Abstract
The aims of this study were to investigate the consequences of prolonged patterns of alcohol and marijuana use on white matter integrity and neurocognitive functioning in late adolescence, and examine neurodevelopmental trajectories over three years of regular follow-up visits. Three groups of demographically similar teens received assessments every 1.5 years (controls with consistently minimal substance use, n = 16; teens who gradually increase their heavy episodic drinking n = 17, and continuous binge drinkers with heavy marijuana use, n = 21), including comprehensive neuropsychological evaluations, diffusion tensor imaging, and detailed substance use interviews. One-way ANOVA identified fifteen white matter clusters that significantly differed between groups at 3-year follow-up, ages 19–22; controls consistently demonstrated higher values of tissue integrity across fiber tracts. Repeated measures ANOVA revealed significant declines in white matter integrity from baseline to 3-year follow-up in the subsample of substance users, along with poorer global neurocognitive performance in alcohol users with heavy marijuana use by the 18-month follow-up. Findings suggest healthier brain white matter microstructure and better neurocognitive performance for teens free from heavy alcohol and marijuana use. Long-term engagement in these substances may adversely influence white matter and increase vulnerability for development of neuropathology purported to underlie future risk-taking and addictive behaviors.
Keywords: Adolescence, Alcohol, Diffusion Tensor Imaging, Cannabis, Brain, Cognition
1. Introduction
Substance use is typically initiated during adolescence, with rates of use increasing into young adulthood. Alcohol is the most commonly used substance, with 70% of high school seniors having at least tried alcohol, 51% being drunk at least once, and most alarmingly, 22% engaging in binge drinking in the past two weeks (i.e., ≥4 drinks on one occasion for females and ≥5 drinks for males; (Johnston et al., 2012). Marijuana is the second most commonly used substance, with 46% of high school seniors endorsing lifetime use and 23% endorsing past month use (Johnston et al., 2012).
These high rates of substance use are concerning, as the adolescent brain is continuing to undergo significant maturation well into young adulthood (~25 years) (Stiles and Jernigan, 2010). These dynamic changes include substantial cortical remodeling (e.g., decreases in gray matter volume and neural pruning) and increases in white matter volume (e.g., myelination and/or axonal caliber) (Yakovlev and Lecours, 1967; Giedd et al., 1999; Gogtay et al., 2004; Stiles and Jernigan, 2010). It is suspected that ongoing myelination during adolescence facilitates faster and more efficient neural transmission and cognitive processing (Giedd, 2008; Schmithorst and Yuan, 2010; Lebel et al., 2012) which in turn is related to better behavioral performance (Nagy et al., 2004; Fryer et al., 2008; Qiu et al., 2008; Schmithorst and Yuan, 2010). Therefore, understanding the effects of substance use on white matter maturation is important, as aberrant development could have significant behavioral implications.
In adults, chronic alcohol use has been consistently associated with abnormalities in white matter volume and integrity (Kril et al., 1997; Pfefferbaum et al., 2000; Pfefferbaum and Sullivan, 2005; Pfefferbaum et al., 2006). While less research has been done with adolescent populations, preliminary cross-sectional findings suggest that adolescents with alcohol use disorders have reduced white matter microstructural integrity (i.e., reduced fractional anisotropy) in the corpus callosum when compared to non-using teens (Tapert et al., 2003). In a study of adolescents who did not meet criteria for alcohol abuse or dependence, but did endorse significant binge drinking, widespread reductions in white matter integrity were found throughout the brain and were significantly related to greater lifetime hangover and higher estimated peak blood alcohol levels (McQueeny et al., 2009). While these findings were consistent with the adult literature, the pervasive effect of binge drinking on white matter in this sample was surprising, given the adolescent binge drinkers were relatively healthy, had limited binge drinking histories, and did not meet criteria for an alcohol use disorder. The microstructural changes reflected in reduced fractional anisotropy (FA) in these areas could underlie neurocognitive deficits that have previously been associated with alcohol use during adolescent brain maturation (Tapert et al., 2002; Squeglia et al., 2009)
Marijuana is the second most commonly used intoxicant in adolescence, second to alcohol (Johnston et al., 2012); therefore it is important for clinical studies on adolescent marijuana users to examine both alcohol and cannabis use concomitantly. In a cross-sectional study of adolescents who frequently binge drink and use marijuana, Bava et al. (2009) found that substance-using adolescents showed decreased FA, or white matter integrity, in fronto-parietal tracts when compared to non-using adolescents (Bava et al., 2009). In one of few longitudinal investigations examining combined alcohol and marijuana use, the same research group found that more alcohol use over 1.5 year follow-up was associated with poorer white matter at follow-up (decreased FA), controlling for marijuana use and baseline white matter health (Bava et al., 2013). Together, findings suggest that deleterious effects might be more attributable to binge drinking than marijuana, as preliminary studies suggest that cannabis may have a lesser effect on white matter development during adolescence (Delisi et al., 2006) and may actually serve a neuroprotective role in attenuating the negative effects of heavy alcohol use on white matter integrity during this critical maturation period (Jacobus et al., 2009), although this remains quite speculative. As existing white matter studies from our laboratory and others are largely cross-sectional, it is unclear if differences between substance users and controls predated the onset or escalation of alcohol and marijuana use, or emerged as a result of substance use. Furthermore, findings on the longer-term outcomes and neurodevelopmental trajectories (e.g., white matter) resulting from repeated consumption of these commonly abused substances from adolescence to young adulthood are still inconclusive due to limited prospective investigations.
The aims of this three-year prospective study were to investigate the prolonged effects of both (1) binge drinking and (2) combined marijuana use and binge drinking on white matter integrity and neurocognitive functioning in adolescents ages 16–22. Based on previous findings, we hypothesized that continued binge drinking and marijuana use during mid- to late-adolescence would be associated with worsened white matter integrity, or decreased FA. We predicted that individuals continuing to engage in continuous heavy episodic alcohol and marijuana use over the three time points would show greater white matter tract disorganization compared to non-using demographically similar controls by approximately 20 years of age, and that these white matter abnormalities would also be associated with poorer neurocognitive functioning and white matter trajectories over three time points.
