Abstract
Introduction:
The activities involving phlebotomy, a critical task for obtaining diagnostic blood samples, are poorly studied as regards the major sources of errors and the procedures related to laboratory quality control. The aim of this study was to verify the compliance with CLSI documents of clinical laboratories from South America and to assess whether teaching phlebotomists to follow the exact procedure for blood collection by venipuncture from CLSI/NCCLS H03-A6 - Procedures for the Collection of Diagnostic Blood Specimens by Venipuncture might improve the quality of the process.
Materials and methods:
A survey was sent by mail to 3674 laboratories from South America to verify the use of CLSI documents. Thirty skilled phlebotomists were trained with the CLSI H03-A6 document to perform venipuncture procedures for a period of 20 consecutive working days. The overall performances of the phlebotomists were further compared before and after the training program.
Results:
2622 from 2781 laboratories that did answer our survey used CLSI documents to standardize their procedures and process. The phlebotomists’ training for 20 days before our evaluation completely eliminated non-conformity procedures for: i) incorrect friction of the forearm, during the cleaning of the venipuncture site to ease vein location; ii) incorrect sequence of vacuum tubes collection; and iii) inadequate mixing of the blood in primary vacuum tubes containing anticoagulants or clot activators. Unfortunately the CLSI H03-A6 document does not caution against both unsuitable tourniquet application time (i.e., for more than one minute) and inappropriate request to clench the fist repeatedly. These inadequate procedures were observed for all phlebotomists.
Conclusion:
We showed that strict observance of the CLSI H03-A6 document can remarkably improve quality, although the various steps for collecting diagnostic blood specimens are not a gold standard, since they may still permit errors. Tourniquet application time and forearm clench should be verified by all quality laboratory managers in the services. Moreover, the procedure for collecting blood specimens should be revised to eliminate this source of laboratory variability and safeguard the quality.
Keywords: phlebotomy, blood specimen collection, tourniquet application time, CLSI documents, pre-analytic variability, extra- analytical variability
Introduction
The interest in quality improvement and patient safety has been the focus of several national and international initiatives, which have globally led to substantial improvements (1–6). The vast majority of errors in laboratory diagnostics are concentrated in the extra-analytical phase (2,7–13). The pre-analytical phase is described as the dark side of the moon in diagnostic process. Errors in pre-analytical phase generate further work or additional investigation that may cause unnecessary procedures for patients and cost to the health care systems (14,15). Preanalytical issues have downstream impact on the use of laboratory resources, hospital costs and overall quality of care. The clinical laboratory results are an essential part of the healthcare delivery. It has been estimated that 60 up to 70% of medical decisions and procedures, such as drug prescriptions, assessments prior to and in the course of further investigations or dialysis, are strongly dependent upon laboratory data (16). Nowadays many procedures are performed and/or oriented by non-laboratory professionals (e.g. nurses, non-technician personnel and administrative staff). A superficial knowledge of the importance of details such as a) adequate fasting time before blood collection (17); b) tourniquet application time (18–24) c) use of appropriate tubes (25–27) and additives (28); d) a series of factors or conditions closely associated with the specimen collection, such as inadequate fulfilling to the rigorous criteria of correct blood drawing, use of tubes containing different additive and/or anti coagulants, incomplete filling, inadequate mixing of the tubes or hemolysis (29–35) are able by themselves either singularly or collectively to strongly influence many laboratory results and thereby affect the diagnostic outcome, the follow-up or even the treatment of the patients. Since 1977, the Clinical Laboratory Standard Institute (CLSI) has recognized the need to put significant attention toward the pre-examination components of laboratory testing, including the correct collection and handling of blood specimens (36). In 2009 Simundic et al. (37) applied a cross-sectional multicentric survey study in some developing European countries and Mexico, aimed at assess the quality of the extra-analytical phase of laboratory activities. This survey showed that the phlebotomy is the most critical activity in the extra-analytical phase (37). The procedures involving phlebotomy, critical for obtaining diagnostic blood specimens, are poorly studied as regards the major sources of errors and the procedures related to quality control process (19). The aim of this study was to verify the compliance with CLSI documents of clinical laboratories from South America and to assess whether teaching phlebotomists to follow the exact procedure for blood collection by venipuncture from CLSI/NCCLS H03-A6 - Procedures for the Collection of Diagnostic Blood Specimens by Venipuncture (36) might improve the quality of the process.
Materials and methods
Data collected
A survey was sent in September 2011 by mail to 3674 laboratories from South America to verify the use of CLSI documents. The questions were:
-
1)
Do you use standardized operating procedures in all your laboratory activities?
-
2)If yes, what steps of your laboratory process are based on CLSI guidelines?
- ( ) pre-analytical
- ( ) analytical
- ( ) post-analytical
- ( ) my processes are not based in CLSI guidelines
-
3a)
If you marked “my processes are not based in CLSI guidelines”, then specify where your procedures are based.
