Abstract
Avoiding recurrent injury in sports-related concussion (SRC) requires understanding the neural mechanisms involved during the time of recovery after injury. The decision for return-to-play is one of the most difficult responsibilities facing the physician, and so far this decision has been based primarily on neurological examination, symptom checklists, and neuropsychological (NP) testing. Functional magnetic resonance imaging (fMRI) may be an additional, more objective tool to assess the severity and recovery of function after concussion. The purpose of this study was to define neural correlates of SRC during the 2 months after injury in varsity contact sport athletes who suffered a SRC. All athletes were scanned as they performed an n-back task, for n=1, 2, 3. Subjects were scanned within 72 hours (session one), at 2 weeks (session two), and 2 months (session three) post-injury. Compared with age and sex matched normal controls, concussed subjects demonstrated persistent, significantly increased activation for the 2 minus 1 n-back contrast in bilateral dorsolateral prefrontal cortex (DLPFC) in all three sessions and in the inferior parietal lobe in session one and two (α≤0.01 corrected). Measures of task performance revealed no significant differences between concussed versus control groups at any of the three time points with respect to any of the three n-back tasks. These findings suggest that functional brain activation differences persist at 2 months after injury in concussed athletes, despite the fact that their performance on a standard working memory task is comparable to normal controls and normalization of clinical and NP test results. These results might indicate a delay between neural and behaviorally assessed recovery after SRC.
Key words: : concussion, DLPFC, fMRI, n-back task, working memory
Introduction
The Centers for Disease Control and Prevention estimate that 300,000 sports-related concussions (SRC) occur annually in the United States.1 The study included only concussions for persons who sustained loss of consciousness (LOC), which has been reported to occur in just 8%2 or 19.2%3 of sports-related concussions. Given the fact that athletes tend not to report their injury, a more accurate estimate may be that between 1.6 and 3.8 million SRC occur each year in the United States including injuries for which no medical treatment is sought.4 At the same time, the annual rate of diagnosed concussions over the past 10 years in high school sports rose by 16.5%,5 which may partly reflect an increased awareness by parents and coaches through increased media attention to concussive injury in sports, as well as better diagnosis and detection by clinicians. Nevertheless, the huge incidence of SRC in adolescents and young adults calls for a full understanding of the neural correlates and consequences of this condition.
According to the most recent consensus statement,6 concussion is considered a brain injury (caused by a direct blow to the head, neck, or face), involving a complex pathophysiological process, induced by biomechanical forces, typically resulting in the rapid onset of short-lived impairment of neurological function that resolves spontaneously. The authors of this statement affirm that: “a concussion may result in neuropathologic injury, but the acute clinical symptoms largely reflect a functional disturbance rather than structural injury.”
This definition is questionable in light of recent neuroimaging research results. Although clinical and cognitive symptoms may subside after approximately 2 weeks in most athletes who sustain a concussion, alteration of physiological brain measures persist. For example, magnetic resonance spectroscopy (MRS) studies have demonstrated neurometabolic alterations lasting up to 1 month post-injury.7–9 Structural changes from repetitive concussive head impacts have been reported in ice hockey players over the course of a single season,10 in athletes with prolonged symptoms,11 as well as in adolescents exhibiting close to normal Sports Concussion Assessment Tool (SCAT) 2 scores at ≤2 months postinjury.12
One methodology for evaluating the neural consequences of mild traumatic brain injury (mTBI) is blood oxygenation level dependent (BOLD) functional magnetic resonance imaging (fMRI), which allows non-invasive evaluation of brain activity based on hemodynamic response to task demands13–15 The imaging contrast in fMRI results from the higher ratio of oxy- to deoxyhemoglobin in local draining veins that accompanies neuronal activation, which in turn changes local magnetic susceptibility because of properties of hemoglobin.13,16 fMRI can reveal brain pathology, often enjoying more power than standard clinical measures.17–19 This makes fMRI an attractive marker for recovery of function after mTBI. fMRI can also be repeated safely, allowing successive measurements.20,21
Recent fMRI studies have revealed alterations of the BOLD signal after mTBI when subjects perform working memory, sensory-motor, attention, and other neurocognitive tasks. Specifically, differences in brain activation under varying cognitive load (0, 1, 2, and 3-back conditions in the n-back task) were identified in subjects with mTBI.21 During the first month after injury, patients with mTBI, presenting with a Glasgow Coma Scale (GCS) score of 13–15, demonstrated a significant increase of activation in bilateral frontal and parietal regions during 1-back and 2-back tasks. An fMRI study22 using a finger sequencing task in four football players with concussion and four uninjured player controls revealed significantly increased activity in lateral frontal, superior, and inferior parietal, and bilateral cerebellar regions within the week after injury. No significant differences in NP test performance were reported between pre-injury and 1 week post-injury conditions. Similarly, larger cluster sizes in the parietal cortex and right dorsolateral prefrontal cortex (DLPFC) and significantly larger BOLD signal percent change in the right hippocampus were identified in athletes with concussion as they performed a spatial memory navigation task in a virtual environment.23
On the other hand, fewer task-related activations in athletes with mTBI who had persistent symptoms were found compared with normal controls, when they performed a verbal memory task at one month after injury. In particular, working memory task-related BOLD signal changes were significantly decreased in the DLPFC in all athletes with concussion who had persistent symptoms.24 A subsequent study25 reported increased activation peaks in the left temporal lobe in response to a verbal memory task when comparing athletes with concussion and persistent symptoms (at 1 month post-injury) with normal elite athletes; this is in addition to decreased activity in the prefrontal areas.
The present study investigates the neural correlates of functional recovery after SRC during the 2 months after injury in male and female varsity level collegiate athletes. Specifically, we track changes of cortical activation at 2 days, 2 weeks, and 2 months post-injury in contact sports athletes who had sustained a concussion without LOC. The first time point (2 days post-injury) was chosen to assess brain function during the acute phase of concussive injury. Whereas neurometabolic changes during the first 3–6 days post-injury are well documented,8,9 much less is known about neural function in the acute phase. The 2-week time point was chosen to assess functional brain activation at a time of recovery when the majority of concussions (80–90%) are thought to resolve (cognitive function returning to normal).6 The third time point (2 months post-injury) provided the opportunity to track patterns of recovery in subjects whose brain activation continued to be atypical at 2 weeks (compared with normal control subjects) and whose NP test results had not returned to baseline at 2 weeks post-injury.
Based on the studies discussed previously (suggesting alterations in DLPFC, parietal, and temporal areas), we designed a working memory task inspired by Hockey and associates,26 that allows assessment of the frontoparietal network during the 2 months after injury. We asked whether atypical brain activation in response to memory load persisted after persons with SRC were symptom free and tested neuropsychologically within normal range. Our results provide information about the appropriate timing of return to play and substantiate the idea that fMRI is a useful tool for the assessment and management of concussion.
