Abstract
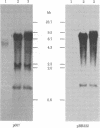
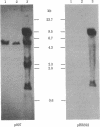
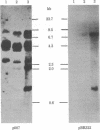
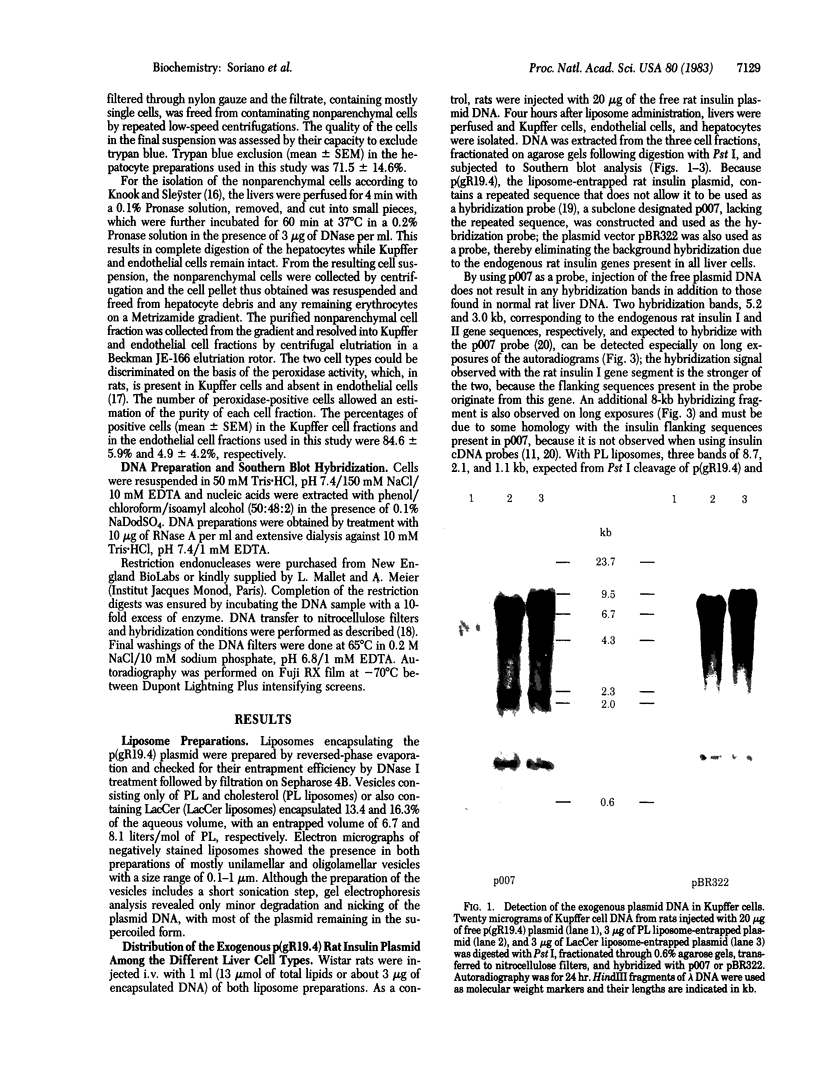
A plasmid containing the rat preproinsulin I gene was entrapped in large liposomes and intravenously administered to rats. Four hours after inoculation, the livers were processed for the isolation of hepatocytes. Kupffer cells, and endothelial cells, DNA was purified, and the exogenous DNA was detected in the different cell DNA preparations by Southern blotting. By using liposomes consisting of phospholipids and cholesterol, Kupffer cells were shown to be, on a per cell basis, the primary target for gene incorporation. In an attempt to target the liposomes to other liver cells, a glycolipid, lactosylceramide, was included in the lipid bilayer of the liposomes; this resulted in a substantial increase in the proportion of the exogenous gene in the hepatocytes and mainly in the endothelial cells, with a simultaneous decrease of this proportion in the Kupffer cells. Thus, it is shown that inclusion of a specific glycolipid within the bilayer of the liposomes may direct the DNA-containing vesicles to specific cell types in the liver.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Pictet R., Rutter W. J. Analysis of the regions flanking the human insulin gene and sequence of an Alu family member. Nucleic Acids Res. 1980 Sep 25;8(18):4091–4109. doi: 10.1093/nar/8.18.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke J. S., Simon G. T. Electron microscopy of the spleen. I. Anatomy and microcirculation. Am J Pathol. 1970 Jan;58(1):127–155. [PMC free article] [PubMed] [Google Scholar]
- Cordell B., Bell G., Tischer E., DeNoto F. M., Ullrich A., Pictet R., Rutter W. J., Goodman H. M. Isolation and characterization of a cloned rat insulin gene. Cell. 1979 Oct;18(2):533–543. doi: 10.1016/0092-8674(79)90070-9. [DOI] [PubMed] [Google Scholar]
- Fraley R., Straubinger R. M., Rule G., Springer E. L., Papahadjopoulos D. Liposome-mediated delivery of deoxyribonucleic acid to cells: enhanced efficiency of delivery related to lipid composition and incubation conditions. Biochemistry. 1981 Nov 24;20(24):6978–6987. doi: 10.1021/bi00527a031. [DOI] [PubMed] [Google Scholar]
- Ghosh P., Das P. K., Bachhawat B. K. Targeting of liposomes towards different cell types of rat liver through the involvement of liposomal surface glycosides. Arch Biochem Biophys. 1982 Jan;213(1):266–270. doi: 10.1016/0003-9861(82)90461-1. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G., Senior J. The phospholipid component of small unilamellar liposomes controls the rate of clearance of entrapped solutes from the circulation. FEBS Lett. 1980 Sep 22;119(1):43–46. doi: 10.1016/0014-5793(80)80994-x. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Wilson G., Ashwell G., Stukenbrok H. An electron microscope autoradiographic study of the carbohydrate recognition systems in rat liver. I. Distribution of 125I-ligands among the liver cell types. J Cell Biol. 1979 Oct;83(1):47–64. doi: 10.1083/jcb.83.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knook D. L., Sleyster E. C. Separation of Kupffer and endothelial cells of the rat liver by centrifugal elutriation. Exp Cell Res. 1976 May;99(2):444–449. doi: 10.1016/0014-4827(76)90605-4. [DOI] [PubMed] [Google Scholar]