-
3b)
If you have checked the above preanalytical option, do you currently employ the CLSI H03-A6 document (36) to standardize your procedures for blood collection by venipuncture?
-
4)
If yes, do your phlebotomists perform the blood collection by venipuncture following the exact venipuncture procedure from page 5 item 8 of CLSI H03-A6 document (36)?
-
5)
If not, what did you change in this procedure? And why did you change this procedure?
All the evaluated laboratories signed a formal consent to participate in this study, all laboratory identification was sealed and the project was approved by our Internal Review Board.
Phlebotomy training program
Thirty phlebotomists from São Paulo state, Brazil, previously evaluated (38) were invited to participated in this study. Each phlebotomist was trained individually to perform exactly the venipuncture procedure from CLSI H03-A6 document (36). The phlebotomy training program was realized during 8 hours where the importance of each step of the procedure was explained (Table 1). Only one external/expert auditor from DICQ (39) trained all phlebotomists in one month (from October to November 2011). DICQ is a National System of Accreditation from Brazilian Society of Clinical Analyses. This accreditation system is based on ISO 15189 (40). After the training, all phlebotomists were monitored for twenty working days, to guarantee the assimilation of the correct procedures for the collection of diagnostic blood specimens, in conformity with the CLSI H03-A6 document (Table 1). Only after this period of time the phlebotomists participating in the present study were revaluated. This period of time is considered sufficient by quality laboratory’s managers for incorporating new procedures. Obviously we chose to train and revaluate the same thirty phlebotomists previously assessed by Lima-Oliveira et al. (38), because we were aware of the delicateness of the workday routine of these professionals.
Table 1.
Procedures for the collection of diagnostic blood specimens by venipuncture from CLSI H03-A6 document (36) used during phlebotomy training program.
| Steps | Procedures | Importance of the procedures |
|---|---|---|
| i | prepare accession order | to guarantee patient identity assurance (11–13,41) |
| ii | approach and indentify the patient; sanitize hands | |
| iii | verify the patient’s fasting status or diet restrictions, as appropriate, and inquire | fasting status is a important source of variability (17,42,43) |
| if the patient has a latex sensitivity; select appropriate gloves and tourniquet | to prevent allergic reaction and/or anaphylactic shock attributed to latex allergy (44–46) | |
| iv | assemble necessary supplies and select appropriate tubes according to the requests | to prevent errors in laboratory medicine induced by supplies and addictives such anticoagulants and clot activators (26–28,47–49) |
| v | position the patient | to eliminate possible interferences of blood distribution due to different posture (50) |
| vi | apply the tourniquet and select the venipuncture site and vein | See discussion |
| vii | put on gloves | preventing phlebotomists’ exposure to potentially infectious blood pathogens (51,52) |
| viii | cleanse the venipuncture site and allow to dry | cleaning prevents infection by skin microorganisms, waiting for drying prevents hemolysis (32,35) |
| ix | perform venipuncture; once blood flow begins, request the patient to open his/her hand | See discussion |
| x | fill tubes using the correct order of draw | to prevent errors by cross contamination between addictives (53–56) |
| xi | release and remove the tourniquet | See discussion |
| xii | place the gauze pad over the puncture site | safe feature for preventing phlebotomists’ exposure to potentially infections by bloodbome pathogens (51,52). applying pressure to the site is a efficient prevention of bruise (57) |
| xiii | remove the needle, activate any safety feature, and dispose of the device | |
| xiv | apply pressure to the site, making sure bleeding has stopped, and then bandage the arm | |
| xv | label the tubes and record the time of collection; some facilities also specify phlebotomist | to reduce missing identification and guarantee the traceability of the process (11,12,40,41) |
| identification on the tubes | ||
| xvi | observe special handling requirements (if any required) | to guarantee diagnostic blood specimens stability (58–61) |
| xvii | send properly labeled blood collection tubes to the appropriate laboratories |
Evaluation of the phlebotomist performance
To assess the performance of phlebotomists during the collection of diagnostic blood specimens the check list (Table 2) previously used by Lima-Oliveira et al. was followed (38). This check list allowed the evaluation of whether procedure for blood collection by venipuncture from CLSI H03-A6 document (36) was able to improve the quality process, or if it introduced greater variability and consequently more errors in clinical laboratory testing.
Table 2.
Checklist to assess the performance of phlebotomists during collection of diagnostic blood specimens by venipuncture.
| Procedure | Verification | |
|---|---|---|
| Tourniquet application time | Patient I | ____seconds |
| Patient II | ____seconds | |
| Patient III | ____seconds | |
| Patient IV | ____seconds | |
| Patient V | ____seconds | |
|
| ||
| Did the phlebotomist inappropriately request to the patient to clench the fist repeatedly? | 1 Yes ( ) | 2 No ( ) |
|
| ||
| Did the phlebotomist make the friction procedure of the forearm, during the cleaning of the venipuncture site, to avoid venous stasis? | 1 Yes ( ) | 2 No ( ) |
|
| ||
| Did the phlebotomist use the correct sequence of vacuum tubes during blood collection? | 1 Yes ( ) | 2 No ( ) |
|
| ||
| What was the sequence of tubes used by the phlebotomist?* | ||
| Did the phlebotomist correctly homogenize the diagnostic blood specimens? | 1 Yes ( ) | 2 No ( ) |
This item is evaluated only if the answer to item 4 was “no”.
