Abstract
Phlebotomy is one of the most complex medical procedures in the diagnosis, management and treatment of patients in healthcare. Since laboratory test results are the basis for a large proportion (60–80%) of medical decisions, any error in the phlebotomy process could have serious consequences. In order to minimize the possibility of errors, phlebotomy procedures should be standardised, well-documented and written instructions should be available at every workstation. Croatia is one of the few European countries that have national guidelines for phlebotomy, besides the universally used CLSI (Clinical Laboratory Standards Institute) H3-A6 Procedures for the Collection of Diagnostic Blood Specimens by Venipuncture; approved Standard-Sixth Edition (CLSI, 2007) and WHO (World Health Organization) guidelines on drawing blood: best practices in phlebotomy (WHO, 2010). However, the growing body of evidence in importance of preanalytical phase management resulted in a need for evidence based revision and expansion of existing recommendations.
The Croatian Society for Medical Biochemistry and Laboratory Medicine, Working Group for the Preanalytical Phase issued this recommendation. This document is based on the CLSI guideline H3-A6, with significant differences and additional information.
Keywords: phlebotomy; venous blood; recommendations, health planning; laboratory errors; preanalytical phase
Introduction
Today, the analytical phase of laboratory work is perfected through automation. Errors in laboratory medicine occur most frequently in the extra-analytical phase of laboratory testing (1). The preanalytical phase and especially pre-preanalytical phase are major sources of laboratory errors (2, 3). Out of all processes that happen outside the laboratory (e.g. test ordering, patient preparation, blood sampling, transportation of samples), phlebotomy is the most complex and most vulnerable to errors (4). Phlebotomy is a pivotal invasive procedure in diagnosis, management and treatment of patients in healthcare (5). Since a large proportion (60–80%) of medical decisions is based on the results of the laboratory tests (6, 7), any error in the phlebotomy process could have serious consequences primarily for the patients, but also for medical professionals and healthcare system in general (8).
Phlebotomy is a complex procedure and includes a large number of steps. Small variations in each of them can create a large cumulative effect. In addition, there is a large heterogeneity in the education of medical staff performing phlebotomy (nurses, laboratory technicians, phlebotomists), introducing another layer of variability into the system (9). Therefore, in order to minimize the possibility of errors, this procedure should be standardised, well-documented and written instructions should be available at every workstation.
Two key documents are used as phlebotomy guidelines worldwide: CLSI (Clinical Laboratory Standards Institute) H3-A6 Procedures for the Collection of Diagnostic Blood Specimens by Venipuncture; Approved Standard-Sixth Edition (CLSI, 2007) (10) and WHO (World Health Organization) Guidelines on Drawing Blood: Best Practices in Phlebotomy (WHO, 2010) (11). Some European countries are using these widely accepted standards, while some have nationally adopted/adjusted/translated phlebotomy guidelines. Based on the recently published survey of the European Federation of Clinical Chemistry and Laboratory Medicine, Working Group for the Preanalytical Phase (EFLM WG-PA) (9), only seven European countries have national guidelines for phlebotomy: Ireland (12), UK (13), Spain, Slovenia, Sweden (14), Italy and Croatia (15). The main reasons for not having national guidelines are: 1) lack of time or leadership to perform the work or 2) implementation of CLSI or WHO guidelines eliminated the need for national guidelines (9). However, based on the self-reported questionnaire, estimated compliance with the guidelines is poor, indicating the need for continuous education and implementation of existing procedures (16).
Although Croatia is one of the few European countries that already have national guidelines for phlebotomy, the growing evidence supporting the importance of preanalytical phase management resulted in a need for evidence-based revision and an expansion of existing recommendations.
Background and scope
In Croatia, clinical chemistry is a recognized and regulated profession and is almost exclusively practiced by specialists in medical biochemistry and laboratory medicine (17). Only medical professionals work in laboratories. Venous blood sampling is performed in hospital wards, primary care offices and laboratories. While nurses perform blood sampling in hospital wards and primary care dispensaries, in medical laboratories blood sampling is performed by laboratory technicians and bachelors of medical laboratory diagnostics. During high school education for laboratory technicians, students are required to comprehend theoretic and practical principles of phlebotomy, however a specialised course in phlebotomy is not part of the curriculum (9). Phlebotomy is not thought of in higher forms of education in clinical chemistry (undergraduate and graduate), and even specialists are not required to be qualified to perform phlebotomy. In addition, there is no specific training in phlebotomy as a continuous educational recourse. The scope of medical biochemistry and laboratory medicine in Croatia includes clinical biochemistry, haematology, coagulation, toxicology and therapeutic drug monitoring, immunology and molecular diagnostics. Fields outside of the scope of profession are microbiology, blood-banking and cytogenetics (17).
This recommendation was issued by the Croatian Society for Medical Biochemistry and Laboratory Medicine, Working Group for the Preanalytical Phase after a review of relevant literature sources in phlebotomy procedures. Although other medical professionals (i.e. nurses and physicians) can use this document, it is intended primarily for laboratory staff when performing venous blood sampling within medical biochemistry laboratories. This document is based on the CLSI guideline H3-A6 (10), however there are some significant differences. In Croatian laboratories, blood sampling using catheters is never performed; therefore, that part of CLSI H3-A6 guideline is omitted. In addition, since evacuated tubes are generally accepted as standard and are used in almost every laboratory in Croatia, blood sampling with syringes is not described in this document. As stated earlier, microbiology is not within the scope of medical biochemistry in Croatia, thus parts referring to microbiology testing are significantly shortened. Certain procedures are modified according to some recent evidence in the field of preanalytics. Additional information is provided and rationale for modification in comparison with the CLSI H3-A6 guideline is explained in highlighted boxes. In addition, some instructions (i.e. order of draw and requirements for mixing) are specified for manufacturers of phlebotomy supplies that are most commonly used in Croatia: BD - Becton, Dickinson and Company (Franklin Lakes, NJ, USA) and Greiner Bio-One (Kremsmünster, Austria).