Methods
Subjects
Participants in this study included 15 varsity level college students who sustained a SRC (12 male, 3 female; mean age 19.8, standard deviation [SD] 0.94 years). The concussions of all 15 athletes were diagnosed by University Athletic Medicine personnel using the third International Consensus Conference definition.27 History of concussion was obtained through self-report. It should be noted that it is difficult to evaluate number of previous concussions objectively in contact sport athletes, given the limitations and subjectivity of self-report. On this basis, eight subjects had no history of concussion. Five subjects had sustained one previous concussion, one subject had two, and the remaining subject had sustained three previous concussions (mean time since last concussion 2.94, SD 1.99 years). Based on review of medical history and physical examinations performed by University Athletic Medicine personnel, no subject had a history of medical, genetic, or psychiatric disorder.
All subjects with concussion were enrolled in the Princeton University Concussion Program for high-risk sports. This program includes baseline NP testing using Immediate Post-Concussion Assessment and Cognitive Testing (ImPACT)28 and SCAT 2,27 history, and physical examination as well as post-injury evaluation and testing. Post-injury testing included SCAT 2, hybrid NP tests from ImPACT and paper/pencil tests administered within 24–48 hours of injury; test results were interpreted by a consulting neuropsychologist. The following tests were included in the paper pencil tests: (1) Brief Visuospatial Memory Test-Revised,29 (2) Hopkins Verbal Learning Test-Revised,30 (3) Symbol Digit Modalities Test,31 (4) Digit Span Test,32 (5) Trail Making Test,33 (6) The Stroop Test,34 (7) Patient Health Questionnaire,35 and (8) Generalized Anxiety Disorder Test.36
After their most recent SRC, subjects included in this study were evaluated by a certified athletic trainer and team physician. None of the subjects demonstrated symptomatology warranting further assessment by the GCS37 or the use of computed tomography (CT) scan or any other clinical imaging. All subjects tested abnormally on paper/pencil and computerized ImPACT tests28 administered immediately after injury (within 24–48 hours). A given athlete's degree of abnormality was determined through the comparison of post-injury NP test scores to that athlete's baseline scores. In particular, abnormality of ImPACT clinical composites was based on Reliable Change Indices at the 0.8 confidence interval.28,38 After concussion, individualized management and return-to-play decisions were made by the team physician. Typically, athletes are kept out of activity until their symptoms resolve and their balance and NP testing return to pre-injury levels, at which time they are allowed to initiate a return to play progression and resume an exertional program that gradually increases both their level of exertion as well as their risk for contact following the First International Consensus Conference on Concussion guidelines.39 Furthermore, the return to play progression is also adapted to each individual athlete, taking into consideration where he or she is in the competitive season and hence to what type of activities he or she might return.
All athletes with concussion agreed to participate in scanning sessions (fMRI) and repeated SCAT 2 and NP testing assessments within 2 days (session 1), 2 weeks (session 2), and 2 months (session 3) post-injury. Additional NP testing, however, was administered between these time points to determine the return to play for each athlete. Healthy control subjects included 15 sex and age matched non-contact varsity athletes (12 male, 3 female; mean age 19.8, SD 1.73 years). They were in good physical condition with no history of head trauma, psychiatric, neurological, or developmental disorder. For controls, data were analyzed from a single scanning session. Concussion and control subject demographics are presented in Table 1. All participants gave written consent to participate in the study, which was approved by the Princeton University Institutional Review Panel for Human Subjects Research.
Table 1.
Subject Demographics of Athletes with Concussion and Matched Controls
| Concussed | Controls | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Sex | Age | Sport | #Previous concussions | NP normal | Symptom free | Return to play | Sex | Age | Sport |
| 1 | F | 19 | Field hockey | 2 | 13 days | 12 | No return to play | F | 19 | Volleyball |
| 2 | M | 19 | Football | 0 | 2 moa | 14 | No return to play | M | 19 | Crew Heavyweight |
| 3 | M | 19 | Water polo | 0 | 6 days | 6 | 24 days | M | 19 | Volleyball |
| 4 | M | 20 | Football | 1 | 24 daysb | 17 (1st), 77(2nd) | 31 days | M | 21 | Crew lightweight |
| 5 | F | 21 | Basketball | 1 | 9 days | 6 | 9 days | F | 21 | Volleyball |
| 6 | F | 18 | Rugby | 0 | 17daysc | 23 | No return to play | F | 18 | Swimming |
| 7 | M | 21 | Ice hockey | 0 | 3 days | 5 | 12 days | M | 21 | Volleyball |
| 8 | M | 20 | Football | 0 | 6 days | 5 | 15 days | M | 18 | Swimming |
| 9 | M | 20 | Basketball | 1 | 9 days | 6 | 12 days | M | 18 | Squash |
| 10 | M | 19 | Football | 0 | 18 days | 10 | 22 days | M | 18 | Crew heavyweight |
| 11 | M | 21 | Ice hockey | 3 | 15 days | 162 | No return to play | M | 22 | Cross country |
| 12 | M | 20 | Lacrosse | 0 | 17 days | 10 | 23 days | M | 22 | Track |
| 13 | M | 20 | Wrestling | 0 | 11 days | 3 | 18 days | M | 22 | Track |
| 14 | M | 19 | Ice hockey | 1 | 2 days | 4 | 23 days | M | 18 | Crew heavyweight |
| 15 | M | 21 | Sprint foot ball | 1 | 13 days | 7 | 16 days | M | 22 | Track |
Not normal at 2 weeks, not repeated until 2 months since season over.
Returned to play after 1st injury at 31 days, sustained a 2nd concussion and decided not to return to play.
No return to play, season was over.
Working memory paradigm
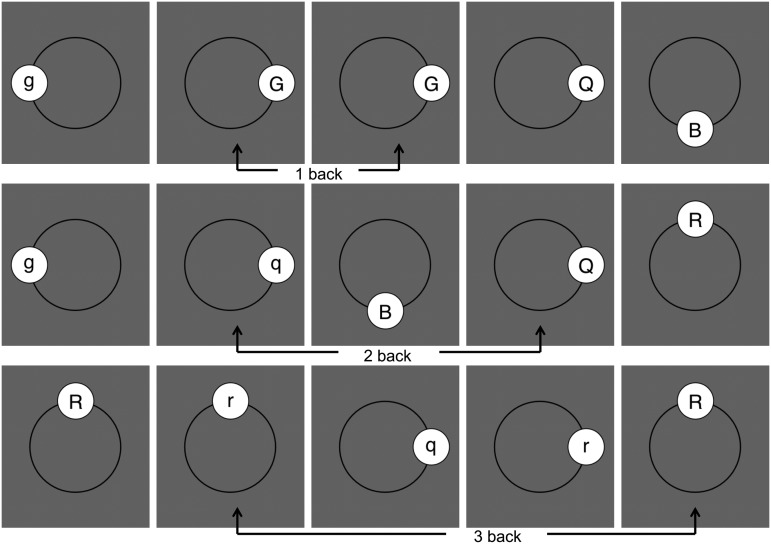
Subjects performed a working memory task inspired by Hockey and colleagues26 during the three fMRI sessions. The task was “n-back” for n=1, 2, 3. Specifically, subjects viewed successive presentations of one of four letters (B, G, Q, and R) chosen randomly in either upper or lower case, appearing at one of the four compass points (Fig. 1). For 1-back, subjects were asked to press a button on a button box when successive images were identical (ignoring case). The 2-back task was the same except that matching images had to be separated by one non-matching image; for 3-back the matching images had to be separated by two non-matching images. Note that matches required that the corresponding letters appear at the same compass point (Fig. 1).