Enumerate the order of the sequence used
Statistical analysis
The Kolmogorov-Smirnov test was used to assess the normality of distribution of tourniquet application time. All parameters in our study were normally distributed. Data were expressed as mean ± standard deviation. Differences were tested by paired Student t-test. Fisher exact test two-tailed, was used to compare qualitative phlebotomy procedures differences between laboratories before and after phlebotomy training program. McNemar Chi-square test for dependent samples was used to compare before-after laboratories training. The values P < 0.05 were considered statistically significant. Statistical analyses were performed with Statistica for Windows, version 8.0 (StatSoft Inc., Tulsa, OK, USA).
Results
Survey
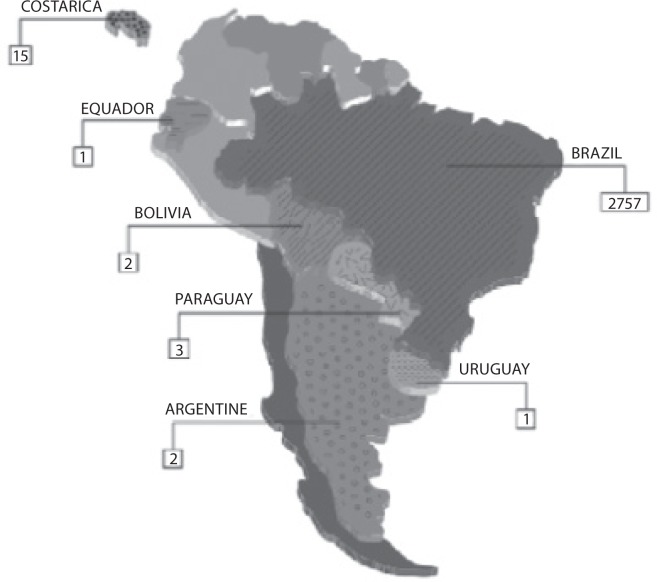
The answers from 2781 laboratories were received throughout the study period (i.e., 60 days), that is ∼76% of the total previously predicted (Figure 1A). After this period the collection of data was stopped. The results of the survey are shown in Figure 1B.
Figure 1.
Representativeness of CLSI documents in South American
Figure 1A: Geographic distribution of evaluated laboratories by survey.
All evaluated countries are showed textured. The absolute number represents the group of laboratories evaluated by countries.
Figure 1B:
Survey results.
Labs: laboratories.
Phlebotomy training program
The training of phlebotomists for 20 days before our evaluation completely eliminated a series of non-conformity, including i) incorrect friction on the forearm during the cleaning of the venipuncture site to produce venous stasis and ease vein location; ii) incorrect sequence of vacuum tubes collection (i.e., incorrect order of draw); and iii) inadequate mixing of blood in primary vacuum tubes containing anticoagulants or clot activators (Table 3). Regarding tourniquet time (Table 4) the overall mean ± SD was 118 ± 1 s. Private laboratories applied the tourniquet for significantly shorter times than public laboratories (87 ± 1 s vs. 148 ± 1 s; P < 0.001). All the phlebotomists inappropriately requested the patient to clench the fist repeatedly (i.e., more than twice).
Table 3.
Relevant error sources associated to phlebotomy procedure before and after phlebotomy training program.
| Error description | Laboratories before training # | Laboratories after training | ||||||
|---|---|---|---|---|---|---|---|---|
| All (N = 30) | Public (N = 15) | Private (N = 15) | P | All (N = 30) | Public (N = 15) | Private (N = 15) | P | |
| Inappropriate request to the patient to clench the fist repeatedly | 25/30 | 14/15 | 11/15 | 0.329 | 29/30* | 15/15 | 14/15 | 1.000 |
| Inadequate friction procedure during the cleaning of the venipuncture site | 27/30 | 13/15 | 14/15 | 1.000 | 0/30** | 0/15 | 0/15 | --- |
| Incorrect sequence of vacuum tubes | 26/30 | 13/15 | 12/15 | 1.000 | 0/30** | 0/15 | 0/15 | --- |
| Incorrect mixing of vacuum tubes | 25/30 | 15/15 | 10/15 | 0.042 | 0/30** | 0/15 | 0/15 | --- |
Public – public laboratories; private – private laboratories. Comparison public-private laboratories, P-value, Fisher exact test two-tailed.
P = 0.113 and
P<0.001: comparison of all laboratories before-after training, McNemar Chi-square test.
---, not calculated; # date previously published (38).
Table 4.