Materials
Before performing venipuncture it is essential to properly prepare the workplace and have undisturbed access to all necessary supplies. A well-organized workplace ensures continuous workflow of all procedures. All supplies should only be used until the declared expiry date. Each phlebotomy site should contain following materials:
Written procedure for blood sampling
Alcoholic (ethanol, isopropyl alcohol) and non-alcoholic (benzine) disinfectants
Test request form
Evacuated blood collection tubes with various additives and volume sizes
Safe-sharp needles of different gauge size, based on the physical characteristics of the vein, location of the vein and volume of drawn blood
Winged blood collection sets
Needle holders
Tourniquets
Cotton pads
Adhesive bandages or tapes
Gloves
Container for disposal of used needles after venipuncture
Ice water
Foil
Water bath at 37 °C.
Procedure
Sanitizing hands
The phlebotomist should sanitize his hands immediately before the first contact with the patient, using warm water and soap or disinfectant gels, tissues or foams according to the nationally accepted guidelines (18). This approach ensures that all surfaces touched by phlebotomists prior to venipuncture are free of pathogens.
Inspecting test request form
It is advisable to modify the test request form according to ISO 15189 requirements (19). Request form should include, but not be limited to, the following information:
Name, surname, gender, date of birth and contact details (address, telephone number) of the patient and unique identifier (health insurance number or personal identification number)
Identification of the doctor who requested examinations and contact details (address of primary healthcare provider or hospital ward full name)
Requested tests
Any clinically relevant information about the patient, his preparation and therapy in order to properly conduct examinations and interpret the results.
In comparison with the CLSI H3-A6 guideline, we have separated data that should be present on the test request form for the samples drawn outside the laboratory.
For blood samples drawn outside the laboratory, test request form should, in addition, contain following items:
Date and time of sample collection;
Name of the medical staff who performed collection;
Type of primary sample (i.e. full blood, serum, plasma), additive (if any), and where relevant, anatomic origin of the sample.
Identifying the patient
Identification of the patient is the key point in patient laboratory processing (20, 21). Proper identification relies on at least two independent identifications (22). Data from the request form should never be solely used for identification. Patient identification should be performed according to following instructions:
Phlebotomist should ask the patient to state the full name, address and/or birth date. The questions should be phrased as such: “Please state your name” and “Please state your date of birth;” not “Are you John Smith?” and “Is your birth date June 1, 1977”
Compare obtained information with the information on the request form
Any discrepancies should be reported, record ed and resolved before sample collection.
There are circumstances that may limit proper patient identification, such as unconscious or semi-conscious patients, children that are too young, deaf and cognitively impaired patients or non-native speakers. In such cases, identification of the patient must be completed with the assistance of ward nurse, legal guardian, parent or accompanying person. All data must be identical with the data on the request form and the name of the person who verified identity must be documented.
Verifying patient preparation for venipuncture
This section brings additional information in comparison with the CLSI guideline H3-A6. CLSI guideline only verifies the patient’s fasting status. It is recommended that laboratory staff verify the patient’s preparation for laboratory testing, including specific preparation for certain tests. Lack of this information can result in acquiring an inadequate sample for analysis. Results from unsuitable specimens are not reliable.
Patient preparation before sample collection is essential for accurate test results. Depending on requested examinations, patient preparation usually involves special diet restrictions or therapy protocols. Depending on the tests ordered, a list of questions that the phlebotomist should ask the patient before venipuncture is provided in Table 1. If the patient is not properly prepared for sample collection, the phlebotomist should inform him of the effects on the laboratory results and postpone the venipuncture giving the patient all information needed for proper preparation. If, for some reason, phlebotomy cannot be postponed, observed nonconformity should be documented on the request form and comment provided on the laboratory report.
TABLE 1.
Verification of patient’s preparation for venipuncture.
| Test | Information on | Question | Reason |
|---|---|---|---|
| All | Fasting | When did you have your last meal? | A large proportion of laboratory tests are influenced by dietary intake (23). Also, lipemia of the sample can cause interference in measurement even for analytes that are not influenced by fasting status (24). |
| All | Fasting | Only water drinking is allowed prior to venipuncture. Did you have anything else to drink this morning? |
Most drinks contain sugar that could influence the results of glucose measurement. In addition, a large proportion of drinks contain active substances that can affect laboratory tests with known (i. e. enzyme induction or inhibition, substrate competition) and unknown mechanisms (25). |
| All | Fasting, therapy | Are you taking any vitamins, food supplements, herbal or natural based medicines? | These supplements may have known or unknown biological effects, medicinal effects, or even technical effects on measuring (e.g. vitamin C in faecal occult blood test, riboflavin in dipstick urine testing). Any information about taking these supplements should be documented (26,27). |
| All | Patient position | Were you resting for 15 minutes prior to blood sampling? | If patient is excited and is not rested prior to blood sampling, increased production of hormones (i.e. catecholamines and corticosteroids) can alter concentration of a large number of proteins, lipids and carbohydrates (26). |
| Coagulation tests | Therapy | Are you receiving any kind of anticoagulant therapy? | PT INR should be measured prior to taking OAT. Also, thrombophilia screening tests (LAC, protein C, protein S, APCR) cannot be performed if the patient is already receiving OAT (28). |
| Female hormones | Menstrual cycle | What is the current day of your menstrual cycle? | Concentration of female hormones depends on the day of the menstrual cycle (26). |
| Homocysteine | Dietary habits | Are you fasting for 12 hours? Did you have protein-rich meal within last 48 hours? | Protein-rich meals can cause falsely elevated homocysteine concentration (29). |
| Iron | Therapy | Were you receiving any oral or intravenous supplements containing iron within the last 10 days? | Consumption of iron supplements or too little time after discontinuing of taking those preparations causes falsely elevated iron concentration (26). |
| Lipid status | Dietary habits | When did you have your last meal? Did you have any changes in diet within the last two weeks? Did you consume alcohol within the last 48 hours? Were you engaged in extreme physical activity within the last 48 hours? | For the measurement of the lipid status, compliance to the recommended protocol is required since any discrepancies influence results of the measurement and, in most cases, cause falsely elevated concentrations of lipids (30). |
| Postprandial glucose | Therapy | Do you have your usual therapy with you (i. e. insulin or oral hypoglycaemics)? | When performing measurement of postprandial glucose, the patient should simulate an everyday meal and therapy regime. If receiving oral hypoglycaemics, the patient should take their meal and therapy after sampling for fasting glucose has been done. Deviation from the usual protocol can cause variations in the result of the test (31). |
| Therapeutic drug monitoring | Therapy | When did you take last dose of the drug? What is the name of the drug you are receiving? | Therapeutic drug monitoring should be done after the drugs are at a steady state and blood collection performed immediately prior to taking the next dose of the drug (32). The time of the application of the previous dose will help to interpret results of the test. Errors in interpretation can occur if the sample is obtained at the wrong time (33). |
| Thyroid hormones (T4, free T4) | Therapy | When did you take last dose of levothyroxine? | Levothyroxine should not be taken in the morning before blood sampling is done, since hormones cause falsely elevated concentration of T4/freeT4 (34). |
PT INR - prothrombin time, international normalized ratio; OAT - oral anticoagulant therapy; LAC - lupus anticoagulant; APCR - activated protein C resistance
Preparing the supplies for blood sampling
In comparison with the CLSI H3-A6 guideline, the use of blood sampling devices other than evacuated blood tubes is not described. Therefore, syringes are not listed as necessary equipment. In addition, since Croatia is a European Union member, an EU Council Directive 2010/32/EU (35) applies for handling sharp medical devices and is added and referenced in the text.