FIG. 1.
Illustration of 1-back, 2-back, 3-back tasks.
Subjects performed 10 blocks for each of 1-back, 2-back, and 3-back. The 30 blocks were individually randomized and divided into three runs of 10 blocks. Runs were separated by a few minutes. At the start of each block, subjects viewed a screen displaying the upcoming n-back task for 2 sec, followed by a blank screen for an additional 2 sec. They then viewed 20 images, each presented for 500 ms, followed by an interstimulus interval of 1200 ms. Between each block, there was a 18.5 sec rest period.
fMRI image acquisition
MR images were acquired on a 3T Siemens (Erlangen, Germany) Skyra whole body scanner with a 16 channel, phase array coil (Siemens). Siemens Skyra EPI PACE sequence was used for fMRI data acquisition with the following parameters: 36 axial slices, 3×3×3 mm3 voxel resolution, repetition time (TR)=2020 ms, echo time (TE)=30 ms, flip angle=76 degrees, field of view=192 mm.
A single contiguous run comprised 284 volumes with an acquisition time of 9 min and 34 sec incorporating 10 randomized blocks of n-back trials with 18.5 sec rest intervals. Three volumes (3 TRs) were incorporated into the task paradigm to ensure syncing with the scanner pulse trigger. Before triggering the start of the run acquisition, additional hidden dummy volumes (3 TRs) were acquired by the scanner for magnetic stabilization. A total of three such runs comprised one fMRI imaging session. The n-back trials were programmed in a MATLAB (Mathworks, Natick, MA) environment using Psychophysics Toolbox extensions.40–42 To support downstream image registration to the MNI (Montreal Neurological Institute) brain atlas and to align functional data across subjects and sessions, a high resolution T1-weighted MPRAGE image was acquired at the end of each fMRI session: 192 sagittal slices, 0.90×0.94×0.94 mm3 voxel resolution, TR=1900 ms, TE=2.13 ms, flip angle=9 degrees, field of view=240 mm, and a total anatomical scan time of 4 min, 26 sec.
A total of 175 volumes of resting state images with identical imaging parameters as in the working memory task were also acquired before the fMRI n-back task sequence. During image acquisition (5 min, 54 sec), subjects were asked to stay awake but keep their eyes closed. Resting state data were acquired for all 15 subjects with concussion at 2 weeks and 2 months. For the 2 days post-injury time point, however, only 12 subjects completed their resting state scan. All 15 controls completed the resting state protocol.
fMRI image data analysis
All imaging data were processed using standard, General Linear Modeling (GLM) based block design routines in FSL-FEAT43 (version 4.1.9). Preprocessing steps included spatial and temporal smoothening with a full width at half maximum filter (FWHM) of 6 mm (2 voxel spacing), high pass filter cutoff set to 110 sec, and motion correction via the included MCFLIRT algorithm. “First Level” analyses GLM used a double-gamma hemodynamic response function to model the three original n-back task conditions (1 to 3-back, with respect to the 15 sec rest intervals). Additional contrasts (2-1, 3-1, with respect to the 1-back task) were set up based on the original task conditions for each fMRI run comprising 10 blocks. Individual fMRI runs were coregistered to the MNI 152 standard brain atlas via a 12 degrees of freedom (DOF) linear registration search in FEAT, using the associated high resolution MPRAGE volume. A “Second Level” analysis via a “Fixed Effects” model was used to collapse the three discrete runs in each session, resulting in beta volumes for the 2-1 and 3-1 contrasts. Post-hoc analyses were run on the derived contrasts in the AFNI44 environment, using 3d analysis of variance (3dANOVA3). All analyses were conducted in fMRI space.
To reduce the number of multiple comparisons, a gray matter mask of the imaged volume was obtained through binary thresholding45 of the average 152 gray matter T1 volume (included in the FSL suite version 4.1.9) of the co-registered MNI 152 atlas. The ventricles were additionally masked (mask included in the FSL suite 4.1.9). Noise in the fMRI dataset was computed from the First Level residuals of the GLM. AFNI subroutines 3dFWHMx and 3dClustSim were used to compute cluster size thresholds to address the issue of multiple comparisons correction. For this dataset, the cluster size threshold was 31 voxels (in fMRI resolution) with a corresponding p value threshold of 0.005 for an alpha correction of 0.01. In this article, we report results of the between-group 2-1 contrast.
Amplitude of Low Frequency Fluctuation (ALFF) was chosen as a measure of resting state neuronal activity.46–48 Whole brain ALFF was computed using the acquired resting state data, via AFNI and FSL 4.1.9 functions with a script adapted from www.nitrc.org/projects/fcon_1000. The T1-weighted MPRAGE volume served as the anatomical reference for each subject. The FWHM was set to 6 mm, the frequency band of interest was selected to range from 0.01 to 0.1 Hz, and the standard space was the 3 mm, MNI 152 T1 volume matching the spatial resolution of the resting scan data.
Results
Behavioral data
Each subject (concussed and control) was scored on the three n-back tasks via the accuracy statistic: number of correct detections (hits) minus number of incorrect responses (false alarms). The mean accuracy for each group at each of the three time points on each of the three n-back tasks is presented in Table 2. The t tests revealed no significant differences between the two groups (concussed vs. control) at any of the three time points with respect to any of the three n-back tasks.
Table 2.
Mean Accuracy (Hits Minus False Alarms) for Controls and Subjects with Concussion during 1-Back, 2-Back, and 3-Back Tasks
| 1-back M (SD) | 2-back M (SD) | 3-back M (SD) | |
|---|---|---|---|
| Controls | 35.20 (2.88) | 28.73 (4.61) | 13.33 (5.12) |
| Concussed Session 1 | 36.07 (3.15) | 26.53 (5.94) | 12.40 (4.00) |
| Concussed Session 2 | 35.07 (3.43) | 28.93 (7.58) | 16.64 (7.08) |
| Concussed Session 3 | 35.21 (4.41) | 28.36 (8.24) | 16.36 (8.34) |
SD, standard deviation.
For each group individually, 3-back was more difficult than 2-back, which was more difficult than 1-back (all comparisons by paired t test, p<0.05 in all cases). These results suggest that concussed and control subjects were comparably challenged by the n-back paradigm. Of the 15 subjects with concussion, all but two tested in the normal range on the ImPACT and paper/pencil tasks by the date of their second scanning session, or a few days later (see column 6 in Table 1).