Effect of phlebotomy training program on tourniquet application time
| Tourniquete time | |||||
|---|---|---|---|---|---|
| Laboratories | Phlebotomists |
Before training (s) mean ± SD |
After training (s) mean ± SD |
Difference (s) | P |
| 1 Public | 1 | 93 ± 40 | 156 ± 3 | 63 | 0.018 |
| 2 | 73 ± 23 | 154 ± 1 | 81 | 0.027 | |
| 3 | 85 ± 18 | 154 ± 2 | 69 | 0.002 | |
|
| |||||
| 2 Public | 4 | 108 ± 12 | 144 ± 1 | 36 | <0.001 |
| 5 | 100 ± 22 | 140 ± 1 | 40 | <0.001 | |
| 6 | 111 ± 18 | 141 ± 1 | 30 | 0.017) | |
|
| |||||
| 3 Public | 7 | 120 ± 10 | 153 ± 2 | 33 | 0.001 |
| 8 | 110 ± 11 | 150 ± 1 | 40 | <0.001 | |
| 9 | 92 ± 23 | 149 ± 1 | 57 | <0.001 | |
|
| |||||
| 4 Public | 10 | 122 ± 10 | 145 ± 1 | 23 | 0.036 |
| 11 | 115 ± 8 | 144 ± 2 | 29 | 0.026 | |
| 12 | 112 ± 6 | 146 ± 1 | 34 | 0.001 | |
|
| |||||
| 5 Public | 13 | 80 ± 16 | 147 ± 1 | 67 | <0.001 |
| 14 | 78 ± 12 | 146 ± 2 | 68 | <0.001 | |
| 15 | 75 ± 20 | 147 ± 1 | 72 | <0.001 | |
|
| |||||
| 1 Private | 16 | 86 ± 7 | 97 ± 1 | 11 | 0.035 |
| 17 | 80 ± 13 | 92 ± 1 | 12 | 0.001 | |
| 18 | 72 ± 12 | 90 ± 1 | 18 | 0.001 | |
|
| |||||
| 2 Private | 19 | 68 ± 10 | 87 ± 2 | 19 | <0.001 |
| 20 | 66 ± 8 | 84 ± 1 | 18 | <0.001 | |
| 21 | 69 ± 11 | 85 ± 1 | 16 | <0.001 | |
|
| |||||
| 3 Private | 22 | 47 ± 6 | 83 ± 2 | 36 | <0.001 |
| 23 | 62 ± 6 | 81 ± 1 | 19 | 0.016 | |
| 24 | 75 ± 8 | 80 ± 1 | 6 | 0.037 | |
|
| |||||
| 4 Private | 25 | 51 ± 7 | 83 ± 2 | 32 | <0.001 |
| 26 | 67 ± 6 | 85 ± 3 | 18 | <0.001 | |
| 27 | 73 ± 6 | 87 ± 1 | 14 | <0.001 | |
|
| |||||
| 5 Private | 28 | 80 ± 16 | 95 ± 1 | 15 | 0.026 |
| 29 | 78 ± 12 | 90 ± 2 | 12 | 0.001 | |
| 30 | 75 ± 20 | 93 ± 1 | 18 | <0.001 | |
date before training were previously published (38).
Discussion
Our survey shows that CLSI documents are widely used in South America as 2622 from 2781 laboratories appear compliant with these documents to standardize their procedures. The CLSI mission is to develop best practices in clinical and laboratory testing, as well as promoting their use worldwide, using a consensus-driven process that balances the viewpoints of industry, government and healthcare professionals (62). Lima-Oliveira et al. previously showed that phlebotomists’ procedures from private- and public laboratories are not harmonized (38). The results of this study shows that the venipuncture procedure training following CLSI H03-A6 document (36) was ideal to harmonize the activities both within-laboratory and between-laboratories (Table 3). This CLSI document standardized important steps; a critical analyze of the importance from each step are show in table 1. As reported, seldom the expert phlebotomist concludes the collection of diagnostic blood specimens within sixty seconds of tourniquet application or even more (38). From a practical point of view, the tourniquet induced venous stasis promotes the exit of water, diffusible ions and low molecular weight substances from the vessel thereby increasing the concentration of various blood analytes at the punctured site thus potentially influencing the laboratory results interpretation (18). More so, when the vascular microenvironment is subjected to both hypoxia and concurrent stasis, accumulation of some bioproducts ensues, such as protons that have the potential to promote changes in laboratory parameters (63). It is noteworthy that the time of tourniquet application was increased significantly (P < 0.05) in all phlebotomist evaluated after training with CLSI H03-A6 document (36). Several concurrent causes might contribute to lengthen the tourniquet time even over 3 minutes, such as a difficult location of an appropriate venous access, the selection of the most suited blood collection system, the insertion of the needle into the vein, the collection of many tubes (18), but here we verified that the procedure from CLSI H03-A6 document(36) per se increased the tourniquet time application. In such case, the caring physicians unaware of the real patient situation might abstain from appropriate treatments as a consequence of venous stasis (18–21,23,24) caused by venipuncture procedure from CLSI H03-A6 document (36). Paradoxically, while the CLSI H03-A6 document (36) advises that the tourniquet application should not exceed one minute, on the other hand the standardization of the various activities according to the document itself entails a tourniquet time of more than one minute. Based on our results, we suggest to put on gloves (step vii), to cleanse the venipuncture site and to allow to dry (step viii) before applying the tourniquet and selecting the venipuncture site and vein (step vi). Moreover we recommend to release and remove the tourniquet (step xi) immediately when the first tube start to fill. These proposals will help to reduce the tourniquet application time and consequently to eliminate important source of errors e.g. venous stasis and hemolysis (18–21,23,24,32,34,35). We have also shown that private laboratories continue to display a significantly lower time of blood collection than public facilities after the training period (i.e., 87.6 ± 1.6 s vs. 147.1 ± 1.9 s; P < 0.001). A reliable explanation for this is that private labs have more ergonomic furnitures in blood collection rooms. Several studies and laboratory quality management documents showed that the clenching of the forearm before venipuncture modifies the concentration of several analytes in blood, especially potassium (this is probably due to hemolysis) (32,64–66). Unfortunately the laboratories staff that collect blood still request “pumping” to aid venipuncture. It has however been reported earlier that this unnecessary activity can be eliminated since a suitable vein access can be reliably identified by using a transilluminator device (18–20,67–69).