All required materials must be assembled prior to venipuncture and according to requested tests. Only supplies with an appropriate expiry date must be available to the phlebotomist and the handling procedure must minimize the possibility of occupational exposure. The supplies that should be available are:
Evacuated blood collection tubes with various additives. Tubes of various volume sizes should be available. Tube volume size should be adjusted to the number of requested tests, since excessive blood drawing volume can cause anaemia in patients if the blood sampling is done frequently (36).
Needles of appropriate gauge size, based on the physical characteristics of the vein, location of the vein and volume of drawn blood. Different gauge sizes should be available. Using inadequate needle gauge size can cause hemolysis of the sample (37). According to EU Council Directive 2010/32/EU (35) all medical devices should be safety-engineered in order to increase safety of healthcare workers and minimize risk of professional injury.
Winged blood collection sets. These sets are used for blood drawing in children or in patients with week and damaged veins.
Needle holders
Tourniquet
Cotton pads
Alcoholic (ethanol, isopropyl alcohol) and non-alcoholic (benzene) disinfectants
Adhesive bandages or tapes
Gloves - it is mandatory to use single-use gloves
Container for disposal of used needles after venipuncture. This container should also be in accordance with EU Council Directive 2010/32/EU (35).
Labelling the tubes
Although CLSI guideline H3-A6 resolutely states that tubes must be labelled after the collection, there is no undisputable evidence to support that recommendation. Therefore, this document recommends tube labelling just after identification of the patient and verification of his appropriate preparation for the laboratory testing and prior to venipuncture procedure. If the tube is to be labelled after venipuncture, it should be done in front of the patient, while he is still sitting in front of the phlebotomist. Otherwise, there is possibility that the tube will be left unlabelled.
Several pieces of data should be documented for each venipuncture:
Patient’s first and last name
Patient’s date of birth
Laboratory identification number
Patient’s health insurance identification number
Time and date of sampling
Identification of phlebotomist.
Not all of them have to be listed on the tube, but they all have to be documented in a way so that the tube is traceable and unambiguously connected to the patient, collected sample or phlebotomist. It is recommended that the tube is labelled with a barcode, as barcodes can contain all abovementioned information. If not on the tube, this information should be recorded in the paper documentation or laboratory information system.
It is recommended that the tube is identified with at least two independent pieces of information. The more information present on the tube, the more the patient’s safety is increased and the possibility of patient identification errors is reduced.
Positioning the patient
Venipuncture should always be performed on the patient sitting or lying down. For patient safety assurance, it is advisable that chairs have arm holders. The arm must be supported by the armrest. During the venepuncture, the patient should not have any foreign objects in the mouth (chewing gum, thermometer etc.).
Putting on gloves
CLSI H3-A6 guideline recommends putting gloves on after applying tourniquet. However, there is evidence that the time of tourniquet application on patient’s hand is longer than the recommended 60 seconds (38). In order to reduce potential errors that may occur because of prolonged blood stasis (39–41), we propose putting on gloves prior to tourniquet application.
Before each venipuncture, the phlebotomist should put on gloves. The phlebotomist should use new gloves for each patient.
Applying tourniquet
A tourniquet is used for easier location of the vein. It increases intravascular pressure and makes veins more visible, which reduces the possibility of erroneous puncture and possible damage of local arteries and nerves. Tourniquets should never be applied for longer than one minute, in order to avoid local hemoconcentration that may result in erroneous laboratory findings. When the tourniquet is applied for longer than one minute, it should be released and reapplied after minimum two minutes. Tourniquets should be applied between 7 and 10 cm above the venipuncture site. If the skin has lesions where tourniquet is to be applied a different venipuncture site must be considered. Alternatively, a tourniquet can be applied over the gauze pad, tissue or patient’s clothes. For better visualization of the preferred puncture site, the patient can form a fist. However, the patient should not pump his hand vigorously. It should be noted that the tourniquet must not be applied when specific tests are required, because blood stasis can significantly alter concentrations of certain analytes: lactate (10), ammonia (42), albumin (43) and calcium (43).
Selecting venipuncture site
The preferred venipuncture sites are antecubital veins that lie near the skin surface. When those veins are not available, venipuncture can be performed on the veins on the back of the hand. Veins on the underside of the wrist are not recommended for venipuncture, because the arteries and tendons are too close to the skin surface. Arterial punctures should never be considered as an alternative for difficult venipuncture without consulting the physician first. Blood should never be drawn from sites of extensive scaring, on the hand on the side on which mastectomy was performed, hematoma (if not avoidable the sample should be drawn distal to the hematoma), arm with an intravenous therapy application, cannula, fistula and vascular graft.
