Abstract
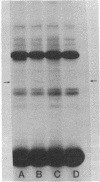
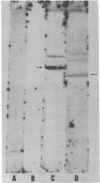
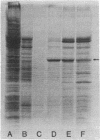
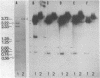
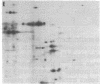
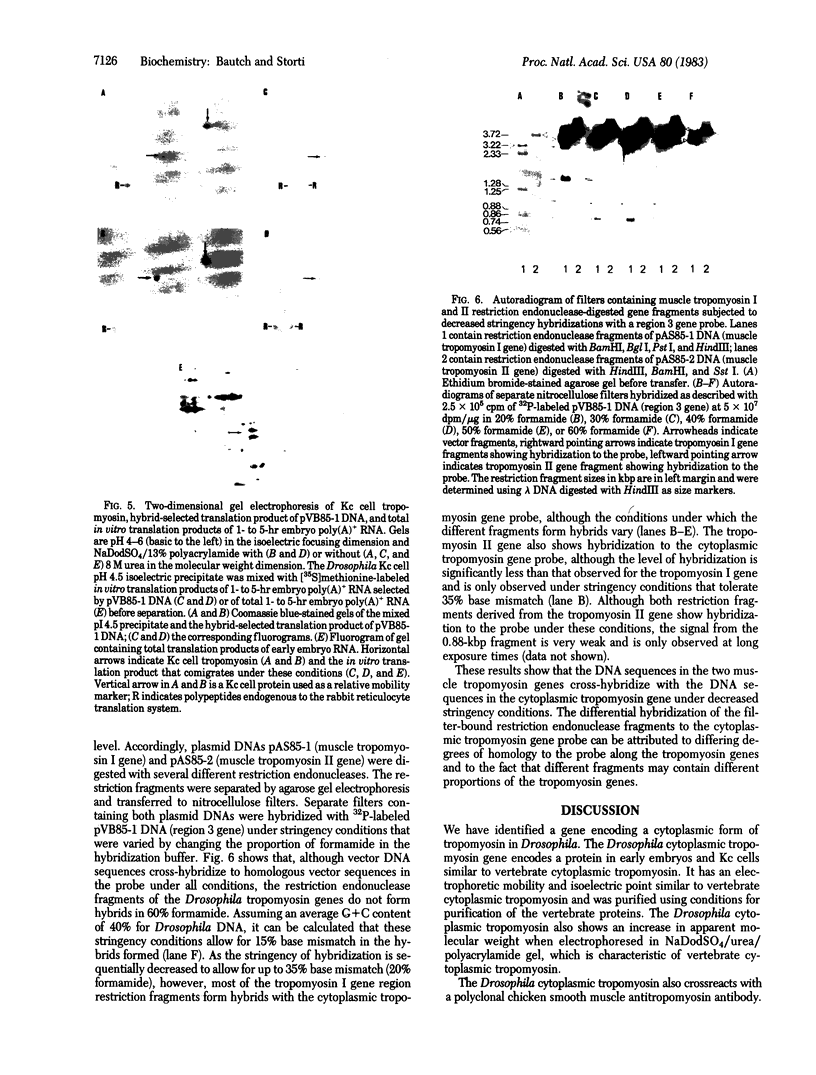
A Drosophila cytoplasmic tropomyosin gene has been identified and is located on the same genomic DNA clone as two Drosophila muscle tropomyosin genes previously identified. A positive hybrid-selection translation assay using the subcloned gene and RNA from non-muscle cell sources yielded a protein with a size (Mr, 31,000) and isoelectric point (5.0) similar to vertebrate cytoplasmic tropomyosin. A modified protocol for the purification of vertebrate cytoplasmic tropomyosin was used to partially purify cytoplasmic tropomyosin from the Drosophila Kc cell line. The Kc cell protein was identified as a cytoplasmic form of tropomyosin on the basis of its size, isoelectric point, and crossreactivity with a polyclonal vertebrate antitropomyosin antibody in a two-step binding assay. The Kc cell cytoplasmic tropomyosin comigrates in two dimensions with the hybrid-selected in vitro translation product of the region 3 gene, and both proteins show a mobility shift in NaDodSO4/urea/polyacrylamide gels that is characteristic of vertebrate tropomyosins. The cytoplasmic tropomyosin gene hybridizes to both Drosophila muscle tropomyosin genes under decreased stringency conditions. This cross-hybridization spans several internal restriction endonuclease sites in each muscle tropomyosin gene and indicates an overall partial homology among the three Drosophila tropomyosin genes. These results show that Drosophila tropomyosins are encoded by a closely linked family of differentially regulated genes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett T., Pachl C., Gergen J. P., Wensink P. C. The isolation and characterization of Drosophila yolk protein genes. Cell. 1980 Oct;21(3):729–738. doi: 10.1016/0092-8674(80)90436-5. [DOI] [PubMed] [Google Scholar]
- Bautch V. L., Storti R. V., Mischke D., Pardue M. L. Organization and expression of Drosophila tropomyosin genes. J Mol Biol. 1982 Dec 5;162(2):231–250. doi: 10.1016/0022-2836(82)90524-1. [DOI] [PubMed] [Google Scholar]
- Bullard B., Dabrowska R., Winkelman L. The contractile and regulatory proteins of insect flight muscle. Biochem J. 1973 Oct;135(2):277–286. doi: 10.1042/bj1350277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Cohen C. A tropomyosin-like protein from human platelets. J Mol Biol. 1972 Jul 21;68(2):383–387. doi: 10.1016/0022-2836(72)90220-3. [DOI] [PubMed] [Google Scholar]
- Corces V., Holmgren R., Freund R., Morimoto R., Meselson M. Four heat shock proteins of Drosophila melanogaster coded within a 12-kilobase region in chromosome subdivision 67B. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5390–5393. doi: 10.1073/pnas.77.9.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté G., Lewis W. G., Smillie L. B. Non-polymerizability of platelet tropomyosin and its NH2- and COOH-terminal sequences. FEBS Lett. 1978 Jul 15;91(2):237–241. doi: 10.1016/0014-5793(78)81181-8. [DOI] [PubMed] [Google Scholar]
- Falkner F. G., Saumweber H., Biessmann H. Two Drosophila melanogaster proteins related to intermediate filament proteins of vertebrate cells. J Cell Biol. 1981 Oct;91(1):175–183. doi: 10.1083/jcb.91.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine R. E., Blitz A. L. A chemical comparison of tropomyosins from muscle and non-muscle tissues. J Mol Biol. 1975 Jul 5;95(3):447–454. doi: 10.1016/0022-2836(75)90202-8. [DOI] [PubMed] [Google Scholar]