In conclusion, the wide distribution and implementation of the CLSI H03-A6 document can improve the laboratory quality process, although the steps for collecting diagnostic blood specimens by venipuncture can still not be considered a gold standard, since they might inherently promote errors. The tourniquet application time and forearm clenching should be verified by all quality laboratory managers in the services. Accordingly, the venipuncture procedure should be revised to eliminate this source of laboratory errors and safeguard the quality throughout the total testing process.
Acknowledgments
We are grateful to all quality laboratory managers that agreed with this re-evaluation and to their phlebotomists. Our special thanks for Mr. Stefano Ferrante for designing Fig. 1.
Footnotes
Potential conflict of interest
None declared.
References
- 1.McCay L, Lemer C, Wu AW. Laboratory safety and the WHO World Alliance for Patient Safety. Clin Chim Acta. 2009;404:6–11. doi: 10.1016/j.cca.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Lippi G, Fostini R, Guidi GC. Quality improvement in laboratory medicine: extra-analytical issues. Clin Lab Med. 2008;28:285–94. doi: 10.1016/j.cll.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Wagar EA, Yuan S. The laboratory and patient safety. Clin Lab Med. 2007;27:909–30. viii–ix. doi: 10.1016/j.cll.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Lippi G, Plebani M, Simundic AM. Quality in laboratory diagnostics: from theory to practice. Biochem Med. 2010;20:126–30. [Google Scholar]
- 5.Lippi G, Simundic AM, Mattiuzzi C. Overview on patient safety in healthcare and laboratory diagnostics. Biochem Med. 2010;20:131–43. [Google Scholar]
- 6.Muller MM. Quality and diagnostic perspectives in laboratory diagnostics. Biochem Med. 2010;20:144–6. [Google Scholar]
- 7.Lippi G. Governance of preanalytical variability: travelling the right path to the bright side of the moon? Clin Chim Acta. 2009;404:32–6. doi: 10.1016/j.cca.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 8.Lippi G, Guidi GC. Risk management in the preanalytical phase of laboratory testing. Clin Chem Lab Med. 2007;45:720–7. doi: 10.1515/CCLM.2007.167. [DOI] [PubMed] [Google Scholar]
- 9.Lippi G, Guidi GC, Mattiuzzi C, Plebani M. Preanalytical variability: the dark side of the moon in laboratory testing. Clin Chem Lab Med. 2006;44:358–65. doi: 10.1515/CCLM.2006.073. [DOI] [PubMed] [Google Scholar]
- 10.Bonini P, Plebani M, Ceriotti F, Rubboli F. Errors in laboratory medicine. Clin Chem. 2002;48:691–8. [PubMed] [Google Scholar]
- 11.Carraro P, Plebani M. Errors in a stat laboratory: types and frequencies 10 years later. Clin Chem. 2007;53:1338–42. doi: 10.1373/clinchem.2007.088344. [DOI] [PubMed] [Google Scholar]
- 12.Plebani M, Carraro P. Mistakes in a stat laboratory: types and frequency. Clin Chem. 1997;43:1348–51. [PubMed] [Google Scholar]
- 13.Lippi G, Simundic A-M. Total quality in laboratory diagnostics. It’s time to think outside the box. Biochem Med. 2010;20:5–8. [Google Scholar]
- 14.Stankovic AK, DeLauro E. Quality improvements in the pre-analytical phase: focus on urine specimen workflow. MLO Med Lab Obs. 2010;42:20, 22, 24–7. [PubMed] [Google Scholar]
- 15.Prusa R, Doupovcova J, Warunek D, Stankovic AK. Improving laboratory efficiencies through significant time reduction in the preanalytical phase. Clin Chem Lab Med. 2010;48:293–6. doi: 10.1515/CCLM.2010.036. [DOI] [PubMed] [Google Scholar]
- 16.Hallworth M, Hyde K, Cumming A, Peake I. The future for clinical scientists in laboratory medicine. Clin Lab Haematol. 2002;24:197–204. doi: 10.1046/j.1365-2257.2002.00455.x. [DOI] [PubMed] [Google Scholar]
- 17.Lippi G, Lima-Oliveira G, Salvagno GL, Montagnana M, Gelati M, Picheth G, et al. Influence of a light meal on routine haematological tests. Blood Transfus. 2010;8:94–9. doi: 10.2450/2009.0142-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Mangueira C, Sumita N, et al. New ways to deal with known preanalytical issues: use of transilluminator instead of tourniquet for easing vein access and eliminating stasis on clinical biochemistry. Biochem Med. 2011;21:152–9. doi: 10.11613/bm.2011.024. [DOI] [PubMed] [Google Scholar]