There are two basic vein distributions in antecubital fossa, H and M shaped patterns. Names of the patterns are based on the shapes that form most prominent veins of that area, cephalic, median chepalic, median cubital, median basilic and basilic vein (Figure 1).
FIGURE 1.
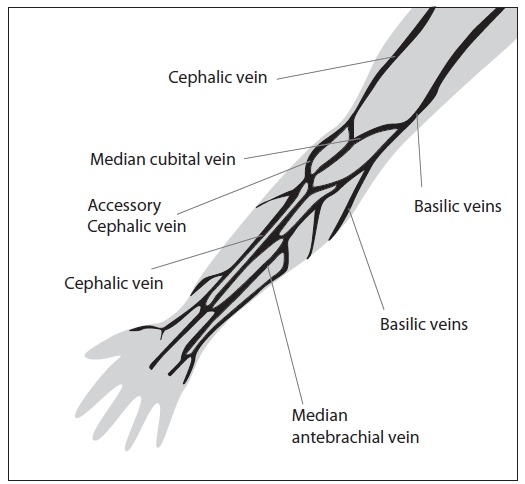
Veins of the Forearm.
The selection of the vein for blood collection must be done carefully. Median cubital and median veins should be considered first for venipuncture. If those veins are not accessible, the cephalic vein should be considered next. Basilic veins are to be considered last for venipuncture, as they are too close to the brachial artery and median nerve. Any form of discrepancy from recommended venipuncture procedure should be documented. There should be written procedures available for dealing with the accidental artery puncture or nerve damage.
With the aid of the tourniquet, the phlebotomist should palpate the selected vein with the index finger. The thumb should not be used, because it has a pulse beat. Palpation also helps in differentiating veins from arteries.
Cleaning venipuncture site
After selecting the vein, the venipuncture site must be properly disinfected, in order to avoid microbial contamination of the patient and collected sample. The skin is cleaned with sterile cotton or gauze pads with 70% isopropyl alcohol or ethanol. The skin should be cleansed with circular motions from centre to periphery.
CLSI guideline H3-A6 states that disinfectant should be left to dry before performing venipuncture in order to avoid possible hemolysis and avoid patient discomfort. However, evidence has shown that this procedure is often unattended in practise (44). In addition, there is no evidence that hemolysis rate will increase if the venipuncture is done immediately after applying disinfectant (45). Therefore, we recommend that, in order to reduce time of tourniquet application, it is not necessary to wait for disinfectant to dry.
When blood alcohol determination is requested, a non-alcoholic based disinfectant should be used, often ether or benzine. When there are difficulties with venipuncture and the vein must be palpated again, the skin should be cleansed again. Special consideration must be given to the duration of tourniquet application.
Performing venipuncture
The recommended blood collection sets are those with evacuated tube systems.
This recommendation does not suggest blood drawing using syringes; therefore, this drawing technique is omitted in comparison to CLSI guideline H3-A6.
For the proper venipuncture technique, the manufacturer’s instructions should be followed. Venipuncture should be performed as follows:
Place the appropriate needle into the needle holder.
Position the patient’s arm in a downward position to prevent reflux from the collection tube into the vein.
Hold the patient’s arm distal to the puncture site. Use the thumb to anchor the vein 2.5 to 5 cm below the venipuncture site.
Inform the patient that venipuncture is about to occur.
Insert the needle at the angle 30° or less and keep it stable in the vein.
Connect the first tube onto the needle.
As soon as blood starts to fill the tube, release the tourniquet.
Allow the tube to fill until blood flow ceases. This ensures correct blood volume and ratio of blood to additive.
Remove the full tube from the needle and replace it with the next tube (if any).
When collecting several tubes, always follow the recommended order of draw (see section Order of draw).
Always remove the last tube from the needle holder before removing the needle from the vein.
Place a cotton or gauze pad on the wound and remove the needle from the vein.
Dispose of the needle.
Inform patient to press cotton or gauze pad on the wound.
Bandage the venipuncture site (see section Bandaging the skin after venipuncture).
When all the tubes are drawn, mix the tubes according to recommendations (see section Tube mixing).
Order of draw
When collecting more than one tube special attention has to be given to order of draw. This recommendation is crucial, in order to avoid additive or anticoagulant carryover and to avoid potential tissue contamination. Failure to follow this procedure could result in significant errors (46, 47).
The recommended order of draw for different types of tubes (with or without gel, with or without additive) is shown in Table 2.
TABLE 2.
| Tube/Additive | Top colour | Top colour legend | |
|---|---|---|---|
| Blood culture tube | Various | ||
| Glass or plastic non-additive serum tube (without clot activator) | Red1, white2 | ||
| Coagulation tube | Light blue1,2 | ||
| Serum tube | Gel separator tube | Red1, gold1,2 | |
| Tube without gel | Red1,2 | ||
| Tube with thrombin based clot activator | Orange1 | ||
| Heparin tube | With gel separator | Green1,2 | |
| Without gel separator | |||
| EDTA tubes | Without gel separator | Lavander1,2 | |
| With gel separator | Lavander1,2, white2 | ||
| ESR tubes | Black1,2 | ||
| Glycolysis inhibitor tubes | Gray1,2 | ||
| Other tubes that contain anticoagulants or additives | |||
Top colours are adjusted to most commonly used manufacturers in Croatia:
BD (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) and
Greiner Bio-One (Grainer Bio-One, Kremsmünster, Austria).
EDTA - ethylenediaminetetraacetic acid; ESR - erythrocyte sedimentation rate.
Clot activators or tissue factors released during venipuncture can affect coagulation testing. Therefore, the coagulation tube has to be drawn before any other tube with additive.
CLSI guideline H3-A6 recommends that a discard tube should be drawn if coagulation assays other than prothrombin time, international normalized ratio and activated partial thromboplastin time should be determined. However, there is published evidence in the literature that there is no difference in the results of other specialized coagulation tests determined with and without discard tube (50–53). Therefore, this recommendation does not support CLSI H3-A6 recommendation of drawing a discard tube for specialized coagulation tests.
However, when using winged blood collection set and any coagulation test is requested, then the discard tube has to be drawn prior to the coagulation tube. This ensures proper anticoagulant and blood ratio, which can be compromised because air in the system can cause under filling of coagulation tube (54).