2. Methods
2.1 Participants
Adolescents (N = 54; ages 16–19 at baseline) were recruited from local high schools and then followed for three years (i.e., at baseline and 18-months and 3-years post baseline (36-months)) (Medina et al., 2007a; Jacobus et al., 2009; McQueeny et al., 2009; Bava et al., 2010b). Adolescents were classified into one of three groups at 3-year follow-up based on self-report: those engaging in heavy episodic alcohol use (i.e., binge drinking, ≥4 drinks on one occasion for females and ≥5 drinks for males) and marijuana use (BG+MJ, n = 21), binge drinking only (BG, n = 17), and control teens consistently reporting (over the course of three years) minimal alcohol or marijuana use (CON, n = 16) by early adulthood. Binge drinkers were required to have at least 3 binge episodes since the follow-up visit (controls reported no binge episodes), range was approximately 3–300 binge drinking episodes for the BG and BG+MJ groups. Importantly, we selected teens whose substance use patterns remained relatively stable over the course of three years and compared them to demographically-similar controls (see Table 1) to best characterize the longer term effects of binge drinking and marijuana use by late adolescence/early adulthood (ages 19–22) at the three-year time point. This study builds on our cross-sectional work (Bava et al., 2009; Jacobus et al., 2009), and the first longitudinal study examining the influence of substance use on brain white matter (i.e., Bava et al., 2013), by adding a third wave of data at a 3-year follow-up, and is our first longitudinal study to separately evaluate binge drinkers from those with marijuana and alcohol use from adolescence to early adulthood.
Table 1.
Demographic characteristics of participants at 3-year follow-up, unless noted otherwise
| CON n = 16 M(SD) |
BG n = 17 M(SD) |
BG+MJ n = 21 M(SD) |
|
|---|---|---|---|
| Age (range), Baseline | 17.9 (16–19) | 17.9 (16–19) | 17.9 (16–19) |
| Age (range), Year 1.5 | 19.4 (17–20) | 19.3 (17–20) | 19.4 (17–20) |
| Age (range), Year 3 | 20.9 (19–22) | 20.9 (19–22) | 20.9 (19–21) |
| % Male | 50% | 64% | 62% |
| % Caucasian | 56% | 88% | 76% |
| Grade Point Average | 3.1 (0.6) | 3.5 (0.3) | 3.2 (0.6) |
| Household Income (range) | 85K (21–183K) | 147K (50–320K) | 135K (24–420K) |
| %Family History negative for substance disorder | 44% | 59% | 29% |
| Vocabulary T-score at baseline | 57.7 (10.9) | 60.7 (8.5) | 59.9 (8.7) |
| Beck Depression Inventory Total, Baseline | 1.7 (2.8) | 1.8 (2.2) | 2.8 (3.5) |
| Beck Depression Inventory Total, Year 1.5 | 1.6 (2.8) | 2.3 (2.9) | 2.2 (2.8) |
| Beck Depression Inventory Total, Year 3 | 1.2 (1.3) | 1.6 (2.7) | 3.2 (5.3) |
| Spielberger State Anxiety T-score, Baseline | 37.4 (5.0) | 37.1 (4.3) | 39.0 (6.7) |
| Spielberger State Anxiety T-score, Year 1.5 | 38.7 (5.2) | 38.1 (6.9) | 35.8 (5.3) |
| Spielberger State Anxiety T-score, Year 3 | 36.7 (6.1) | 34.3 (3.9) | 38.1 (7.0) |
| Externalizing T-score†‡ | 46.2 (7.0) | 42.0 (9.7) | 53.3 (7.5) |
| Internalizing T-score | 39.8 (8.0) | 40.6 (9.7) | 44.6 (10.7) |
| Age of initiation of regular alcohol consumption | - | 14.0 (7.9) | 14.0 (6.3) |
| Total Binge Drinking Episodes | - | 70.1 (75.2) | 99.9 (105.3) |
| Cigarettes smoked per day, past 1.5 years †‡ | 0.1 (0.3) | 0.0 (0.0) | 0.8 (1.5) |
BG+MJ > CON; p < 0.05
BG+MJ > BG; p < 0.05
Due to MRI scanner upgrades early in the study protocol, of the 54 teens identified at three-year follow-up who reported consistent substance use (or non-use) histories (i.e., reported the same pattern of use over three years) those individuals with valid diffusion tensor imaging (DTI) data at baseline included 39 individuals (BG+MJ, n = 16, BG, n = 17, CON, n = 6) and those individuals with valid DTI data at 18-month follow-up included 46 individuals (BG+MJ, n = 18, BG, n = 17, CON, n = 11). All subjects had valid substance use and neuropsychological data at baseline, 18-month, and 3-year follow-up, with the exception of 3 individuals who were not available for an 18-month follow-up neuropsychological assessment (1 control and 2 BG+MJ; please see Figure 1 for clarification on study timeline and detailed sample sizes for measures at each time point). Exclusionary criteria were: history of a lifetime DSM-IV Axis I disorder (other than cannabis or alcohol abuse or dependence), history of learning disability, history of neurological disorder or head trauma with loss of consciousness >2 minutes, history of a serious physical health problem, complicated or premature birth including prenatal substance use; uncorrectable sensory impairments; left handedness; MRI contraindications, and use of psychoactive medications at project intake.
Figure 1.
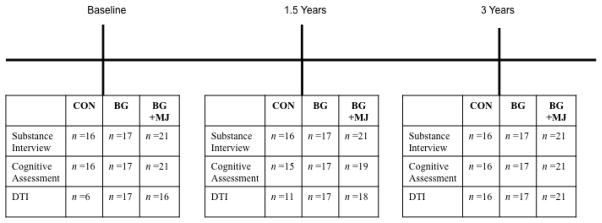
Timeline of study procedures and corresponding sample sizes for valid data at each time point.