- Kolb-Bachofen V., Schlepper-Schäfer J., Vogell W., Kolb H. Electron microscopic evidence for an asialoglycoprotein receptor on Kupffer cells: localization of lectin-mediated endocytosis. Cell. 1982 Jul;29(3):859–866. doi: 10.1016/0092-8674(82)90447-0. [DOI] [PubMed] [Google Scholar]
- Kooistra T., Duursma A. M., Bouma J. M., Gruber M. Endocytosis and breakdown of ribonuclease oligomers by sinusoidal rat liver cells in vivo. I. Effect of size. Biochim Biophys Acta. 1979 Oct 4;587(2):282–298. doi: 10.1016/0304-4165(79)90361-1. [DOI] [PubMed] [Google Scholar]
- Lomedico P., Rosenthal N., Efstratidadis A., Gilbert W., Kolodner R., Tizard R. The structure and evolution of the two nonallelic rat preproinsulin genes. Cell. 1979 Oct;18(2):545–558. doi: 10.1016/0092-8674(79)90071-0. [DOI] [PubMed] [Google Scholar]
- Nicolau C., Le Pape A., Soriano P., Fargette F., Juhel M. F. In vivo expression of rat insulin after intravenous administration of the liposome-entrapped gene for rat insulin I. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1068–1072. doi: 10.1073/pnas.80.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano R. E., Takeichi M. Adhesion of phospholipid vesicles to Chinese hamster fibroblasts. Role of cell surface proteins. J Cell Biol. 1977 Aug;74(2):531–546. doi: 10.1083/jcb.74.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roerdink F., Dijkstra J., Hartman G., Bolscher B., Scherphof G. The involvement of parenchymal, Kupffer and endothelial liver cells in the hepatic uptake of intravenously injected liposomes. Effects of lanthanum and gadolinium salts. Biochim Biophys Acta. 1981 Sep 18;677(1):79–89. doi: 10.1016/0304-4165(81)90148-3. [DOI] [PubMed] [Google Scholar]
- SINGLETON W. S., GRAY M. S., BROWN M. L., WHITE J. L. CHROMATOGRAPHICALLY HOMOGENEOUS LECITHIN FROM EGG PHOSPHOLIPIDS. J Am Oil Chem Soc. 1965 Jan;42:53–56. doi: 10.1007/BF02558256. [DOI] [PubMed] [Google Scholar]
- Schaefer-Ridder M., Wang Y., Hofschneider P. H. Liposomes as gene carriers: efficient transformation of mouse L cells by thymidine kinase gene. Science. 1982 Jan 8;215(4529):166–168. doi: 10.1126/science.7053567. [DOI] [PubMed] [Google Scholar]
- Soriano P., Szabo P., Bernardi G. The scattered distribution of actin genes in the mouse and human genomes. EMBO J. 1982;1(5):579–583. doi: 10.1002/j.1460-2075.1982.tb01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanjer H. H., Scherphof G. L. Targeting of lactosylceramide-containing liposomes to hepatocytes in vivo. Biochim Biophys Acta. 1983 Sep 21;734(1):40–47. doi: 10.1016/0005-2736(83)90072-x. [DOI] [PubMed] [Google Scholar]
- Szoka F., Jr, Papahadjopoulos D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4194–4198. doi: 10.1073/pnas.75.9.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall D. A., Wilson G., Hubbard A. L. The galactose-specific recognition system of mammalian liver: the route of ligand internalization in rat hepatocytes. Cell. 1980 Aug;21(1):79–93. doi: 10.1016/0092-8674(80)90116-6. [DOI] [PubMed] [Google Scholar]
- Wong T. K., Nicolau C., Hofschneider P. H. Appearance of beta-lactamase activity in animal cells upon liposome-mediated gene transfer. Gene. 1980 Jul;10(2):87–94. doi: 10.1016/0378-1119(80)90126-2. [DOI] [PubMed] [Google Scholar]