Needle disposing
Disposable equipment used in collection procedure has to be properly removed according to manufacturer’s recommendations. Needles have to be immediately disposed into the sharps container according to local regulations (35).
Any potential injuries or blood contamination of the personnel during or after the phlebotomy process should be handled according to institution policies. Written documents explaining procedures should be available at each phlebotomy site.
Tube mixing
Since all tubes generally contain some kind of additive (anticoagulant or clot activator), all of them have to be properly mixed immediately after collection. The exception is a glass BD serum tube with clot activator (all Greiner Bio-One tubes are plastic and all should be mixed). Recommendations for proper tube mixing given by two manufacturers commonly used in our country are given in Table 3. Mixing cycle stands for one full inversion of the tube (180°) and returning the tube to the initial position (Figure 2). Full inversion is when the air bubble moves from one end of the tube to the other.
TABLE 3.
Recommendations for tube mixing by two manufacturers commonly used in Croatia - BD (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) and Greiner Bio-One (Kremsmünster, Austria).
| Manufacturer | Number of tube inversions | Tube type (additive) |
|---|---|---|
| Grainer Bio-One tube mixing | 3–4 | Coagulation test (citrate tubes) |
| 8 | Serum tubes with clot activator or gel, plasma heparin or EDTA tubes with clot activator or gel, whole blood EDTA tubes, ESR tubes, trace elements tubes (all kind), blood group tubes (ACD additive), tubes with glycolysis inhibitor | |
|
| ||
| BD tube mixing | 0 | Glass serum tube with clot activator |
| 3–4 | Coagulation test (citrate tubes) | |
| 5 | Plastic serum tube with clot activator and gel | |
| 8 | Serum tube with thrombine based clot activator, plasma heparin or EDTA tubes with clot activator or gel, whole blood EDTA tubes, ESR tubes, trace elements tubes (all kind), blood group tubes (ACD additive), tubes with glycolysis inhibitor | |
EDTA - ethylenediaminetetraacetic acid; ESR - erythrocyte sedimentation rate; ACD - acid citrat dextrose; BD - Becton, Dickinson and Company).
FIGURE 2.
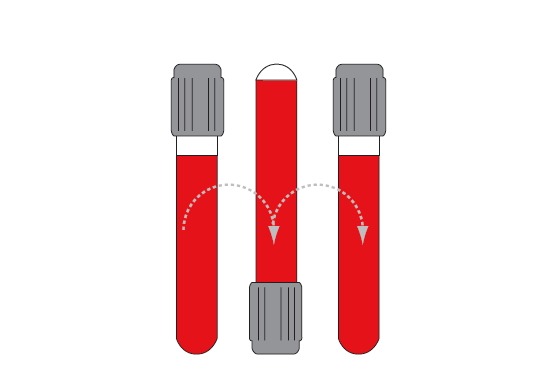
One mixing cycle.
Bandaging the skin after venipuncture
After blood collection, the phlebotomist should gently put cotton or gauze pads over the venipuncture site and then remove the needle. The patient has to hold the pad on the vein and should not bend their arm. After 30 seconds to 1 minute (when all tubes are properly mixed), the phlebotomist should bandage the arm and advise the patient to hold the bandage for at least 15 minutes. In case of prolonged (longer than 5 minutes) or excessive bleeding, the physician should be noted and more pressure should be applied until bleeding stops. Personnel involved in phlebotomy should be educated to help patient in case of emergencies like fainting, nausea, vomiting or convulsions. There should be written procedure for handling such cases.
Removing gloves
Dispose of the gloves after venipuncture.
Recording additional information
Laboratory staff should record any nonconformity that occurs during the phlebotomy according to laboratory protocol.
Special handling requirements
Some specimens need special handling procedures after blood collection. There are thermolabile analytes or those that are sensitive to light exposure. Analytes that require special handling during transportation to the laboratory are listed in Table 4.
TABLE 4.
Analytes that require special handling procedures after blood collection.
| Special handling of specimens | Source | Comments |
|---|---|---|
| Light sensitive analytes | ||
|
| ||
| Amphotericin B | (55) | Transport specimens wrapped in a foil immediately after collection and store protected from the light until analysis. |
| Bilirubin | (10) | |
| Carotene | (55) | |
| Chlordiazepoxide | (55) | |
| Chlorpromazine | (55) | |
| Isoniazid | (55) | |
| Methotrexate | (56) | |
| Porphobilinogen | (55) | |
| Porphyrins | (10) | |
| Pyridoxal 5-phosphate | (55) | |
| Rifampin | (55) | |
| Thioridazine | (55) | |
| Trifluoperazine | (55) | |
| Vitamin A | (55) | |
| Vitamin B1 | (55) | |
| Vitamin B2 | (55) | |
| Vitamin B3 (niacin) | (55) | |
| Vitamin C | (55) | |
| Vitamin E | (55) | |
| Vitamin K1 | (55) | |
|
| ||
| Specimen Chilling | ||
|
| ||
| Ammonia Lactate | (10) | To chill a specimen, place it immediately in a mixture of ice and water (ice slurry not big ice cubes). Do not put the specimen in direct contact with ice or dry ice to avoid hemolysis. Chilling whole blood specimens for longer than two hours is contraindicated for determination of potassium levels. |
| Pyruvate | (10) | |
| Gastrin | (10) | |
| Homocysteine | (10) | |
| Renin | (29) | |
| Parathyroid hormone | (57) | |
| Catecholamines | (10) | |
| Adrenocorticotropic | (10) | |
| hormone | (57) | |
| Free fatty acid | (57) | |
| Acetone | (57) | |
| ACE | (57) | |
|
| ||
| Specimen Transportation at 37 °C | ||
|
| ||
| Cold agglutinin | (10) | For determination of cold agglutinins, an EDTA tube should be used. For cryofibrinogen and cryoglobulins, use tubes that do not contain any additives, all collection supplies must be pre-warmed, keep the sample at water bath heated at 37°C until serum can be separated from the cells, separate the serum from the cells within 1 hour of collection. |
| Cryofibrinogen | (10) | |
| Cryoglobulins | (10) | |
ACE – angiotensin-converting enzyme. Reference numbers are presented in parenthesis.