Imaging
For the imaging analysis, in each group we computed for each voxel the difference in beta values in response to the 2-back minus 1-back tasks (using the latter as baseline). We did not use 3-back for this purpose because of its relatively low performance scores and high variability in both groups. We identified all brain areas that manifested significant between-group differences in each of the three sessions for the 2-back minus 1-back contrast (3dClustSim corrected α≤0.01). In every one of these areas, subjects with concussion demonstrated significantly increased activity compared with their age and sex matched controls.
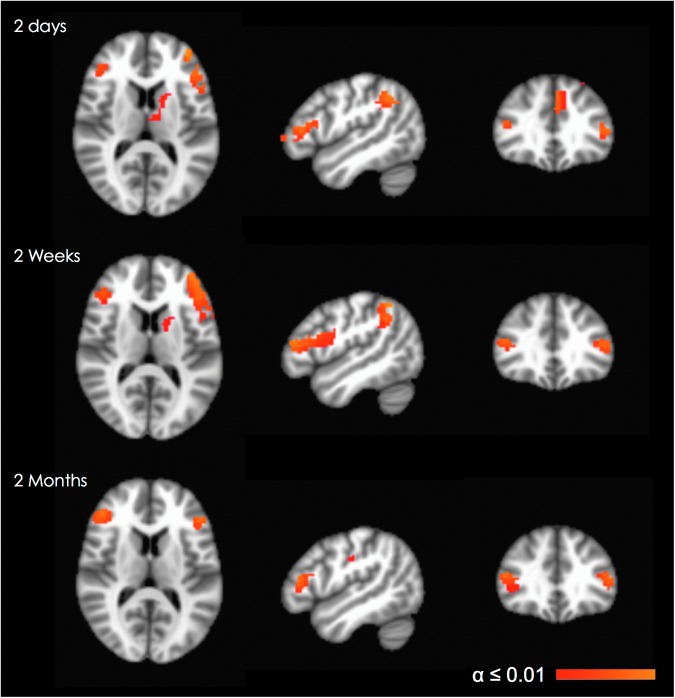
Turning to activation contrasts in particular sessions, subjects with concussion demonstrated significantly higher activation in 11 clusters in session 1, compared with only six clusters in session two and session three (Table 3). Thus, when performing a working memory task at a comparable level of performance to normal controls, subjects with concussion significantly increased their activation in a greater number of brain areas immediately after injury as compared with 2 weeks and 2 months post-injury. The areas showing group differences in activation for all three sessions were limited to left and right prefrontal areas (BA46, extending into BA10 in the left hemisphere). Restricting attention to just sessions one and two, there were significant group differences in the left inferior parietal (supramarginal) gyrus (BA40) as well (Fig. 2). These findings suggest that subjects with concussion demonstrated persistent significantly increased activation not only in the first phase of recovery, but also at 2 weeks and 2 months post-injury.
Table 3.
MNI Coordinates of the Voxel with the Minimum P Value for Each Significant Cluster (between Group Comparison for the 2 Minus 1 N-Back Contrast, α≤0.01, Corrected)*
| MNI coordinates, min. p value | ||||
|---|---|---|---|---|
| ROI | Cluster size voxel count (1 voxel=3×3×3 mm3) | x | y | z |
| Controls vs. concussed session 1 | ||||
| Left inferior frontal gyrus, BA10 extending into BA 46 | 253 | −36 | 57 | 0 |
| Left cingulate gyrus BA31 | 212 | 0 | −24 | 42 |
| Left inferior parietal (supramarginal) gyrus BA40 | 166 | −36 | −66 | 51 |
| Left medial frontal/cingulate gyrus BA6, BA9 | 140 | 0 | 24 | 51 |
| Left middle frontal gyrus BA8 | 76 | −33 | 18 | 57 |
| Left thalamus | 58 | −3 | −6 | 6 |
| Right inferior parietal (angular) gyrus, BA39 | 55 | 39 | −69 | 42 |
| Right postcentral gyrus BA3 | 35 | 24 | −39 | 66 |
| Right superior frontal gyrus BA6 | 34 | 27 | 12 | 63 |
| Right superior frontal gyrus BA11 | 33 | 27 | 60 | −12 |
| Right inferior frontal gyrus BA46 | 32 | 48 | 33 | 18 |
| Controls vs. concussed session 2 | ||||
| Left inferior frontal gyrus BA10 extending into BA46/44 | 339 | −39 | 51 | 15 |
| Left inferior parietal (supramarginal) gyrus BA40 | 134 | −42 | −54 | 54 |
| Right inferior frontal gyrus BA46 | 46 | 48 | 33 | 18 |
| Left caudate | 43 | −12 | 6 | 12 |
| Right caudate | 42 | 15 | 15 | 6 |
| Left medial frontal gyrus BA8 | 41 | −3 | 24 | 42 |
| Controls vs. concussed session 3 | ||||
| Left cingulate gyrus BA31 | 105 | 0 | −24 | 42 |
| Right inferior frontal gyrus, BA46 | 96 | 45 | 39 | 12 |
| Right precentral BA4 | 87 | 33 | −24 | 63 |
| Left inferior frontal gyrus BA46 | 60 | −48 | 33 | 12 |
| Left parahippocampal (lentiform, putamen) | 47 | −18 | −3 | −18 |
| Left precentral | 33 | −63 | −21 | 24 |
The corresponding cluster voxel count is also listed.
ROI, region of interest; MNI, Montreal Neurological Institute.
FIG. 2.
Between group comparison (concussed–controls), for the 2 minus 1 n-back contrast, demonstrated persistent, significantly increased (α≤0.01, corrected) activity in bilateral, dorsolateral prefrontal areas throughout all three time points and in the left inferior parietal area within 72 hours and at 2 weeks post-injury. Images are shown in radiological convention (right=subject's left) for slice coordinates: x=−48 mm, y=32 mm, z=12 mm. Color image is available online at www.liebertpub.com/neu
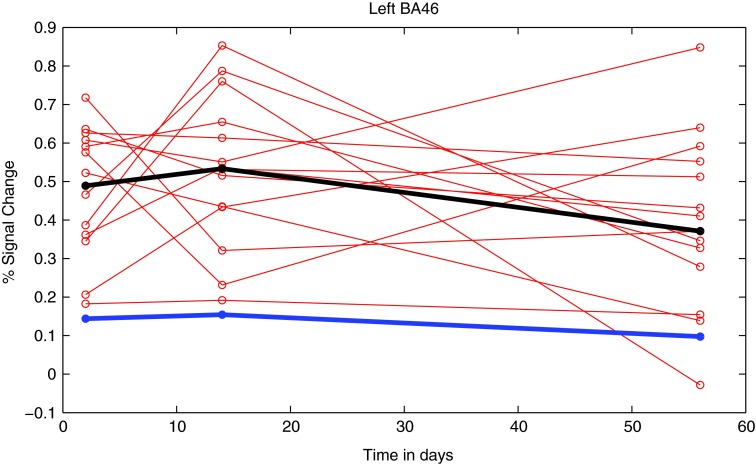
To study individual differences, we proceeded as follows. We computed the average percent signal change of the voxels in a given subject's brain that were mapped to the left BA46/BA10 cluster that was revealed by the between-group contrast (thresholded at α≤0.01, corrected). Figure 3 shows the trajectory of these averages for each subject with concussion over the three sessions; the median value of the corresponding averages for control subjects is shown as well. The figure reveals the variability in recovery for subjects with concussion along with a suggestion of overall nonlinearity (increased hyperactivity from session one to two followed by decline). Group differences, however, were swamped by the variability in activation from session one to three; indeed, there was no main effect of session number for any of the neural areas revealed by the 2-back minus 1-back contrast.
