin-derived mesenchymal stem cell proliferation rate in different growth conditions. Cytotechnology 2008; 58:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kinoshita N, Tsuda M, Hamuy R, Nakashima M, Nakamura-Kurashige T, Matsuu-Matsuyama M, Hirano A, and Akita S: The usefulness of basic fibroblast growth factor for radiation-exposed tissue. Wound Repair Regen 2012; 20:91. [DOI] [PubMed] [Google Scholar]
- 56.Anscher MS, Thrasher B, Rabbani Z, Teicher B, and Vujaskovic Z: Antitransforming growth factor-beta antibody 1D11 ameliorates normal tissue damage caused by high-dose radiation. Int J Radiat Oncol Biol Phys 2006; 65:876. [DOI] [PubMed] [Google Scholar]
- 57.Xavier S, Piek E, Fujii M, Javelaud D, Mauviel A, Flanders KC, Samuni AM, Felici A, Reiss M, Yarkoni S, Sowers A, Mitchell JB, Roberts AB, and Russo A: Amelioration of radiation-induced fibrosis: inhibition of transforming growth factor-beta signaling by halofuginone. J Biol Chem 2004; 279:15167. [DOI] [PubMed] [Google Scholar]
- 58.Bey E, Prat M, Duhamel P, Benderitter M, Brachet M, Trompier F, Battaglini P, Emou I, Gourven M, Tissedre F, Crea S, Mansour CA, de Revel T, Carsin H, Gourmelon P, and Lataillade JJ: Emerging therapy for improving wound repair of severe radiation burns using local bone marrow-derived stem cell administrations. Wound Repair Regen 2010; 18:50. [DOI] [PubMed] [Google Scholar]
- 59.Akita S, Yoshimoto H, Akino K, Ohtsuru A, Hayashida K, Hirano A, Suzuki K, and Yamashita S: Early experiences with stem cells in treating chronic wounds. Clin Plast Surg 2012; 39:281. [DOI] [PubMed] [Google Scholar]
- 60.Agay D, Scherthan H, Forcheron F, Grenier N, Herondin F, Meineke V, and Drouet M: Multipotent mesenchymal stem cell grafting to treat cutaneous radiation syndrome: development of a new minipig model. Exp Hematol 2010; 38:945. [DOI] [PubMed] [Google Scholar]
- 61.Roche S, Delorme B, Oostendorp RA, Barbet R, Caton D, Noel D, Boumediene K, Papadaki HA, Cousin B, Crozet C, Milhavet O, Casteilla L, Hatzfeld J, Jorgensen C, Charbord P, and Lehmann S: Comparative proteomic analysis of human mesenchymal and embryonic stem cells: towards the definition of a mesenchymal stem cell proteomic signature. Proteomics 2009; 9:223. [DOI] [PubMed] [Google Scholar]
- 62.Akita S, Yoshimoto H, Ohtsuru A, Hirano A, and Yamashita S: Autologous adipose-derived regenerative cells are affective for chronic intractable radiation injuries. Radiat Prot Dosimetry 2012; 151:656. [DOI] [PubMed] [Google Scholar]
- 63.Utsunomiya T, Shimada M, Imura S, Morine Y, Ikemoto T, Mori H, Hanaoka J, Iwahashi S, Saito Y, and Iwaguro H: Human adipose-derived stem cells: potential clinical applications in surgey. Surg Today 2011; 41:18. [DOI] [PubMed] [Google Scholar]