- 19.Lima-Oliveira G, Salvagno GL, Lippi G, Montagnana M, Scartezini M, Picheth G, et al. Elimination of the venous stasis error for routine coagulation testing by transillumination. Clin Chim Acta. 2011;412:1482–4. doi: 10.1016/j.cca.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Scartezini M, Guidi GC, et al. Transillumination: a new tool to eliminate the impact of venous stasis during the procedure for the collection of diagnostic blood specimens for routine haematological testing. Int J Lab Hematol. 2011;33:457–62. doi: 10.1111/j.1751-553X.2011.01305.x. [DOI] [PubMed] [Google Scholar]
- 21.Lippi G, Salvagno GL, Montagnana M, Guidi GC. Short-term venous stasis influences routine coagulation testing. Blood Coagul Fibrinolysis. 2005;16:453–8. doi: 10.1097/01.mbc.0000178828.59866.03. [DOI] [PubMed] [Google Scholar]
- 22.Gren B. Incorrect guidelines for venipuncture affect the analytical results. Scand J Clin Lab Invest. 2009;69:815–6. doi: 10.3109/00365510903307970. [DOI] [PubMed] [Google Scholar]
- 23.Lippi G, Salvagno GL, Montagnana M, Brocco G, Guidi GC. Influence of short-term venous stasis on clinical chemistry testing. Clin Chem Lab Med. 2005;43:869–75. doi: 10.1515/CCLM.2005.146. [DOI] [PubMed] [Google Scholar]
- 24.Lippi G, Salvagno GL, Montagnana M, Franchini M, Guidi GC. Venous stasis and routine hematologic testing. Clin Lab Haematol. 2006;28:332–7. doi: 10.1111/j.1365-2257.2006.00818.x. [DOI] [PubMed] [Google Scholar]
- 25.Loh TP, Saw S, Chai V, Sethi SK. Impact of phlebotomy decision support application on sample collection errors and laboratory efficiency. Clin Chim Acta. 2011;412:393–5. doi: 10.1016/j.cca.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 26.Gosselin RC, Janatpour K, Larkin EC, Lee YP, Owings JT. Comparison of samples obtained from 3.2% sodium citrate glass and two 3.2% sodium citrate plastic blood collection tubes used in coagulation testing. Am J Clin Pathol. 2004;122:843–8. doi: 10.1309/7V2K-P1HP-9Q29-BMN2. [DOI] [PubMed] [Google Scholar]
- 27.Kratz A, Stanganelli N, Van Cott EM. A comparison of glass and plastic blood collection tubes for routine and specialized coagulation assays: a comprehensive study. Arch Pathol Lab Med. 2006;130:39–44. doi: 10.5858/2006-130-39-ACOGAP. [DOI] [PubMed] [Google Scholar]
- 28.Lippi G, Salvagno GL, Montagnana M, Guidi GC. Influence of two different buffered sodium citrate concentrations on coagulation testing. Blood Coagul Fibrinolysis. 2005;16:381–3. doi: 10.1097/01.mbc.0000172097.15458.c5. [DOI] [PubMed] [Google Scholar]
- 29.Salvagno GL, Lippi G, Bassi A, Poli G, Guidi GC. Prevalence and type of pre-analytical problems for inpatients samples in coagulation laboratory. J Eval Clin Pract. 2008;14:351–3. doi: 10.1111/j.1365-2753.2007.00875.x. [DOI] [PubMed] [Google Scholar]
- 30.van Geest-Daalderop JH, Mulder AB, Boonman-de Winter LJ, Hoekstra MM, van den Besselaar AM. Preanalytical variables and off-site blood collection: influences on the results of the prothrombin time/international normalized ratio test and implications for monitoring of oral anticoagulant therapy. Clin Chem. 2005;51:561–8. doi: 10.1373/clinchem.2004.043174. [DOI] [PubMed] [Google Scholar]
- 31.Polack B, Schved JF, Boneu B. Preanalytical recommendations of the ‘Groupe d’Etude sur l’Hemostase et la Thrombose’ (GEHT) for venous blood testing in hemostasis laboratories. Haemostasis. 2001;31:61–8. doi: 10.1159/000048046. [DOI] [PubMed] [Google Scholar]
- 32.Simundic AM, Topic E, Nikolac N, Lippi G. Hemolysis detection and management of hemolyzed specimens. Biochem Med. 2010;20:154–9. [Google Scholar]