FIG. 3.
Individual trajectories (red) of the average percent signal change derived for each subject with concussion in each of the three sessions for the cluster in left BA46 (mask derived from the 2 minus 1 contrast, α≤0.01 for each session). The median of the concussed (black) and controls (blue) are overlaid. Color image is available online at www.liebertpub.com/neu
It is important to note that by 2 weeks from injury or shortly thereafter, most athletes were symptom free and tested neuropsychologically within normal range. Most subjects returned to play between 9 and 31 days after injury, with the exception of four subjects who did not return to play either because the season ended too soon or because the subject decided not to resume the sport.
To test whether altered resting state activity might contribute to the observed persistent increased activity in the concussed compared with the normal control group, we assessed mean ALFF values in bilateral DLPFC. The session-specific left BA46 mask from the 2-1 between-group contrast served as the primary region of interest for between-group comparisons. The mask was mirrored for each session to create a BA46 mask for the right hemisphere. The mean ALFF values were examined for both left and right BA46 masks for each session. Pooled t tests were used to investigate between-group differences of the left minus right BA46 as well as between-group differences for each hemisphere separately, using the mean ALFF measures for each session.
None of these t tests produced significant results. That is, we found no between-group differences in resting state in the comparison between left and right BA46; likewise, no differences in mean ALFF reached significance in tests within each hemisphere in any session. These negative results suggest that altered resting state activity does not drive the persistent BOLD signal differences identified in the DLPFC between concussed and normal groups. Rather, it appears that the increased BOLD signal identified in the whole brain analysis for the 2-1 n-back contrast in the participants with concussion can be attributed to a task-specific response to working memory load.
Discussion
The present study examined brain activation, clinical symptoms, and cognitive function based on NP testing in a sample of athletes with concussion within 2 days of injury, then at 2 weeks and 2 months later. Previous fMRI experiments have typically investigated changes in brain activation at just one time point, either during the first weeks after injury49 or at a later stage of recovery.24,25 Exceptions include a study22 in which athletes with concussion who had previously participated in a baseline scan were scanned again within 1 week after injury and another experiment50 in which athletes with concussion were scanned at 1 week after injury and at full clinical recovery. To our knowledge, no previous study has compared brain activation between normal and concussed brains at three temporal landmarks after injury.
Compared with normal control subjects, in response to a verbal/spatial working memory task (n-back), our subjects with concussion manifested significantly higher activity in left and right DLPFC (persisting up to 2 months) and in the left inferior parietal area (persisting up to 2 weeks post-injury). In contrast, the subjects with concussion performed the working memory task at a level of performance that is comparable to that of normal controls. Subjects with concussion also demonstrated abnormally high activation in brain regions beyond DLPFC and the inferior parietal area. The number of hyperactive brain regions was greater in the days after injury than at 2 weeks and 2 months.
In scanning sessions one and two, the largest clusters of activation demonstrating significant between-group differences were located in the left frontoparietal network, with hyperactivation persisting bilaterally in the DLPFC through session three. The hyperactive regions we observed in subjects with concussion are typically activated in the normal population only for high load conditions in the n-back task.51,52
The hyperactivation in bilateral DLPFC and inferior parietal areas is in close agreement with the results of previous working memory fMRI studies in athletes with SRC.22,49,50 It also corresponds to findings involving subjects with mTBI who had a GCS score of 13–15.21 It should be mentioned, however, that significantly decreased BOLD signal (hypoactivation) was reported in the DLPFC when athletes, diagnosed with post-concussive syndrome, performed a working memory task.24,25 These opposite findings are likely because of methodological differences between the two studies. The studies differed in the choice of working memory tasks, timing of fMRI scan (the average was 4–5 months after injury), and history of multiple concussions.24,25 Moreover, the subjects24 exhibited prolonged symptoms for several months after injury, whereas in our sample, only one subject was symptomatic beyond 2 months.
Beyond SRC, the majority of imaging studies on working memory report differences in brain activation (increased activation in prefrontal regions and elsewhere) when comparing healthy controls with clinical populations with severe TBI or other neurological disorders.53–56 In cases of mild brain dysfunction, where performance on the working memory task may not differ between the experimental and control groups, additional recruitment of neural resources has usually been interpreted as neural compensation in the face of cognitive deficits.21,53,57,58 This hypothesis posits transient alteration of brain function to support task performance without permanent alteration of the underlying brain structure.
Neural compensation may be contrasted with the hypothesis of brain reorganization, which assumes that additional DLPFC recruitment reflects structural alteration and changes in functional networks associated with working memory.54,59–61 Consistent with neural reorganization, DTI studies provide preliminary evidence of alteration in white matter fiber tracts in persons with SRC. These studies involved athletes with prolonged symptoms11 among college varsity football players62 and male ice hockey players.10 The latter study was prospective, assessing brain structure in players in a pre- and post-season DTI scan.10
Yet another interpretation has been articulated, termed latent support hypothesis.63 According to this idea, the hyperactivation of the prefrontal cortex observed in mTBI is representative of the engagement of additional cognitive and attentional resources to meet the task demands in a challenged neural system.64,65 Neither structural alteration nor bolstering cognitive functioning is posited. Instead, the latent support hypothesis63 suggests that the hyperactivation of the prefrontal cortex and (depending on the task demands) in the inferior parietal areas26,51,56,66 is from increased cognitive and attention control similar to what is observed in load manipulations with healthy controls.51,52,67
Returning to the data presented here, the fact that no between-group differences in BOLD signal were found in bilateral DLPFC cortex during the resting state suggests that hyperactivation of the DLPFC is task driven. In turn, this discourages the thesis of structural brain reorganization, consistent instead with either neural compensation or latent support. Obviously, the relative merits of the foregoing hypotheses (specifically, the extent of structural brain reorganization after concussion) can only be resolved through future longitudinal studies based on both fMRI and DTI. Special attention should be devoted to possible structural alterations in the deep white matter.10,11,62
In the present study, the marked variability in the individual athlete's pattern of change in brain activation was unexpected, and its cause remains unclear. Still, most subjects demonstrated a non-linear, altered pattern of brain activation compared with their normal controls with a trend toward increased hyperactivity in the DLPFC at two weeks followed by a decline toward 2 months post-injury. In contrast, with one exception, all athletes with concussion tested neuropsychologically within normal range between 2–3 weeks after injury. Ten subjects returned to play between 2–3 weeks, and one subject returned to play at 1 month after injury. For three of the remaining four subjects, the season ended before being allowed to return to play. The remaining subject decided to discontinue her sport because of prolonged symptoms. The persistence of brain hyperactivation despite the fact that the athletes have returned to the normal range in NP testing (and often resumed play) raises the concern that athletes might be exposed to further concussive (and sub-concussive) shocks before the brain is fully recovered.