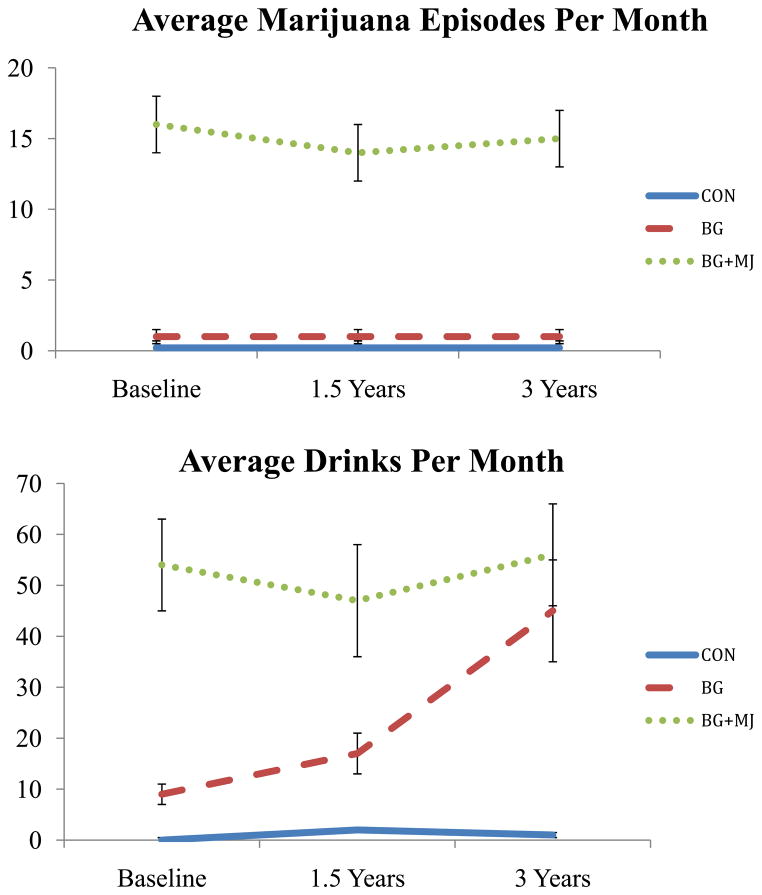
At project enrollment, marijuana users were required to have > 200 lifetime marijuana use episodes for eligibility, controls (and binge drinkers) were required to have < 10 lifetime marijuana use episodes. Controls were required to have < 20 lifetime alcohol use episodes and binge drinkers were required to have < 150 lifetime alcohol use episodes (see Figure 2). Approximately 30% of the binge drinkers met criteria for alcohol abuse over the three years of the study and approximately 53% of the marijuana users met criteria for alcohol abuse. Of the marijuana users the vast majority of the sample met criteria for marijuana abuse/dependence throughout the study (95%).
Figure 2.
Substance use characteristics, (N = 54) at baseline, 18-month follow-up (1.5 Years), and 3-year follow-up.
2.2 Procedures
Following recruitment, eligible teens and parents received both written consent and assent forms. Teens underwent weekly toxicology for four weeks prior to beginning the study protocol (and at each follow-up visit) to confirm abstinence at each testing and scan session. Teens received comprehensive neuropsychological evaluations, a DTI session, and detailed substance use interviews at baseline, 18-month follow-up, and 3-year follow-up. Parents were also interviewed to corroborate demographic, psychosocial functioning, and substance use reports of youth. If self-report and collateral data are discrepant, data are coded to reflect the presence of substance use.
2.3 Neuropsychological battery
Standardized neuropsychological tests examining the well-established domains of complex attention, processing speed, verbal memory, visuospatial functioning, and executive functioning (Lezak et al., 2004) were composited from the following neurocognitive tests to reduce the number of dependent variables (Medina et al., 2007a): 1) complex attention: California Verbal Learning Test-II Trial 1 and Trial 1–5 Total Recall (CVLT-II; (Delis et al., 2001)); Wechsler Adult Intelligence Scale- Third Edition (WAIS-III, (Wechsler, 1997b)) Digit Symbol, Arithmetic, and Digit Span Backward subtests; Paced Auditory Serial Addition Test (PASAT; (Gronwall, 1974)); 2) processing speed: Delis-Kaplan Executive Function System Trail Making Test Number Sequencing, Letter Sequencing, and Motor Speed subtests (DKEFS, (Delis and Kaplan, 2000)); 3) verbal memory: Wechsler Memory Scale-Third Edition (WMS-III, (Wechsler, 1997a)) Logical Memory I, II, and Recognition; CVLT-II Short and Long Delay Free Recall, CVLT-II Recognition (Delis et al., 2001); 4) visuospatial functioning: Rey-Osterrieth Complex Figure Copy and Delay Accuracy (Rey-O, (Rey and Osterrieth, 1993)), Wechsler Abbreviated Scale of Intelligence Block Design subtest (WASI, (Wechsler, 1999)); and 5) executive functioning: DKEFS Trial Making Test Number-Letter Switching, Tower Test Achievement and Accuracy, and Verbal Letter Fluency subtests (Delis and Kaplan, 2000).
After developing composite categories, individual z-scores were created for each subject for each neuropsychological variable at each time point based on the whole sample of adolescents and corresponding standard deviations (N = 54 for baseline and 3-year follow-up, N = 51 for 18-month follow-up). Next, z-scores within each domain were averaged to create the final composite z-score for each of the five domains at each time point. A global neurocognitive functioning z-score was also created for each time point by averaging the composite scores for each cognitive domain, resulting in a global neurocognitive functioning z-score for baseline, 18-month follow-up, and 3-year follow-up. Cronbach’s alpha coefficients were used to assess internal consistency (α > .50, values ranged from .50–.80). Individual neuropsychological performance variables were standardized for age and sex prior to being converted to z-scores (i.e., CVLT-II, WAIS-III, DKFS, WMS-III)(Medina et al., 2007a).
2.4 Substance use assessments and mental health assessment
The Customary Drinking and Drug Use Record was used to assess quantity and frequency of lifetime alcohol, marijuana, cigarette, and other illicit drugs (Brown et al., 1998) including amphetamines, hallucinogens, cocaine, opiates, benzodiazepines, ecstasy, ketamine, and GHB. The Timeline Followback was used to assess self-reported substance use in the 28 days prior to each scan session (Sobell and Sobell, 1992). The Beck Depression Inventory (BDI) (Beck, 1978) and Spielberger State Trait Anxiety Inventory (STAI) (Spielberger et al., 1970) assessed depressive symptoms and state anxiety. The Adult Self-Report (ASR), part of the Achenbach assessment system, was completed by participants at 3-year follow-up to assess internalizing and externalizing psychopathology (Achenbach and Rescorla, 2001).
2.5 Diffusion tensor imaging (DTI)
Participants were imaged at all three time points on a 3T General Electric CXK4 short bore Excite-2 MR system with an 8-channel phase-array head coil (General Electric Medical System, Milwaukee, WI, USA) at the University of California, San Diego. A 10-second scout session was included in the protocol to assure good head placement. DTI encoding included a single-shot dual spin echo excitation sequence averaged over four volumes (15 directions, TE/TR = 93/12,000ms, FOV 240 mm, matrix = 128x128, 36 contiguous slices, 3 mm slice thickness, b-value = 2000 s/mm2). Two field maps (TE/TR = 3.8/1,000 ms) were collected and applied to account for inhomogenities in the magnetic field (Andersson and Kare, 2002).
All DTI data were corrected for eddy current distortion and head motion using Functional Magnetic Resonance of the Brain (FMRIB) Software Package FSL (Smith et al., 2004). Affine registration with six degree of freedom was used to correct for eddy current distortions and head motion by registration of the 15 direction files to the B0 image file (FLIRT-FMRIB’s Linear Registration Tool) (Jenkinson et al., 2002). FSL’s PRELUDE (Phase Region Expanding Labeler for Unwrapping Discrete Estimates) (Jenkinson, 2003) and FUGUE (FMRIB’s Utility for Geometrically Unwarping EPIs) (Jenkinson and Smith, 2001) tools were used to correct for field distortions.