- 33.Salvagno GL, Lippi G, Gelati M, Guidi GC. Hemolysis, lipaemia and icterus in specimens for arterial blood gas analysis. Clin Biochem. 2012;45:372–3. doi: 10.1016/j.clinbiochem.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Lippi G, Salvagno GL, Montagnana M, Brocco G, Guidi GC. Influence of hemolysis on routine clinical chemistry testing. Clin Chem Lab Med. 2006;44:311–6. doi: 10.1515/CCLM.2006.054. [DOI] [PubMed] [Google Scholar]
- 35.Koseoglu M, Hur A, Atay A, Cuhadar S. Effects of hemolysis interferences on routine biochemistry parameters. Biochem Med (Zagreb) 2011;21:79–85. doi: 10.11613/bm.2011.015. [DOI] [PubMed] [Google Scholar]
- 36.Clinical Laboratory Standards Institute . Procedures for the collection of diagnostic blood specimens by venipuncture. CLSI H3-A6 document. 6th ed. Wayne, PA: Clinical Laboratory Standards Institute; 2007. [Google Scholar]
- 37.Simundic AM, Bilic-Zulle L, Nikolac N, Supak-Smolcic V, Honovic L, Avram S, et al. The quality of the extra-analytical phase of laboratory practice in some developing European countries and Mexico - a multicentric study. Clin Chem Lab Med. 2011;49:215–28. doi: 10.1515/CCLM.2011.034. [DOI] [PubMed] [Google Scholar]
- 38.Lima-Oliveira G, Guidi GC, Salvagno GL, Montagnana M, Rego FGM, Lippi G, et al. Is phlebotomy part of the dark side in the clinical laboratory struggle for quality? Laboratory Medicine. 2012;43:17–21. [Google Scholar]
- 39.National System of Accreditation - Brazilian Society of Clinical Analyses . Manual for accreditation of quality management system for clinical laboratories. 5th ed. Brazilian Society of Clinical Analyses; 2011. http://www.dicq.org.br/pdfs/manual_dicq_2011.pdf. [Google Scholar]
- 40.International Organization for Standardization . Medical laboratories — Particular requirements for quality and competence ISO document 15189. 2nd ed. Geneva, Switzerland: International Organization for Standardization; 2007. [Google Scholar]
- 41.Plebani M. Laboratory errors: how to improve pre- and post-analytical phases? Biochem Med (Zagreb) 2007;17:5–9. [Google Scholar]
- 42.ADA Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2010;33:S62–S9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohn JS, McNamara JR, Cohn SD, Ordovas JM, Schaefer EJ. Postprandial plasma lipoprotein changes in human subjects of different ages. J Lipid Res. 1988;29:469–79. [PubMed] [Google Scholar]
- 44.Peixinho CM, Tavares-Ratado P, Gabriel MF, Romeira AM, Lozoya-Ibanez C, Taborda-Barata L, et al. Different in vivo reactivity profile in health care workers and patients with spina bifida to internal and external latex glove surface-derived allergen extracts. Br J Dermatol. 2012;166:518–24. doi: 10.1111/j.1365-2133.2011.10656.x. [DOI] [PubMed] [Google Scholar]
- 45.Meade BJ, Weissman DN, Beezhold DH. Latex allergy: past and present. Int Immunopharmacol. 2002;2:225–38. doi: 10.1016/s1567-5769(01)00175-8. [DOI] [PubMed] [Google Scholar]
- 46.Lee W, Lee JH, Park do J, Kim HH. A case of anaphylactic shock attributed to latex allergy during gastric cancer surgery. J Korean Surg Soc. 2011;81(Suppl 1):S30–3. doi: 10.4174/jkss.2011.81.Suppl1.S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lippi G, Salvagno GL, Montagnana M, Poli G, Guidi GC. Influence of the needle bore size on platelet count and routine coagulation testing. Blood Coagul Fibrinolysis. 2006;17:557–61. doi: 10.1097/01.mbc.0000245300.10387.ca. [DOI] [PubMed] [Google Scholar]
- 48.Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Picheth G, Guidi GC. Different manufacturers of syringes: A new source of variability in blood gas, acid-base balance and related laboratory test? Clin Biochem. 2012;45:683–7. doi: 10.1016/j.clinbiochem.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 49.Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Picheth G, Guidi GC. Pre analytical management: serum vacuum tubes validation for routine clinical chemistry. Biochem Med (Zagreb) 2012;22:180–6. doi: 10.11613/bm.2012.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guder WG, Narayanan S, Wisser H, Zawta B, editors. Diagnostic samples: from the patient to the laboratory: the impact of preanalytical variables on the quality of laboratory results. 4 ed. Weinheim GmbH: Wiley-Blackwell; 2009. [Google Scholar]