- Fyrberg E. A., Kindle K. L., Davidson N., Kindle K. L. The actin genes of Drosophila: a dispersed multigene family. Cell. 1980 Feb;19(2):365–378. doi: 10.1016/0092-8674(80)90511-5. [DOI] [PubMed] [Google Scholar]
- Geisler N., Weber K. Comparison of the proteins of two immunologically distinct intermediate-sized filaments by amino acid sequence analysis: desmin and vimentin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4120–4123. doi: 10.1073/pnas.78.7.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks M., Weintraub H. Tropomyosin is decreased in transformed cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5633–5637. doi: 10.1073/pnas.78.9.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howley P. M., Israel M. A., Law M. F., Martin M. A. A rapid method for detecting and mapping homology between heterologous DNAs. Evaluation of polyomavirus genomes. J Biol Chem. 1979 Jun 10;254(11):4876–4883. [PubMed] [Google Scholar]
- Lewis W. G., Cote G. P., Mak A. S., Smillie L. B. Amino acid sequence of equine platelet tropomyosin. Correlation with interaction properties. FEBS Lett. 1983 Jun 13;156(2):269–273. doi: 10.1016/0014-5793(83)80511-0. [DOI] [PubMed] [Google Scholar]
- Mak A. S., Smillie L. B., Stewart G. R. A comparison of the amino acid sequences of rabbit skeletal muscle alpha- and beta-tropomyosins. J Biol Chem. 1980 Apr 25;255(8):3647–3655. [PubMed] [Google Scholar]
- Matsumura F., Yamashiro-Matsumura S., Lin J. J. Isolation and characterization of tropomyosin-containing microfilaments from cultured cells. J Biol Chem. 1983 May 25;258(10):6636–6644. [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. F., McClelland A., White B. N., Addison C. F., Glover D. M. The molecular cloning of a dispersed set of developmentally regulated genes which encode the major larval serum protein of D. melanogaster. Cell. 1981 Feb;23(2):441–449. doi: 10.1016/0092-8674(81)90139-2. [DOI] [PubMed] [Google Scholar]
- Snyder M., Hunkapiller M., Yuen D., Silvert D., Fristrom J., Davidson N. Cuticle protein genes of Drosophila: structure, organization and evolution of four clustered genes. Cell. 1982 Jul;29(3):1027–1040. doi: 10.1016/0092-8674(82)90466-4. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Digan M. E., Mahowald A. P., Scott M., Craig E. A. Two clusters of genes for major chorion proteins of Drosophila melanogaster. Cell. 1980 Apr;19(4):905–914. doi: 10.1016/0092-8674(80)90082-3. [DOI] [PubMed] [Google Scholar]
- Sánchez F., Natzle J. E., Cleveland D. W., Kirschner M. W., McCarthy B. J. A dispersed multigene family encoding tubulin in Drosophila melanogaster. Cell. 1980 Dec;22(3):845–854. doi: 10.1016/0092-8674(80)90561-9. [DOI] [PubMed] [Google Scholar]
- Talbot K., MacLeod A. R. Novel form of non-muscle tropomyosin in human fibroblasts. J Mol Biol. 1983 Feb 15;164(1):159–174. doi: 10.1016/0022-2836(83)90091-8. [DOI] [PubMed] [Google Scholar]
- Tobin S. L., Zulauf E., Sánchez F., Craig E. A., McCarthy B. J. Multiple actin-related sequences in the Drosophila melanogaster genome. Cell. 1980 Jan;19(1):121–131. doi: 10.1016/0092-8674(80)90393-1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- der Terrossian E., Fuller S. D., Stewart M., Weeds A. G. Porcine platelet tropomyosin. Purification, characterization and paracrystal formation. J Mol Biol. 1981 Nov 25;153(1):147–167. doi: 10.1016/0022-2836(81)90531-3. [DOI] [PubMed] [Google Scholar]