In addition to Table 4, laboratory managers are required to follow any additional manufacturer’s recommendations regarding special handling conditions.
Additional considerations
Phlebotomy for a child younger than six years should be done with previous consultation with physician. Special attention has to be paid to appropriate equipment for venipuncture of children. All before stated steps in phlebotomy procedure must be applied when collecting blood from a child. This recommendation does not refer to collection of capillary blood by skin puncture.
Flowchart
A step-by-step phlebotomy procedure is presented in Figure 3.
FIGURE 3.
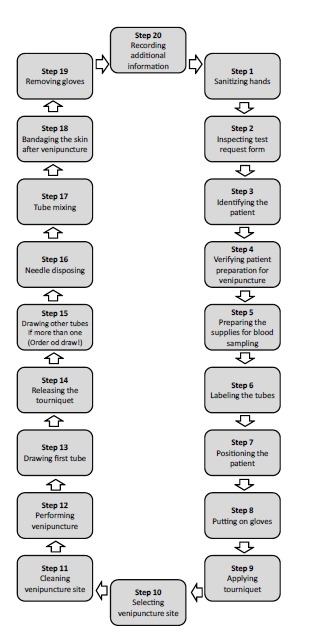
Step-by-step phlebotomy procedure.
Acknowledgments
We thank Clinical Laboratory Standards Institute for granting permission for the use of CLSI’s internationally copyrighted material.
Footnotes
Potential conflict of interest
None declared.
References
- 1.Plebani M. Errors in clinical laboratories or errors in laboratory medicine? Clin Chem Lab Med. 2006;44:750–9. doi: 10.1515/CCLM.2006.123. http://dx.doi.org/10.1515/CCLM.2006.123. [DOI] [PubMed] [Google Scholar]
- 2.Lippi G, Becan-McBride K, Behúlová D, Bowen RAR, Church S, Delanghe JR, et al. Preanalytical quality improvement: in quality we trust. Clin Chem Lab Med. 2013;51:229–41. doi: 10.1515/cclm-2012-0597. http://dx.doi.org/10.1515/cclm-2012-0597. [DOI] [PubMed] [Google Scholar]
- 3.Simundic AM, Nikolac N, Vukasovic I, Vrkic N. The prevalence of preanalytical errors in Croatian ISO 15189 accredited laboratory. Clin Chem Lab Med. 2010;48:1009–14. doi: 10.1515/CCLM.2010.221. http://dx.doi.org/10.1515/cclm.2010.221. [DOI] [PubMed] [Google Scholar]
- 4.Lippi G, Salvagno GL, Montagnana M, Franchini M, Guidi GC. Phlebotomy issues and quality improvement in results of laboratory testing. Clin Lab. 2006;52:217–30. [PubMed] [Google Scholar]
- 5.Lavery I, Ingram P. Venepuncture: best practice. Nurs Stand. 2005;19:55–65. doi: 10.7748/ns2005.08.19.49.55.c3936. http://dx.doi.org/10.7748/ns2005.08.19.49.55.c3936. [DOI] [PubMed] [Google Scholar]
- 6.Forsman RW. Why is the laboratory an afterthought for managed care organizations? Clin Chem. 1996;42:813–6. [PubMed] [Google Scholar]
- 7.Katayev A, Balciza C, Seccombe DW. Establishing reference intervals for clinical laboratory test results: is there a better way? Am J Clin Pathol. 2010;133:180–6. doi: 10.1309/AJCPN5BMTSF1CDYP. http://dx.doi.org/10.1309/AJCPN5BMTSF1CDYP. [DOI] [PubMed] [Google Scholar]
- 8.Green SF. The cost of poor blood specimen quality and errors in preanalytical processes. Clin Biochem. 2013 doi: 10.1016/j.clinbiochem.2013.06.001. [Epub ahead of print] http://dx.doi.org/10.1016/j.clinbiochem.2013.06.001. [DOI] [PubMed]
- 9.Simundic AM, Cornes M, Grankvist K, Lippi G, Nybo M, Kovalevskaya S, et al. Survey of national guidelines, education and training on phlebotomy in 28 European countries: an original report by the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) working group for the preanalytical phase (WG-PA) Clin Chem Lab Med. 2013;51:1–9. doi: 10.1515/cclm-2013-0283. http://dx.doi.org/10.1515/cclm-2013-0283. [DOI] [PubMed] [Google Scholar]
- 10.Clinical Laboratory Standards Institute . Procedures for the Collection of Diagnostic Blood Specimens by. Venipuncture; Approved Standard—Sixth Edition. CLSI document H3-A6. Clinical Laboratory Standards Institute; Wayne, Pennsylvania, USA: 2007. [Google Scholar]
- 11. WHO guidelines on drawing blood: best practices in phlebotomy. Available at: http://whqlibdoc.who.int/publications/2010/9789241599221_eng.pdf. Accessed June 19, 2013.
- 12.Phlebotomists Association of Ireland Ltd Phlebotomy Guidelines. 2010 Available at: http://pairl.ie/doc/PAI_Guidelines_PDF.pdf. Accessed June 19, 2013.
- 13. CHS132 Obtain venous blood samples. Available at: https://tools.skillsforhealth.org.uk/competence/show/html/code/CHS132/. Last accessed July 3, 2013.
- 14. [Blodprov, venös provtagning]. (in Swedish). Available at: http://www.vardhandboken.se/Texter/Blodprov-venosprovtagning/Oversikt/. Last accessed July 3, 2013.
- 15. [Povjerenstvo za stručna pitanja Hrvatske komore medicinskih biokemičara]. [Standardi dobre stručne prakse]. (in Croatian). Available at: http://www.hkmb.hr/povjerenstva/strucna-pitanja.html#1. Accessed June 19, 2013.