Fractional anisotropy was calculated using FMRIB’s Diffusion Toolbox (Behrens et al., 2003). White matter tracts common to all participants were identified using Tract-Based Spatial Statistics (TBSS) (Smith et al., 2006). This involves individual FA maps from all participants from all three time points registered to a standard template in MNI-152 standard space. Images are averaged across participants to create a mean FA image from which a white matter skeleton is derived that represents common tracts to all participants. FA values were thresholded at FA > 0.2 to minimize partial volume effects (Smith et al., 2006).
2.6 Data analyses
2.6.1 Demographic comparisons
ANOVA and Chi-square tests were run between groups to evaluate differences on demographic variables and to identify appropriate covariates for subsequent analysis.
2.6.2 DTI analyses
Whole brain voxelwise statistical analysis of variance (AFNI 3dANOVA) identified between-group differences across all subjects at the 3-year follow-up assessment (approximately age 20). Multiple comparisons were corrected with voxel probability and cluster size thresholding using Monte Carlo simulations. Only clusters ≥21 μl (21 contiguous 1 x 1 x 1 mm voxels) with an individual voxel effect of α<0.01 were interpreted, yielding a brain-wise α<0.05 of finding such a cluster under the null hypothesis. Next, all significant clusters were extracted for follow-up repeated measures analysis in IBM SPSS Statistics Version 20. Specifically, mean FA values within each cluster showing statistically significant between-group differences at 3-year follow-up were extracted from each individual at each time point to examine the white matter trajectories of these statistically significant clusters from baseline to 18-month follow-up, and 18-month follow-up to 3-year follow-up. Due to limited valid DTI data at baseline for several controls (n =6) repeated measures analysis was performed for BG and BG+MJ only to help elucidate the white matter trajectories of these regions in our substance users over the course of three years.
2.6.3 Neuropsychological performance
Repeated measures analysis of variance (ANOVA) examining the within group and between group effects of global neurocognitive functioning were examined at each time point, as well as the group by time interaction effect. If a main effect or interaction was found to be significant, the analysis was followed-up and re-ran with each individual domain score to determine what cognitive ability was driving the relationship.
2.6.4 Secondary analyses
A series of bivariate regression analyses were conducted to evaluate if white matter indices were related to either neuropsychological global composite scores and/or change in composite scores at each corresponding time point. If significant bivariate correlations were identified, then hierarchical linear regressions were run specifying the neurocognitive composite score as the dependent variable, the identified white matter index on step 1 (FA value) and group, and the corresponding interaction term with group (white matter x group) on step 2 in order to understand if the relationship between white matter (WM) and neuropsychological performance was moderated by group status.
Bivariate correlations were also examined between global neuropsychological performance at each time point and total drinking episodes since last follow-up visit, total marijuana use episodes since last follow-up visit, average alcohol and marijuana use per month since last follow-up visit, and total other substance use episodes since last follow-up visit (e.g., cocaine, heroin, ecstasy) for each corresponding time point.
3. Results
Groups did not differ on age, gender, or emotional functioning at 3-year follow-up (ps > 0.05, see Table 1). Groups differed on externalizing symptoms, as BG+MJ reported more externalizing behaviors in the past six months compared to BG and CON (p < 0.05). Groups differed on substance use episodes besides marijuana and alcohol, as expected. Specifically, BG+MJ reported more other-drug use episodes and cigarette use (p < 0.05) compared to CON and BG.
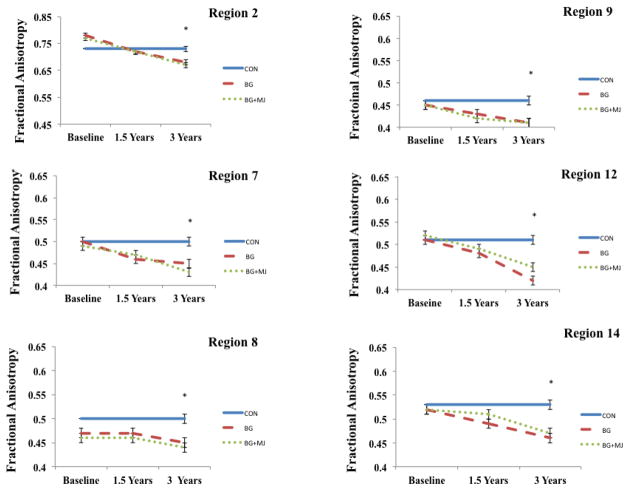
One-way ANOVA identified fifteen clusters within projection, association, and interhemispheric fiber tracts that differed between groups at the 3-year follow-up (see Table 2, ps < 0.01, corrected). Follow-up pairwise comparisons (Bonferroni corrected) identified higher FA values in the control group as compared to BG and BG+MJ groups across 14 significant clusters. In one cluster (cluster 15), higher FA values were found in the control compared to BG only, and higher FA values were found in BG+MJ compared to BG. Limited differences were found between the user groups, except for one cluster in the right uncinate fasciculus in which BG+MJ had higher values compared to BG (see Table 2).
Table 2.
White matter clusters demonstrating significant between-group differences at 3-year follow-up and corresponding pairwise comparisons (ps< 0.01, corrected); 3-year follow-up includes 54 individuals (BG+MJ, n = 21; BG, n = 17, CON, n = 16).