- 51.Ionescu G, Negut M, Combiescu AA. Biosafety and biosecurity in the medical laboratory. Update and trends. Bacteriol Virusol Parazitol Epidemiol. 2007;52:91–9. [PubMed] [Google Scholar]
- 52.Ishak R, Linhares AC, Ishak MO. Biosafety in the laboratory. Rev Inst Med Trop Sao Paulo. 1989;31:126–31. doi: 10.1590/s0036-46651989000200011. [DOI] [PubMed] [Google Scholar]
- 53.Rushing J. Drawing blood with vacuum tubes. Nursing. 2004;34:26. doi: 10.1097/00152193-200401000-00024. [DOI] [PubMed] [Google Scholar]
- 54.Majid A, Heaney DC, Padmanabhan N, Spooner R. The order of draw of blood specimens into additive containing tubes not affect potassium and calcium measurements. J Clin Pathol. 1996;49:1019–20. doi: 10.1136/jcp.49.12.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lippi G, Salvagno GL, Brocco G, Guidi GC. Preanalytical variability in laboratory testing: influence of the blood drawing technique. Clin Chem Lab Med. 2005;43:319–25. doi: 10.1515/CCLM.2005.055. [DOI] [PubMed] [Google Scholar]
- 56.Calam RR, Cooper MH. Recommended “order of draw” for collecting blood specimens into additive-containing tubes. Clin Chem. 1982;28:1399. [PubMed] [Google Scholar]
- 57.Godwin PG, Cuthbert AC, Choyce A. Reducing bruising after venepuncture. Qual Health Care. 1992;1:245–6. doi: 10.1136/qshc.1.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clinical Laboratory Standards Institute . Collection, Transport, and Processing of Blood Specimens for Testing Plasma-Based Coagulation Assays and Molecular Hemostasis Assays. CLSI H21-A5 document. 5th ed. Wayne, PA: Clinical Laboratory Standards Institute; 2008. [Google Scholar]
- 59.Salvagno GL, Lippi G, Montagnana M, Franchini M, Poli G, Guidi GC. Influence of temperature and time before centrifugation of specimens for routine coagulation testing. Int J Lab Hematol. 2009;31:462–7. doi: 10.1111/j.1751-553X.2008.01058.x. [DOI] [PubMed] [Google Scholar]
- 60.Lippi G, Lima-Oliveira G, Nazer SC, Moreira ML, Souza RF, Salvagno GL, et al. Suitability of a transport box for blood sample shipment over a long period. Clin Biochem. 2011;44:1028–9. doi: 10.1016/j.clinbiochem.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 61.Clinical Laboratory Standards Institute . Procedures for the handling and processing of blood specimens for common laboratory tests. CLSI H18-A4 document. 4th ed. Wayne, PA: Clinical Laboratory Standards Institute; 2010. [Google Scholar]
- 62.Clinical Laboratory Standards Institute Mission of Clinical and Laboratory Standards Institute. 2012 Available at: http://www.clsi.org/Content/NavigationMenu/AboutCLSI/VisionMissionandValues/Vision_Mission_Value.htm. Accessed February 16, 2012.
- 63.Dennis SC, Gevers W, Opie LH. Protons in ischemia: where do they come from; where do they go to? J Mol Cell Cardiol. 1991;23:1077–86. doi: 10.1016/0022-2828(91)91642-5. [DOI] [PubMed] [Google Scholar]
- 64.Kaplan LA, Pesce AJ. Clinical Chemistry. Theory, analysis, correlation. 3rd ed. St. Louis: Mosby; 1996. [Google Scholar]
- 65.Baer DM, Ernst DJ, Willeford SI, Gambino R. Investigating elevated potassium values. MLO Med Lab Obs. 2006;38:24, 26, 30–1. [PubMed] [Google Scholar]
- 66.Young DS. Effects of preanalytical variables on clinical laboratory tests. 3ed. Washington: AACC Press; 2007. [Google Scholar]
- 67.Katsogridakis YL, Seshadri R, Sullivan C, Waltzman ML. Veinlite transillumination in the pediatric emergency department: a therapeutic interventional trial. Pediatr Emerg Care. 2008;24:83–8. doi: 10.1097/PEC.0b013e318163db5f. [DOI] [PubMed] [Google Scholar]
- 68.Kuhns LR, Martin AJ, Gildersleeve S, Poznanski AK. Intense transillumination for infant venipuncture. Radiology. 1975;116:734–5. doi: 10.1148/116.3.734. [DOI] [PubMed] [Google Scholar]
- 69.Weiss RA, Goldman MP. Transillumination mapping prior to ambulatory phlebectomy. Dermatol Surg. 1998;24:447–50. doi: 10.1111/j.1524-4725.1998.tb04186.x. [DOI] [PubMed] [Google Scholar]