- 16.Lipley N. Staff require training in phlebotomy to ensure accurate blood test results. Emerg Nurse. 2011;19:9. doi: 10.7748/en2011.11.19.7.9.p6835. http://dx.doi.org/10.7748/en2011.11.19.7.9.p6835. [DOI] [PubMed] [Google Scholar]
- 17.Simundic AM, Topic E, Cvoriscec D, Cepelak I. Clinical chemistry and laboratory medicine in Croatia: regulation of the profession. Biochem Med. 2011;21:15–21. doi: 10.11613/bm.2011.005. http://dx.doi.org/10.11613/BM.2011.005. [DOI] [PubMed] [Google Scholar]
- 18.Kalenić S, Budimir A, Bosnjak Z, Acketa L, Belina D, Benko I, et al. [Smjernice za higijenu ruku u zdravstvenim ustanovama]. (in Croatian) Lijec Vjesn. 2011;133:155–70. [PubMed] [Google Scholar]
- 19.International Organization for Standardization ISO 15189:2012 Medical laboratories -- Requirements for quality and competence Medical laboratories -- Requirements for quality and competence. 2012. [DOI] [PubMed]
- 20.Henneman PL, Fisher DL, Henneman EA, Pham TA, Mei YY, Talati R, et al. Providers do not verify patient identity during computer order entry. Acad Emerg Med. 2008;15:641–8. doi: 10.1111/j.1553-2712.2008.00148.x. http://dx.doi.org/10.1111/j.1553-2712.2008.00148.x. [DOI] [PubMed] [Google Scholar]
- 21.Schulmeister L. Patient misidentification in oncology care. Clin J Oncol Nurs. 2008;12:495–8. doi: 10.1188/08.CJON.495-498. http://dx.doi.org/10.1188/08.CJON.495-498. [DOI] [PubMed] [Google Scholar]
- 22.The Joint Commission Two Patient Identifiers - NPSG - Goal 1 - 01.01.01. Available at: http://www.jointcommission.org/standards_information/npsgs.aspx. Last accessed July 2, 2013.
- 23.Lima-Oliveira G, Salvagno GL, Lippi G, Gelati M, Montagnana M, Danese E, et al. Influence of a regular, standardized meal on clinical chemistry analytes. Ann Lab Med. 2012;32:250–6. doi: 10.3343/alm.2012.32.4.250. http://dx.doi.org/10.3343/alm.2012.32.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calmarza P, Cordero J. Lipemia interferences in routine clinical biochemical tests. Biochem Med. 2011;21:160–6. doi: 10.11613/bm.2011.025. http://dx.doi.org/10.11613/BM.2011.025. [DOI] [PubMed] [Google Scholar]
- 25.Won CS, Oberlies NH, Paine MF. Mechanisms underlying food-drug interactions: inhibition of intestinal metabolism and transport. Pharmacol Ther. 2012;136:186–201. doi: 10.1016/j.pharmthera.2012.08.001. http://dx.doi.org/10.1016/j.pharmthera.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burtis CA, Ashwood ER, Bruns DE, editors. Tietz Fundamentals of Clinical Chemistry. 6th edition. St Louis, MO: Saunders/Elsevier; 2008. [Google Scholar]
- 27.Dasgupta A. Review of abnormal laboratory test results and toxic effects due to use of herbal medicines. Am J Clin Pathol. 2003;120:127–37. doi: 10.1309/P024-K7VR-DDPJ-CTVN. http://dx.doi.org/10.1309/P024K7VRDDPJCTVN. [DOI] [PubMed] [Google Scholar]
- 28.Favaloro EJ, Funk DM, Lippi G. Pre-analytical Variables in Coagulation Testing Associated With Diagnostic Errors in Hemostasis. LabMedicine. 2012;43:1–10. [Google Scholar]
- 29.Hortin GL. Homocysteine: Clinical Significance and Laboratory Measurement. Lab Med. 2006;37:551–3. http://dx.doi.org/10.1309/93G5JG1BF44N65BQ. [Google Scholar]
- 30.Cooper GR, Myers GL, Smith SJ, Schlant RC. Blood lipid measurements. Variations and practical utility. JAMA. 1992;267:1652–60. http://dx.doi.org/10.1001/jama.267.12.1652. [PubMed] [Google Scholar]
- 31.American Diabetes Association Postprandial Blood Glucose. Diabetes Care. 2001;24:775–8. doi: 10.2337/diacare.24.4.775. http://dx.doi.org/10.2337/diacare.24.4.775. [DOI] [PubMed] [Google Scholar]
- 32.Lucas C, Donovan P. ‘Just a repeat’ - When drug monitoring is indicated. Aust Fam Physician. 2013;42:18–22. [PubMed] [Google Scholar]
- 33.Sidwell A, Barclay M, Begg E, Moore G. Digoxin therapeutic drug monitoring: an audit and review. N Z Med J. 2003;116:U708. [PubMed] [Google Scholar]
- 34.Ain KB, Pucino F, Shiver TM, Banks SM. Thyroid hormone levels affected by time of blood sampling in thyroxine-treated patients. Thyroid. 1993;3:81–5. doi: 10.1089/thy.1993.3.81. http://dx.doi.org/10.1089/thy.1993.3.81. [DOI] [PubMed] [Google Scholar]
- 35.Official Journal of the European Union EU Council Directive 2010/32/EU. Prevention of Sharps Injuries in the Hospital and Healthcare Sector. Official Journal of the European Union, No. L134 of 1. 2010 Jun;:66–72. [Google Scholar]
- 36.Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med. 2005;20:520–4. doi: 10.1111/j.1525-1497.2005.0094.x. http://dx.doi.org/10.1111/j.1525-1497.2005.0094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dugan L, Leech L, Speroni KG, Corriher J. Factors affecting hemolysis rates in blood samples drawn from newly placed IV sites in the emergency department. J Emerg Nurs. 2005;31:338–45. doi: 10.1016/j.jen.2005.05.004. http://dx.doi.org/10.1016/j.jen.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Picheth G, Guidi GC. Impact of the phlebotomy training based on CLSI/NCCLS H03-a6 - procedures for the collection of diagnostic blood specimens by venipuncture. Biochem Med. 2012;22:342–51. doi: 10.11613/bm.2012.036. http://dx.doi.org/10.11613/BM.2012.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lippi G, Salvagno GL, Montagnana M, Brocco G, Guidi GC. Influence of short-term venous stasis on clinical chemistry testing. Clin Chem Lab Med. 2005;43:869–75. doi: 10.1515/CCLM.2005.146. http://dx.doi.org/10.1515/CCLM.2005.146. [DOI] [PubMed] [Google Scholar]
- 40.Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Manguera CL, Sumita NM, et al. New ways to deal with known preanalytical issues: use of transilluminator instead of tourniquet for easing vein access and eliminating stasis on clinical biochemistry. Biochem Med. 2011;21:152–9. doi: 10.11613/bm.2011.024. http://dx.doi.org/10.11613/BM.2011.024. [DOI] [PubMed] [Google Scholar]
- 41.Cengiz M, Ulker P, Meiselman HJ, Baskurt OK. Influence of tourniquet application on venous blood sampling for serum chemistry, hematological parameters, leukocyte activation and erythrocyte mechanical properties. Clin Chem Lab Med. 2009;47:769–76. doi: 10.1515/CCLM.2009.157. http://dx.doi.org/10.1515/CCLM.2009.157. [DOI] [PubMed] [Google Scholar]
- 42.Ammonia Available at: http://www.clinlabnavigator.com/ammonia.html. Last accessed July 15, 2013.