| Anatomical Region | Volume (μl) | MNI Coordinates* | Effect Size (Cohen’s d†) CON > BG |
Effect Size (Cohen’s d†) CON > BG+MJ |
Effect Size (Cohen’s d†) BG+MJ > BG |
||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| 1. Splenium (medial) | 212 | 3.4 | −34.9 | 16.5 | 1.6 | 1.7 | - |
| 2. Genu (medial) | 59 | 4.4 | 27.7 | 2.6 | 1.3 | 1.5 | - |
| 3. (Inferior) Fronto-occipital Fasciculus (L) | 52 | −19.2 | 20.2 | −7.9 | 1.0 | 1.5 | - |
| 4. Anterior Thalamic Radiations (R) | 38 | 13.6 | −12.8 | 14.2 | 1.3 | 1.4 | - |
| 5. Uncinate Fasciculus (L) | 36 | −26.0 | 28.5 | −8.5 | 1.5 | 1.5 | - |
| 6. Uncinate Fasciculus (R) | 34 | 36.5 | 47.2 | −7.4 | 1.3 | 1.7 | - |
| 7. Superior Longitudinal Fasciculus (R) | 32 | 46.5 | −7.5 | 25.5 | 1.2 | 1.9 | - |
| 8. Anterior Limb Internal Capsule (L) | 30 | −22.3 | 20.3 | 3.4 | 1.2 | 1.2 | - |
| 9. Anterior Corona Radiata (R) | 28 | 21.8 | 24.5 | −4.5 | 1.4 | 1.5 | - |
| 10. Superior Corona Radiata (L) | 27 | −19.6 | −10.0 | 4.8 | 1.3 | 1.3 | - |
| 11 Posterior Limb Internal Capsule (R) | 26 | 19.2 | −10.0 | 4.8 | 0.9 | 1.0 | - |
| 12. Superior Longitudinal Fasciculus (R) | 25 | 31.3 | −22.9 | 37.2 | 1.2 | 1.2 | - |
| 13 Anterior Corona Radiata (R) | 24 | 19.5 | 35.7 | 16.9 | 1.1 | 1.1 | - |
| 14. Superior Longitudinal Fasciculus (R) | 22 | 41.5 | −7.0 | 28.9 | 1.3 | 0.9 | - |
| 15. Uncinate Fasciculus (R) | 21 | 26.0 | 27.9 | −10.1 | 1.3 | - | 1.2 |
Coordinates of the center of mass,
Effect sizes computed from sample means and standard deviations
A repeated measures ANOVA was used to examine the white matter trajectories of these clusters in the user groups only (i.e., BG and BG+MJ) over the course of three-year follow-up intervals (see Figure 3). Given the limited control data available at baseline (n = 6), these individuals were not included in the repeated measures analysis, although their FA data from 3-year follow-up was included in Figure 3 (across all time points) to provide a reference point. We found a main effect of time in all clusters examined within the users F2,31 = 9.9 to 36.1; ps < 0.01, excluding cluster 8 (anterior limb of internal capsule) (no main effect of group or group by time interactions were found). Follow-up comparisons (Bonferroni corrected) revealed decreased FA from baseline to 3-year follow-up in all clusters, excluding clusters 2 and 8. Decreases from 18-month follow-up to 3-year follow-up were seen in cluster 2, as well as cluster 4 and 14 (ps < 0.01).
Figure 3.
Selected regions demonstrating between group differences at year 3. Repeated measures ANOVA performed within user groups only at all three time points. Controls not included in repeated measures analysis, FA values from 3-year follow-up represented at each time point for controls and included in figure as reference point only.
* p < 0.01, corrected, between group differences at 3-year follow-up
A repeated-measures ANOVA was also used to examine global neurocognitive functioning at baseline, 18-month follow-up and 3-year follow-up, which revealed a group by time interaction (F(4, 96) = 2.6, p = 0.04), whereas subtle global neurocognitive differences were observed between groups at the 18-month follow-up between BG+MJ and BG (p = 0.01) that was no longer observed at 3-year follow-up. Prior to a substantial alcohol use increase, those binge drinkers who would later transition to even heavier episodic drinking showed higher global cognitive functioning scores compared to marijuana users at 1.5 years. Follow-up analyses corrected for multiple comparisons did not reveal a particular domain (e.g., executive functioning, processing speed, etc.) driving this relationship (ps > 0.05, see Figure 4).
Figure 4.
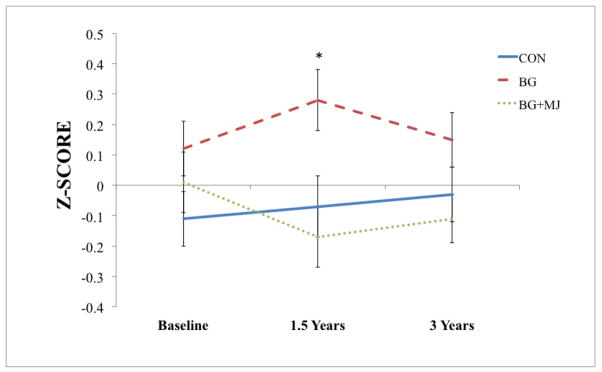
Observed global neuropsychological performance at baseline, 18-month follow-up and 3-year follow-up (Controls (CON), n = 15; Binge Drinkers (BG), n = 17; Binge Drinkers with Heavy Marijuana Use (BG+MJ), n = 19)
* p < 0.01
There were no significant relationships between global neurocognitive performance at each time point and corresponding WM clusters or global neurocognitive performance at each time point and dose-dependent substance use variables using both transformed or untransformed substance use variables (ps > 0.05); however we saw a relationship between change in global neurocognitive functioning from 18-month to 3-year follow-up- and WM integrity in the right superior longitudinal fasciculus (r = .38, p < 0.01), as improved global cognitive functioning from 18-month follow-up to year 3 follow-up was associated with better white matter integrity at year 3 follow-up; this relationship was not moderated by group status and remained significant after controlling for baseline performance (see Figure 5).
Figure 5.
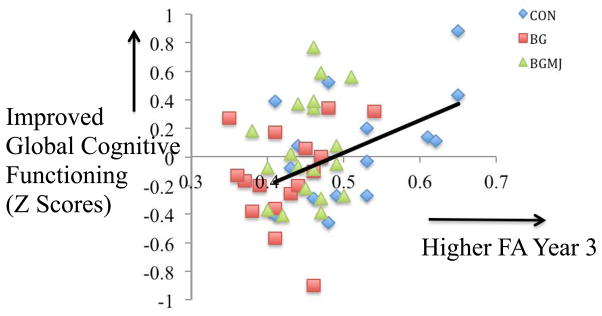
White matter integrity in the right superior longitudinal fasciculus at three-year follow-up and change in global neuropsychological functioning from 18-month follow-up to three-year follow-up (N = 51; Controls (CON), n = 15; Binge Drinkers (BG), n = 17; Binge Drinkers with Heavy Marijuana Use (BG+MJ), n = 19)
4. Discussion
In this investigation, we hypothesized that adolescents who engage in both heavy episodic drinking and/or marijuana use over the course of three years would demonstrate poorer white matter integrity by approximately 20 years of age, compared to controls with minimal substance use histories by late adolescence/early adulthood. We found evidence to support this hypothesis in 15 white matter clusters, including association fiber tracts (e.g., superior longitudinal fasciculus, uncinate fasciculus), projection fiber tracts (e.g., regions of the corona radiata), and interhemispheric tracts (e.g., splenium, genu). Notably, we found a main effect of time for all significant clusters for the users, as most clusters showed significantly decreased FA from baseline to 3-year follow-up. Further, we found preliminary evidence that poorer global neurocognitive performance, as measured by a change from 18-month follow-up to 3-year follow-up was related to poorer white matter integrity within a large white matter tract connecting frontal and parietal brain regions (i.e., superior longitudinal fasciculus) at approximately 20 years of age.