The discrepancy between normal NP test results at 2 weeks and hyperactivation in the DLPFC up to 2 months after injury might be explained by lack of sensitivity of the NP testing battery (ImPACT, paper pencil tests). Along with the resolution of symptoms and normalization of balance deficits, NP testing, especially hybrid testing protocols, are currently the best practices available for determining when athletes have recovered from SRC. Of course, the persistent hyperactivation in DLPFC until 2 months post-injury raises the question of whether brain activation will ever normalize. The clarification of this issue seems vital for deciding when it is safe to return to play. In particular, correlations of NP test results with associated brain measures should be investigated further. The issue is especially pressing given recent results of neuropathological studies, indicating that repetitive injury might result in neurodegenerative changes later in life.68,69 Given the millions of amateur and professional athletes, as well as military personnel, who are exposed to repetitive concussions, it seems imperative to further elucidate when and if concussion-induced changes in brain activation might remit.
Conclusion
The longitudinal nature of this study advances our understanding of the neural correlates of SRC by demonstrating alteration of brain activation subsequent to a return to normal scores on NP tests. Future research should include longitudinal experimental designs based on larger samples and scanning beyond 2 months post-injury. Ideally, such research would involve a multimodal neuroimaging approach and the use of biomarkers that can be correlated to brain structure and function.
Acknowledgments
We would like to acknowledge the athletic trainers of University Health Services for their assistance with subject recruitment and NP testing. This work was funded by the New Jersey Commission for Brain Injury Research grant No. 10-3217-BIR-E-0, AMSSM Foundation grant No. 005548, the Goldstein Family Fund, and the Peter & Cynthia Kellogg Foundation.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Thurman D.J., Branche C.M., and Sniezek J.E. (1998). The epidemiology of sports-related traumatic brain injuries in the United States: recent developments. J. Head Trauma Rehabil, 13, 1–8 [DOI] [PubMed] [Google Scholar]
- 2.Schulz M.R., Marshall S.W., Mueller F.O., Yang J., Weaver N.L., Kalsbeek W.D., and Bowling J.M. (2004). Incident and risk factors for concussion in high school athletes, North Carolina, 1996–1999. Am. J. Epidemiol. 160, 937– 944 [DOI] [PubMed] [Google Scholar]
- 3.Collins M.W., Iverson G.L., Lovell M.R., McKeag D.B., Norwig J., and Maroon J. (2003). On-field predictors of neuropsychological and symptom deficit following sports-related concussion. Clin. J. Sport Med. 13, 222–229 [DOI] [PubMed] [Google Scholar]
- 4.Langlois J.A., Rutland-Brown W., and Thomas K.E. (2005). Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths. Centers for Disease Control, National Center for Injury Prevention and Control: Atlanta, Georgia: Available at: http://www.cdc.gov/traumaticbraininjury/pdf/Future_of_Registries-a.pdf [Google Scholar]
- 5.Lincoln A.E., Casewell S.V., Almquist J.L., Dunn R.E., Norris J.B., and Hinton R.Y. (2011). Trends in concussion incidence in high school sports: a prospective 11-year study. Am. J. Sports Med. 39, 958–963 [DOI] [PubMed] [Google Scholar]
- 6.McCrory P., Meeuwiss W., Aubry M., Cantu B., Dvorak J., Echemendia R, Engebretsen L., Johnston K., Kutcher J., Rafferty M., Sills A., Benson B.W., Davis G.A., Ellenbogen R., Guskiewicz K.M., Herring S.A., Iverson G.L., Jordan B.D., Kissick J., McCrea M., McIntosh A.S., Maddocks D., Makdissi M., Purcell L., Putukian M., Schneider K., Tator C.H., and Turner M. (2013). Consensus statement on Concussion in Sport—the 4th International Conference on Concussions in Sport held in Zurich, November 2012. Clin. J. Sport Med. 23, 89–117 [DOI] [PubMed] [Google Scholar]
- 7.Vagnozzi R., Signoretti S., Cristofori L., Alessandrini F., Floris R., Isgro E., Ria A., Marziale S., Zoccatelli G., Tavazzi B., Del Bolgia F., Sorge R., Broglio S.P., McIntosh T.K., and Lazzarino G. (2010). Assessment of metabolic brain damage and recovery following mild traumatic brain injury: a multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain 133, 3232–3242 [DOI] [PubMed] [Google Scholar]
- 8.Vagnozzi R., Signoretti S., Floris R., Marziali S., Manara M., Amorini A. M., Belli A., Di Pietro V., D'Urso S., Pastore F.S., Lazzarino G., and Tavazzi B. (2013). Decrease in N-acetylaspartate following concussion may be coupled to decrease in creatine. J. Head Trauma Rehabil. 28, 284–292 [DOI] [PubMed] [Google Scholar]
- 9.Henry L.C., Tremblay S., Boulanger Y., Ellemberg D., and Lassonde M. (2010). Neurometabolic changes in the acute phase after sports concussions correlate with symptom severity. J. Neurotrauma 27, 65–76 [DOI] [PubMed] [Google Scholar]
- 10.Koerte I.K., Kaufman D., Hartl E., Bouix S., Pasternak O., Kubicki M., Rauscher A., Li D.K., Dadachanji S.B., Taunton J.A., Forwell L.A., Johnson A. E, chlin P.S., and Shenton M.E. (2012). A prospective study of physician-observed concussion during a varsity university hockey season: white matter integrity in ice hockey players. Neurosurg. Focus 33, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cubon V.A., Putukian M., Boyer C., and Dettwiler A. (2011). A diffusion tensor imaging study on the white matter skeleton in individuals with sports-related concussion. J. Neurotrauma 28, 189–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Virji-Babu N., Borich M.R., Makan N., Moore T., Frew K., Emery C.A., and Boyd L.A. (2013). Diffusion tensor imaging of sports-related concussion in adolescents. Pediatr. Neurol. 48, 24–29 [DOI] [PubMed] [Google Scholar]