- 43.Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Manguera CL, Sumita NM. New ways to deal with known preanalytical issues: use of transilluminator instead of tourniquet for easing vein access and eliminating stasis on clinical biochemistry. Biochem Med. 2011;21:152–9. doi: 10.11613/bm.2011.024. http://dx.doi.org/10.11613/BM.2011.024. [DOI] [PubMed] [Google Scholar]
- 44.Ćelap I, Nikolac N, Čuljak M, Šimundić AM. [Primjena CLSI smjernica za uzorkovanje venske krvi] (in Croatian) [Simpozij u organizaciji Hrvatskog društva za medicinsku biokemiju i laboratorijsku medicinu (HDMBLM) i tvrtke Abbott Laboratories LOKUS] (Book of abstracts) 2013:31. [Google Scholar]
- 45.Salvagno GL, Danese E, Lima-Oliveira G, Guidi GC, Lippi G. Avoidance to wipe alcohol before venipuncture is not a source of spurious hemolysis. Biochem Med. 2013;23:201–5. doi: 10.11613/BM.2013.023. http://dx.doi.org/10.11613/BM.2013.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fukugawa Y, Ohnishi H, Ishii T, Tanouchi A, Sano J, Miyawaki H, et al. Effect of carryover of clot activators on coagulation tests during phlebotomy. Am J Clin Pathol. 2012;137:900–3. doi: 10.1309/AJCPL3LZZN9WJGZT. http://dx.doi.org/10.1309/AJCPL3LZZN9WJGZT. [DOI] [PubMed] [Google Scholar]
- 47.Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Picheth G, Guidi GC. Incorrect order of draw could be mitigate the patient safety: a phlebotomy management case report. Biochem Med. 2013;23:218–23. doi: 10.11613/BM.2013.026. http://dx.doi.org/10.11613/BM.2013.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. BD Vacutainer ® Order of Draw for Multiple Tube Collections. Available at: http://www.bd.com/vacutainer/pdfs/plus_plastic_tubes_wallchart_orderofdraw_VS5729.pdf. Last accessed July 4, 2013.
- 49. Evacuated Blood Collection System For In Vitro Diagnostic Use. Available at: http://www.gbo.com/documents/980200B_0722001R2_IFU_VenousBloodCollection_11-12-10_US.pdf. Last accessed July 4, 2013.
- 50.Raijmakers MTM, Menting CHF, Vader HL, van der Graaf F. Collection of blood specimens by venipuncture for plasma-based coagulation assays: necessity of a discard tube. Am J Clin Pathol. 2010;133:331–5. doi: 10.1309/AJCP9ATB0AXPFJCC. http://dx.doi.org/10.1309/AJCP9ATB0AXPFJCC. [DOI] [PubMed] [Google Scholar]
- 51.Smock KJ, Crist RA, Hansen SJ, Rodgers GM, Lehman CM. Discard tubes are not necessary when drawing samples for specialized coagulation testing. Blood Coagul Fibrinolysis. 2010;21:279–82. doi: 10.1097/MBC.0b013e3283380d12. http://dx.doi.org/10.1097/MBC.0b013e3283380d12. [DOI] [PubMed] [Google Scholar]
- 52.Serin E, Bugdayci G. Effect of tube filling order on specific coagulation parameters in healthy subjects. Lab Med. 2007;38:556–8. http://dx.doi.org/10.1309/RQ00WJ60-KQXAM9B0. [Google Scholar]
- 53.Lippi G, Guidi GC. Effect of specimen collection on routine coagulation assays and D-dimer measurement. Clin Chem. 2004;50:2150–2. doi: 10.1373/clinchem.2004.036244. http://dx.doi.org/10.1373/clinchem.2004.036244. [DOI] [PubMed] [Google Scholar]
- 54.Favaloro EJ, Lippi G, Raijmakers MT, Vader HL, van der Graaf F. Discard tubes are sometimes necessary when drawing samples for hemostasis. Am J Clin Pathol. 2010;134:851. doi: 10.1309/AJCP9HIFFC6FWKYF. http://dx.doi.org/10.1309/AJCP9HIFFC6FWKYF. [DOI] [PubMed] [Google Scholar]
- 55.MLabs Specimen Collection and Transport Guidelines Available at: http://mlabs.umich.edu/files/pdfs/POL-SPECIMEN_GUIDELINES.pdf. Last accessed July 2, 2013.
- 56.Mayo Clinic Laboratories Protecting Specimens from Light. Available at: http://www.mayomedicallaboratories.com/specimen-transport/domestic/light-protection.html. Last accessed July 2, 2013.
- 57. BD - Special handling of chilled specimens. Available at: http://www.bd.com/vacutainer/pdfs/plus_plastic_tubes_instructions_lightsensitive_VS5732.pdf. Last accessed July 2, 2013.