Interestingly, we did not see substantial differences in white matter integrity between our two user groups, or correlations between neuropsychological performance and substance use severity (e.g., lifetime drinking occasions, marijuana occasions). However, global neurocognitive functioning showed a group by time interaction; at 18-month follow-up, adolescents who engaged in binge drinking demonstrated better global cognitive performance than their marijuana-using counterparts, but this was no longer observed at the 3-year follow-up. While direct dose-dependent relationships with substance use variables were not observed here (linking cumulative use over interscan interval to cognitive performance), the decline in mean cognitive performance among binge drinkers is emerging during a time period when binge drinkers are substantially increasing monthly intake from 17 drinks per month at 18-month follow-up to 45 drinks per month at 3-year follow-up. Thus, it is possible that this increased drinking accounts for a slight decline in their once more superior cognitive performance.
Our findings suggest two important considerations: 1) teens without a history of alcohol use and/or marijuana use show better white matter integrity compared to those with reported substance use histories of alcohol and marijuana (Jacobus et al., 2009); and 2) despite engaging in subdiagnostic binge drinking (as opposed to meeting alcohol dependence criteria), these adolescents show poorer white matter integrity than controls, and similar white matter architectural profiles as teens with even heavier alcohol use histories as well as heavy marijuana use. This finding underscores the potential deleterious effects of heavy episodic alcohol use during adolescent neurodevelopment, and the important role it may play in altering neurodevelopmental trajectories.
Our research has consistently shown poorer white matter integrity in adolescent alcohol users compared to matched controls reflected in decreased FA values (Jacobus et al., 2009; McQueeny et al., 2009; Bava et al., 2010a) during age periods in which the literature widely suggests increasing FA in healthy non-using adolescents as fiber tracts mature (Giorgio et al., 2008; Schmithorst and Yuan, 2010; Stiles and Jernigan, 2010; Tamnes et al., 2010; Lebel et al., 2012). We have also seen alterations in macrostructural integrity (e.g., cortical thickness, (Squeglia et al., 2012b) and functional brain activation patterns (Schweinsburg et al., 2011) in teens with similar alcohol use profiles, as well as declines in cognitive status (Squeglia et al., 2009; Mahmood et al., 2010). In a previous investigation, Jacobus et al. (2009), we found that adolescent alcohol users (approximately 17 years old) demonstrated poorer white matter integrity compared to not only matched controls, but to heavy marijuana users with similar levels of alcohol use.
This finding is important given that in previous investigations (e.g., Bava et al., 2009), our subdiagnostic binge drinkers were not separated from non-using controls (defined only by their lack of marijuana use), so an alcohol effect may have been undetected. Therefore, we concluded that teens with both marijuana and alcohol use histories had poorer white matter integrity compared to those controls with minimal use (including minimal alcohol use/binge drinking). A follow-up cross-sectional investigation (Jacobus et al., 2009) provided preliminary evidence that moderate binge drinkers may, have different outcomes than controls with minimal to no alcohol exposure. Interestingly, Bava et al. (2013) found that cumulative alcohol use in the larger sample (and not marijuana use) predicted poorer follow-up white matter integrity (1.5 year follow-up) in the superior longitudinal fasciculus and thalamic fiber tracts. This is notable given that we saw some evidence for improved cognitive performance associated with better white matter integrity by approximately 20 years of age in present study. While the present follow-up investigation did not find the same surprising differences first seen between our user groups in Jacobus et al. 2009, we observed consistently poorer tissue integrity across the brain by 20 years of age in both user groups compared to non-using controls in several similar brain regions as observed in Bava et al., 2009, 2013 and Jacobus et al., 2009 (e.g., superior longitudinal fasciculus, corona radiata). Given that these individuals engaged in their substance use patterns consistently over three-years (confirmed by in-person assessment at 3 time points), we anticipated the cumulative use to impact white matter health in both of these groups. However it remains surprising that our marijuana users do not show a marked decrease in tissue integrity compared to the binge drinkers given their consistent and heavy co-occurring use. The lack of large differences between the user groups highlights the potentially adverse effects of repeated alcohol consumption on development, and calls attention to the need for more research on the neurotoxic effects of marijuana.
Preclinical studies have shown alcohol use to have neurotoxic effects on the brain in both adult and adolescent animals. Activation of proinflammatory markers may be the result of repeated alcohol intoxication, leading to neurodegeneration and/or inhibition of neurogenesis, important during ongoing neural development (Crews and Nixon, 2009). While it is difficult to say how alcohol and inflammation likely impacts diffusion estimates and white matter per se, FA is speculated to reflect increased coherence and compactness of fiber tracts, and inflammatory processes could lead to changes in the intra/extracellular environment and myelination (i.e., decreased FA) thought important for communication between brain regions and optimal cognitive processing. It is important to point out that pre-existing differences and/or more rapid brain maturation for some individuals (i.e., the substance users), in areas implicated in reward such as subcortical/limbic regions, could be accounting for the observed morphological differences (Berns et al., 2009; Somerville and Casey, 2010; Steinberg, 2010; Sturman and Moghaddam, 2011), lending some to use substances earlier in development and therefore evidence white matter decline more rapidly into early adulthood.
The biological mechanisms of marijuana-related neurotoxicity are not as well characterized. For instance, in our laboratory and others, findings have been mixed. We have seen poorer cognitive performance in adolescent marijuana users (Medina et al., 2007a; Churchwell et al., 2010) alterations in macrostructural volume (Medina et al., 2009; Medina et al., 2010; Lopez-Larson et al., 2011; McQueeny et al., 2011; Cousijn et al., 2012) and to some degree, poorer white matter integrity (Gruber and Yurgelun-Todd, 2005; Bava et al., 2009). However, we have also observed minimal findings in terms of brain structural changes (Block et al., 1999; Medina et al., 2007b) and learning and memory performance (Mahmood et al., 2010). Alterations in the endocannabinoid system, which plays a role in influencing developmental processes by modulating neurotransmitter activity, may lead to changes in neural circuits (neurochemical changes, structural changes), that can impact processes such as synaptogenesis, pruning, and myelination (Rubino and Parolaro, 2008; Realini et al., 2009) resulting in cognitive and emotional changes. While our previous investigation speculated the potential for neuroprotective properties of marijuana from a neurobiological perspective (Jacobus et al., 2009), it is important to point out we do not see strong evidence of neuroprotection in this study, as significant differences between the user groups are not observed here by age 20, and the evidence suggest that neither heavy alcohol or marijuana benefits the developing brain. The limited differences observed between the user groups is interesting, and further research on the neurobiological role of marijuana in combination with other substances (e.g., alcohol) is needed to better understand how marijuana may exert detrimental or protective effects on the brain in general.