- 13.Ogawa S., Lee T.M., Kay A.R., and Tank D.W. (1990). Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. U.S.A. 87, 9868–9872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jezzard P., Matthews P.M., and Smith S.M., eds. (2003). Functional MRI: An Introduction to Methods. Oxford University Press: Oxford, UK [Google Scholar]
- 15.Matthews P.M., and Jezzard P. (2004). Functional magnetic resonance imaging. J. Neurol. Neurosurg. Psychiatry 75, 6–12 [PMC free article] [PubMed] [Google Scholar]
- 16.Pauling L., and Coryell C.D. (1936). The magnetic properties and structure of hemoglobin, oxyhemoglobin and carbonmonoxyhemoglobin. Proc. Natl. Acad. Sci. U.S.A. 22, 210–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karni A., Meyer G., Jezzard P., Adams M.M., Turner R., and Ungerleider L.G. (1995). Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature 377, 155–158 [DOI] [PubMed] [Google Scholar]
- 18.Binder L.M. (1997). A review of mild head trauma. Part II: Clinical implications. J. Clin. Exp. Neuropsychol. 19, 432–457 [DOI] [PubMed] [Google Scholar]
- 19.Bookheimer S.Y., Strojwas M.H., Cohen M.S., Saunders A.M., Pericak-Vance M.A., Mazziotta J.C., and Small G.W. (2000). Patterns of brain activation in people at risk for Alzheimer's disease. N. Engl. J. Med. 343, 450–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansen-Berg H., Dawes H., Guy C., Smith S.M., Wade D., and Matthews P.M. (2002). Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain 125, 2731–2742 [DOI] [PubMed] [Google Scholar]
- 21.McAllister T.W., Saykin A.J., Flashman L.A., Sparling M.B., Johnson S.C., Guerin S.J., Mamourian A.C., Weaver J.B., and Yanofsky N. (1999). Brain activation during working memory one month after mild traumatic brain injury: a functional MRI study. Neurology 53, 1300–1308 [DOI] [PubMed] [Google Scholar]
- 22.Jantzen K.J., Anderson B., Steinberg F.L., and Kelso J.A. (2004). A prospective functional MR imaging study of mild traumatic brain injury in college football players. AJNR Am. J. Neuroradiol. 25, 738–745 [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang K., Johnson B., Pennell D., Ray W., Sebastianelli W., and Slobounov S. (2010). Are functional deficits in concussed individuals consistent with white matter structural alterations: combined fMRI & DTI study. Exp. Brain Res. 204, 57–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J.K., Johnston K.M., Frey S., Petrides M., Worsley K., and Ptito A. (2004). Functional abnormalities in symptomatic concussed athletes: an fMRI study. Neuroimage 22, 68–82 [DOI] [PubMed] [Google Scholar]
- 25.Chen J.K., Johnston K.M., Petrides M., and Ptito A. (2008). Recovery from mild head injury in sports: evidence from serial functional magnetic resonance imaging studies in male athletes. Clin. J. Sport Med. 18, 241–247 [DOI] [PubMed] [Google Scholar]
- 26.Hockey A., and Geffen G. (2004). The concurrent validity and test-retest reliability of a visuospatial working memory task. Intelligence 32, 591–605 [Google Scholar]
- 27.McCrory P., Meeuwisse W., Johnston K., Dvorak J., Aubry M., Molloy M., and Cantu R. (2009). Consensus statement on concussion in sport: the 3rd International Conference on Concussion in Sport held in Zurich, November 2008. Br. J. Sports Med. 43, Suppl 1, 76–90 [DOI] [PubMed] [Google Scholar]
- 28.Lovell M.R., Collins M.W., Podell K., Powell J., and Maroon J. (2007). Immediate post concussion assessment and cognitive testing (version 2.0) NeuroHealth Systems, LLC: Pittsburgh, PA [Google Scholar]
- 29.Benedict R. H., Schretlen D., Groninger L., Dobraski M., and Shpritz B. (1996). Revision of the Brief Visuospatial Memory Test: Studies of normal performance, reliability, and validity. Psychological Assessment 8, 145–153 [Google Scholar]
- 30.Benedict R. H., Schretlen D., Groninger L., and Brandt J. (1998). Hopkins verbal learning test–revised: normative data and analysis of inter-form and test-retest reliability. The Clinical Neuropsychologist, 12, 43–55 [Google Scholar]
- 31.Smith A. (1982). The Symbol Digit Modalities Test Manual. Western Psychological Services: Los Angeles, CA [Google Scholar]
- 32.Williams M. (1991). Cognitive Behavior Rating Scale. Psychological Assessment Resources: Odessa, FL [Google Scholar]
- 33.Partington J.E., and Letter R.G. (1949). Partington's Pathway Test. The Psychological Service Center Bulletin, 1, 9–20 [Google Scholar]
- 34.Stroop J.R. (1935). Studies of interference in serial verbal reactions. J. Exp. Psychol. 18, 643–662 [Google Scholar]
- 35.Kroenke K., and Spitzer R.L. (2002). The PHQ-9: a new depression diagnostic and severity measure. Psych. Ann. 32, 1–7 [Google Scholar]
- 36.Spitzer R.L., Kroenke K., Williams J.B., and Löwe B. (2006). A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch. Intern. Med. 166, 1092–1097 [DOI] [PubMed] [Google Scholar]
- 37.Teasdale G., and Jennett B. (1974). Assessment of coma and impaired consciousness: a practical scale. Lancet 2, 81–84 [DOI] [PubMed] [Google Scholar]
- 38.Iverson G.L., Lovell M.R., and Collins M.W. (2003). Interpreting change on ImPACT following sport concussion. Clin. Neuropsychol. 17, 460–467 [DOI] [PubMed] [Google Scholar]
- 39.Aubry M., Cantu R., Dvorak J., Graf-Baumann T., Johnston K., Kelly J., Lovell M., McCrory P., Meeuswisse W., and Schamasch P. (2002). Summary and agreement statement of the First International Conference on Concussion in Sport, Vienna 2001. Recommendations for the improvement of safety and health of athletes who may suffer concussive injuries. Br. J. Sports Med. 36, 6–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brainard D.H. (1997). The psychophysics toolbox. Spat. Vis. 10, 433–436 [PubMed] [Google Scholar]
- 41.Pelli D.G. (1997). The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat. Vis. 10, 437–442 [PubMed] [Google Scholar]
- 42.Kleiner M., Brainard D., and Pelli D. (2007). What's new in psychtoolbox-3?. Perception 36 ECVP Abstract Supplement.