Limitations of this investigation include modest sample size, limitations of self -report data, and variations in internal consistency of the neurocognitive domains, which may bias statistical estimates. There is also very limited control data at the baseline time point. Additionally, as several correlational analyses were examined, this does increase the risk of Type I error; therefore replication of these findings is important, particularly the preliminary findings between change in cognitive functioning and white matter integrity at 3-year follow-up. Adolescents were assessed after initiation of substance use, therefore pre-existing developmental differences may account for the findings discussed (Norman et al., 2011; Squeglia et al., 2012a), although it is very difficult to disentangle neurodevelopmental changes from alcohol/drug changes. Similarly, existing demographic differences (e.g., race/ethnicity, family history, socioeconomic status) may contribute to the developmental trajectories observed in these groups, and differences in unmeasured factors could contribute to neurocognitive changes, although between group differences were not observed in depression or state anxiety in this sample. Further, the alcohol and marijuana users reported slightly more other lifetime substance use episodes (e.g., limited use episodes of amphetamine use, ecstasy), and while this is unlikely to account for the differences observed it remains a possibility. Additional information on marijuana and alcohol consumption (e.g., if frequently used simultaneously, etc.), along with tobacco use, should be obtained in follow-up studies to address how this may differentially impact neural development.
Future work should focus on understanding and parsing pre-existing developmental differences. Furthermore, family history of an alcohol use disorder as well as gender (Herting et al., 2010; Herting et al., 2012) have both been shown to play a significant role in brain development and future work should focus on to what degree these important factors play a role in microstructural and macrostructral integrity in adolescent substance users. Expansion of this work may also include additional diffusion estimates such as axial, radial, and mean diffusivity, along with the addition of restriction spectrum imaging, which could describe changes in white matter (e.g., changes in myelin and/or intra- and extracellular fluid). Multi-modal imaging is becoming increasingly important as we work to integrate imaging findings, we are hopeful that future investigations will combine structural, functional, and diffusion markers to give us a better understanding of the complex relationships between adolescent alcohol use, marijuana use, and neurodevelopment.
Acknowledgments
This research was made possible by funding from the National Institute on Drug Abuse (R01 DA021182, PI Tapert; F32 DA032188, PI Jacobus) and the National Institute on Alcohol Abuse and Alcoholism (R01 AA013419, PI Tapert).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach T, Rescorla L. Manual for the ASEBA school-age forms & profiles. University of Vermont, Research Center for Children, Youth, and Families; Burlington, Vermont: 2001. [Google Scholar]
- Andersson JL, Kare S. A model-based method for retrospective correction of geometric distortions in diffusoin weighted EPI. Neuroimage. 2002;16:177–199. doi: 10.1006/nimg.2001.1039. [DOI] [PubMed] [Google Scholar]
- Bava S, Frank LR, McQueeny T, Schweinsburg BC, Schweinsburg AD, Tapert SF. Altered white matter microstructure in adolescent substance users. Psychiatry Res. 2009;173:228–237. doi: 10.1016/j.pscychresns.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Jacobus J, Mahmood O, Yang TT, Tapert SF. Neurocognitive correlates of white matter quality in adolescent substance users. Brain Cogn. 2010a;72:347–354. doi: 10.1016/j.bandc.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Thayer R, Jacobus J, Ward M, Jernigan TL, Tapert SF. Longitudinal characterization of white matter maturation during adolescence. Brain Res. 2010b;1327:38–46. doi: 10.1016/j.brainres.2010.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A. Beck Depression Inventory (BDI) Psychological Corporation; San Antonio, TX: 1978. [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Berns GS, Moore S, Capra CM. Adolescent engagement in dangerous behaviors is associated with increased white matter maturity of frontal cortex. PLoS One. 2009;4:e6773. doi: 10.1371/journal.pone.0006773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block RI, O’Leary DS, Ehrhardt JC, Augustinack JC, Ghoneim MM, Arndt S, Hall JA. Effects of frequent marijuana use on brain tissue volume and composition. Brain Imaging. 1999;11:491–496. doi: 10.1097/00001756-200002280-00013. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. J Stud Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Churchwell JC, Lopez-Larson M, Yurgelun-Todd DA. Altered frontal cortical volume and decision making in adolescent cannabis users. Front Psychol. 2010;1:225. doi: 10.3389/fpsyg.2010.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Goudriaan AE. Grey matter alterations associated with cannabis use: results of a VBM study in heavy cannabis users and healthy controls. Neuroimage. 2012;59:3845–3851. doi: 10.1016/j.neuroimage.2011.09.046. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E. Delis-Kaplan Executive Functioning Scale Manual. Psychological Corporation; San Antonio, Texas: 2000. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. 2. The Psychological Corporation; San Antonio, Texas: 2001. [Google Scholar]
- Delisi LE, Bertisch HC, Szulc KU, Majcher M, Brown K, Bappal A, Ardekani BA. A preliminary DTI study showing no brain structural change associated with adolescent cannabis use. Harm Reduct J. 2006;3:17. doi: 10.1186/1477-7517-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer SL, Frank LR, Spadoni AD, Theilmann RJ, Nagel BJ, Schweinsburg AD, Tapert SF. Microstructural integrity of the corpus callosum linked with neuropsychological performance in adolescents. Brain Cogn. 2008;67:225–233. doi: 10.1016/j.bandc.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. The teen brain: insights from neuroimaging. J Adolesc Health. 2008;42:335–343. doi: 10.1016/j.jadohealth.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giorgio A, Watkins KE, Douaud G, James AC, James S, De Stefano N, Matthews PM, Smith SM, Johansen-Berg H. Changes in white matter microstructure during adolescence. Neuroimage. 2008;39:52–61. doi: 10.1016/j.neuroimage.2007.07.043. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronwall DMA. Paced Auditory Serial-Addition Task: A measure of recovery from concussion. Perceptual and Motor Skills. 1974;44:367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Yurgelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Brain Res Cogn Brain Res. 2005;23:107–118. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Herting MM, Maxwell EC, Irvine C, Nagel BJ. The impact of sex, puberty, and hormones on white matter microstructure in adolescents. Cereb Cortex. 2012;22:1979–1992. doi: 10.1093/cercor/bhr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Schwartz D, Mitchell SH, Nagel BJ. Delay discounting behavior and white matter microstructure abnormalities in youth with a family history of alcoholism. Alcohol Clin Exp Res. 2010;34:1590–1602. doi: 10.1111/j.1530-0277.2010.01244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, McQueeny T, Bava S, Schweinsburg BC, Frank LR, Yang TT, Tapert SF. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicol Teratol. 2009;31:349–355. doi: 10.1016/j.ntt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M. Fast, automated, N-dimensional phase-unwrapping algorithm. Magn Reson Med. 2003;49:193–197. doi: 10.1002/mrm.10354. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the future national results on adolescent drug use: Overview of key findings, 2011. Ann Arbor: Institute for Social Research, The University of Michigan; 2012. [Google Scholar]
- Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–998. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012;60:340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. New York: 2004. [Google Scholar]
- Lopez-Larson MP, Bogorodzki P, Rogowska J, McGlade E, King JB, Terry J, Yurgelun-Todd D. Altered prefrontal and insular cortical thickness in adolescent marijuana users. Behav Brain Res. 2011;220:164–172. doi: 10.1016/j.bbr.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood OM, Jacobus J, Bava S, Scarlett A, Tapert SF. Learning and memory performances in adolescent users of alcohol and marijuana: interactive effects. J Stud Alcohol Drugs. 2010;71:885–894. doi: 10.15288/jsad.2010.71.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueeny T, Padula CB, Price J, Medina KL, Logan P, Tapert SF. Gender effects on amygdala morphometry in adolescent marijuana users. Behav Brain Res. 2011;224:128–134. doi: 10.1016/j.bbr.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, Frank LR, Tapert SF. Altered white matter integrity in adolescent binge drinkers. Alcohol Clin Exp Res. 2009;33:1278–1285. doi: 10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: subtle deficits detectable after a month of abstinence. J Int Neuropsychol Soc. 2007a;13:807–820. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Yang TT, Tapert SF. Prefrontal cortex morphometry in abstinent adolescent marijuana users: subtle gender effects. Addict Biol. 2009;14:457–468. doi: 10.1111/j.1369-1600.2009.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Tapert SF. Abnormal cerebellar morphometry in abstinent adolescent marijuana users. Psychiatry Res. 2010;182:152–159. doi: 10.1016/j.pscychresns.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicol Teratol. 2007b;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci. 2004;16:1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, Tapert SF. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug Alcohol Depend. 2011;119:216–223. doi: 10.1016/j.drugalcdep.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Supratentorial profile of white matter microstructural integrity in recovering alcoholic men and women. Biol Psychiatry. 2006;59:364–372. doi: 10.1016/j.biopsych.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Disruption of brain white matter microstructure by excessive intracellular and extracellular fluid in alcoholism: evidence from diffusion tensor imaging. Neuropsychopharmacology. 2005;30:423–432. doi: 10.1038/sj.npp.1300623. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Adalsteinsson E, Lim KO, Moseley M. In vivo detection and functional correlates of white matter microstructural disruption in chronic alcoholism. Alcohol Clin Exp Res. 2000;24:1214–1221. [PubMed] [Google Scholar]
- Qiu D, Tan LH, Zhou K, Khong PL. Diffusion tensor imaging of normal white matter maturation from late childhood to young adulthood: voxel-wise evaluation of mean diffusivity, fractional anisotropy, radial and axial diffusivities, and correlation with reading development. Neuroimage. 2008;41:223–232. doi: 10.1016/j.neuroimage.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Realini N, Rubino T, Parolaro D. Neurobiological alterations at adult age triggered by adolescent exposure to cannabinoids. Pharmacol Res. 2009;60:132–138. doi: 10.1016/j.phrs.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Rey A, Osterrieth PA. Translations of excerpts from Andre Rey’s “Psychological examiniation of traumatic encephalopathy,” and P.A. Osterrieth’s “The complex figure copy test”. In: Corwin J, Bylsma FW, editors. The Clinical Neuropsychologist. 1993. pp. 3–21. [Google Scholar]
- Rubino T, Parolaro D. Long lasting consequences of cannabis exposure in adolescence. Mol Cell Endocrinol. 2008;286:S108–113. doi: 10.1016/j.mce.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Yuan W. White matter development during adolescence as shown by diffusion MRI. Brain Cogn. 2010;72:16–25. doi: 10.1016/j.bandc.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Nagel BJ, Eyler LT, Tapert SF. Neural correlates of verbal learning in adolescent alcohol and marijuana users. Addiction. 2011;106:564–573. doi: 10.1111/j.1360-0443.2010.03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow back. A technique for assessing self-reported alcohol consumption. Humana Press; New York, NY: 1992. [Google Scholar]
- Somerville LH, Casey BJ. Developmental neurobiology of cognitive control and motivational systems. Curr Opin Neurobiol. 2010;20:236–241. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R. Manual for the state-trait anxiety inventory. Consulting Psychologists Press; Palo Alto, CA, USA: 1970. [Google Scholar]
- Squeglia LM, Pulido C, Wetherill RR, Jacobus J, Brown GG, Tapert SF. Brain response to working memory over three years of adolescence: influence of initiating heavy drinking. J Stud Alcohol Drugs. 2012a;73:749–760. doi: 10.15288/jsad.2012.73.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Sorg SF, Schweinsburg AD, Wetherill RR, Pulido C, Tapert SF. Binge drinking differentially affects adolescent male and female brain morphometry. Psychopharmacology (Berl) 2012b;220:529–539. doi: 10.1007/s00213-011-2500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychol Addict Behav. 2009;23:715–722. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A dual systems model of adolescent risk-taking. Dev Psychobiol. 2010;52:216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev. 2010;20:327–348. doi: 10.1007/s11065-010-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman DA, Moghaddam B. The neurobiology of adolescence: changes in brain architecture, functional dynamics, and behavioral tendencies. Neurosci Biobehav Rev. 2011;35:1704–1712. doi: 10.1016/j.neubiorev.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK, Ostby Y, Fjell AM, Westlye LT, Due-Tonnessen P, Walhovd KB. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb Cortex. 2010;20:534–548. doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: neuropsychological functioning over 8 years in youth. J Int Neuropsychol Soc. 2002;8:873–883. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Theilmann RJ, Schweinsburg AD. Reduced fractional anistropy in the splenium of adolescents with alcohol use disorder. Proceedings of the International Society of Magnetic Resonance Medicine. 2003;11:8217. [Google Scholar]
- Wechsler D. Manual for the Wechsler Memory Scale. 3. Psychological Corporation; New York: 1997a. [Google Scholar]
- Wechsler D. WAIS-III Manual. Psychological Corporation; New York: 1997b. [Google Scholar]
- Wechsler D. Manual for the Wechsler Abbreviated Scale of Intelligence. Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional development of the brain in early life. Blackwell Scientific; Oxford, UK: 1967. pp. 3–70. [Google Scholar]