- 43.Smith S.M., Jenkinson M., Wollrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H., Bannister P.R., DeLuca M., Drobnjak I., Flitney D. E., et al. (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23, Suppl 1, S208–S219 [DOI] [PubMed] [Google Scholar]
- 44.Cox R.W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res, 29, 162–173 [DOI] [PubMed] [Google Scholar]
- 45.Otsu N. (1979). A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man. Cybern. 9, 62–66 [Google Scholar]
- 46.Zang Y.F., He Y., Zhu C.Z., Cao Q.J., Sui M.Q., Liang M., Tian L.X., Jiang T.Z., and Wang Y.F. (2007). Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 29, 83–91 [DOI] [PubMed] [Google Scholar]
- 47.Hoptman M.J., Zuo X.N., Butler P.D., Javitt D.C., D'Angelo D., Mauro C.J., and Milham M.P. (2010). Amplitude of low-frequency oscillations in schizophrenia: a resting state fMRI study. Schizophr. Res. 117, 13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kiviniemi V., Jauhiainen J., Tervonen O., Pääkkö E., Oikarinen J., Vainionpää V., Rantala H., and Biswai B. (2000). Slow vasomotor fluctuation in fMRI of anesthetized child brain. Magn. Reson. Med. 44, 373–378 [DOI] [PubMed] [Google Scholar]
- 49.Slobounov S.M., Zhang K., Pennell D., Ray W., Johnson B., Sebastianelli W. (2010). Functional abnormalities in normally appearing athletes following mild traumatic brain injury: a functional MRI study. Exp. Brain Res. 202, 341–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lovell M.R., Pardini J.E., Welling J., Collins M.W., Bakal J., Lazar N., Roush R., Eddy W.F., and Becker J.T. (2007). Functional brain abnormalities are related to clinical recovery and time to return-to-play in athletes. Neurosurgery 61, 352–360 [DOI] [PubMed] [Google Scholar]
- 51.Braver T.S., Cohen J.D., Nystrom L.E., Jonides J., Smith E.E., and Noll D.C. (1997). A parametric study of the prefrontal cortex involvement in human working memory. Neuroimage 5, 49–62 [DOI] [PubMed] [Google Scholar]
- 52.Jaeggi S.M., Seewer R., Nirkko A.C., Eckstein D., Schroth G., Groner R., and Gutbrod K. (2003). Does excessive memory load attenuate activation in the prefrontal cortex? Load-dependent processing in single and dual tasks: functional magnetic resonance imaging study. Neuroimage 19, 210–225 [DOI] [PubMed] [Google Scholar]
- 53.Audoin B., Ibarrola D., Ranjeva J.P., Confort-Gouny S., Malikova I., Ali-Cherif A., Pelletier J., and Cozzone P. (2003). Compensatory cortical activation observed by fMRI during cognitive task at the earliest stage of MS. Hum. Brain Mapp. 20, 51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mainero C., Pantano P., Caramia F., and Pozzilli C. (2006). Brain reorganization during attention and memory tasks in multiple sclerosis: insights from functional MRI studies. J. Neurol. Sci. 245, 93–98 [DOI] [PubMed] [Google Scholar]
- 55.Chiaravalloti N., Hillary F., Ricker J., Christodoulou C., Kalnin A., Liu W.C., Steffener J., and DeLuca J. (2005). Cerebral activation patterns during working memory performance in multiple sclerosis using fMRI. J. Clin. Exp. Neuropsychol. 27, 33–54 [DOI] [PubMed] [Google Scholar]
- 56.Scheibel R.S., Newsome M.R., Troyanskaya M., Steinberg J.L., Goldstein F.C., Mao H., and Levin H.S. (2009). Effects of severity of traumatic brain injury and brain reserve on cognitive-control related brain activation. J. Neurotrauma 26, 1447–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maruishi M., Miyatani M., Nakao T., and Muranaka H. (2007). Compensatory cortical activation during performance of an attention task by patients with diffuse axonal injury: a functional magnetic resonance imaging study. J. Neurol. Neurosurg. Psychiatry 78, 168–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McAllister T.W., Sparling M.B., Flashman L.A., Guerin S.J., Mamourian A.C., and Saykin A.J. (2001). Differential working memory load effects after mild traumatic brain injury. Neuroimage 14, 1004–1012 [DOI] [PubMed] [Google Scholar]
- 59.Levin H.S. (2003). Neuroplasticity following non-penetrating traumatic brain injury. Brain Inj. 17, 665–674 [DOI] [PubMed] [Google Scholar]
- 60.Pantano P., Mainero C., and Caramia F. (2006). Functional brain reorganization in multiple sclerosis: evidence from fMRI studies. J. Neuroimaging 16, 104–114 [DOI] [PubMed] [Google Scholar]
- 61.Sanchez-Carrion R., Fernandez-Espejo D., Junque C., Falcon C., Bargallo N., Roig T., Bernabeu M., Tormos J.M., and Vendrell P. (2008). A longitudinal fMRI study of working memory in severe TBI patients with diffuse axonal injury. Neuroimage 43, 421–429 [DOI] [PubMed] [Google Scholar]
- 62.Henry L.C., Tremblay J., Tremblay S., Lee A., Brun C., Lepore N., Theoret H., Ellemberg D., and Lassonde M. (2011). Acute and chronic changes in diffusivity measures after sports concussion. J. Neurotrauma 28, 2049–2059 [DOI] [PubMed] [Google Scholar]
- 63.Hillary F.G., Genova H.M., Medaglia J.D., Fitzpatrick N.M., Chiou K.S., Wardecker B.M., Franklin R.G., Jr., Wang J., and DeLuca J. (2010). The nature of processing speed deficits in traumatic brain injury: is less brain more? Brain Imaging Behav. 4, 141–154 [DOI] [PubMed] [Google Scholar]
- 64.Hillary F.G. (2008). Neuroimaging of working memory dysfunction and the dilemma with brain reorganization hypotheses. J. Int. Neuropsychol. Soc. 14, 526–534 [DOI] [PubMed] [Google Scholar]
- 65.Slobounov S., Gay M., Johnson B., and Zhang K. (2012). Concussion in athletics: ongoing clinical and brain imaging research controversies. Brain Imaging Behav. 6, 224–243 [DOI] [PubMed] [Google Scholar]
- 66.Owen A.M., McMillan K.M., Laird A.R., and Bullmore E. (2005). N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 25, 46–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Landau S.M., Schumacher E.H., Garavan H., Druzgal T.J., and D'Esposito M. (2004). A functional MRI study of the influence of practice on component processes of working memory. Neuroimage 22, 211–221 [DOI] [PubMed] [Google Scholar]
- 68.McKee AC., Stein T.D., Nowinski C.J., Stern R.A., Daneshvar D.H., Alvarez V.E., Lee H.S., Hall G., Wojtowicz S.M., Baugh C.M., Riley D.O., Kubilus C.A., Cormier K.A., Jacobs M.A., Martin B.R., Abraham C.R., Ikezu T., Reichard R.R., Wolozin B.L., Budson A.E., Goldstein L.E., Kowall N.W., and Cantu R.C. (2013). The spectrum of disease in chronic traumatic encephalopathy. Brain 136, 43–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stern R.A., Riley D.O., Daneshvar D.H., Nowinski C.J., Cantu R.C., and McKee A.C. (2011). Long-term consequences of repetitive brain trauma: chronic traumatic encephalopathy. PM R 3, Suppl 2, S460–S467 [DOI] [PubMed] [Google Scholar]