Abstract
An increasing prevalence of gestational diabetes has become a very challenging task in prenatal care worldwide. International Association of Diabetes and Pregnancy Study Groups (IADPSG) has recently issued recommendations on the diagnosis and classification of hyperglycaemia in pregnancy. These recommendations, the first to provide harmonised, evidence-based criteria for the diagnosis and classification of diabetes in pregnancy, are currently being discussed and accepted worldwide by the relevant authorities. As the acceptance of the proposed criteria has major implications for both clinical and laboratory settings, a concerted action towards necessary changes in practice has to be carefully planned and adjusted to national health-care specificities.
IADPSG criteria have been strongly advocated by the Croatian Perinatology Society, resulting in a new strategy for the detection and diagnosis of hyperglycaemic disorders in pregnancy.
To address the respective laboratory requirements, in April 2012, the Croatian Chamber of Medical Biochemists appointed a Working Group to provide a standardised procedure for the diagnosis of gestational diabetes, applicable to all laboratories involved in prenatal care, in both primary and specialised health-care facilities.
In this paper we discuss key laboratory-related issues regarding succesful implementation of the IADPSG criteria in Croatia.
Keywords: gestational diabetes, IADPSG criteria, clinical laboratory techniques
Introduction
Gestational diabetes mellitus (GDM) is defined as „any degree of glucose intolerance with onset or first recognition during pregnancy“ (1). Inadequate B-cell response to increased insulin requirements during fetal development within physiological, pregnancy-associated insulin resistance, is implicated in the pathophysiology of this common pregnancy complication. The prevalence of GDM is increasing, which is closely associated with a higher prevalence of obesity and a more advanced maternal age; both of these risk factors are involved in the higher prevalence of previously un-diagnosed type 2 diabetes mellitus, which has also become a very important issue in prenatal care (2,3).
Early diagnosis and appropriate management of both GDM and pre-existing diabetes in pregnancy are of the utmost importance in avoiding adverse pregnancy outcomes for both mother and her baby. Although adverse perinatal outcomes associated with the degree of hyperglycaemia in overt diabetes are well known, until recently diagnostic criteria for GDM either referred to the mother-related outcomes, i.e. to the identification of women with a high risk of developing diabetes in pregnancy (4), or were identical to diagnostic criteria for non-pregnant individuals (5).
In any case, diagnostic cut-offs were defined as specific fasting and post-oral glucose-tolerance test load (oGTT) concentrations of plasma glucose; however, both the amount of glucose and the time-points of sampling in oGTT, as well as the diagnostic thresholds for glucose differed significantly between criteria. As a result, screening procedures and management of GDM varied significantly among countries, leading to serious controversies in epidemiological data and clinical practice (3).
In order to clarify the issue of whether maternal hyperglycaemia below diagnostic values for overt diabetes is associated with an increased risk of adverse pregnancy outcomes, a large multicentric study, the Hyperglycaemia and Adverse Pregnancy Outcomes (HAPO) study was conducted, including 25,505 pregnant women (15 centers, 9 countries, all ethnicities) who underwent 75-g oGTT at 24–32 weeks of gestation (6). Their glucose values were analysed and compared to predefined primary and secondary perinatal outcomes. Results revealed a strong, continuous positive association of maternal glucose levels below diagnostic cutoffs for diabetes with primary outcomes: birth weight for gestational age above 90th percentile, primary cesarean delivery, clinically diagnosed neonatal hypoglycemia, and cord-blood-serum C-peptide levels above 90th percentile.
IADPSG Recommendations
International Association of Diabetes and Pregnancy Study Groups (IADPSG), an umbrella organisation with a primary focus on enhancing collaboration between various regional/national organisations dealing with diabetes and pregnancy issues, has recently issued recommendations on the diagnosis and classification of hyperglycaemia in pregnancy (7). Diagnostic criteria for GDM and overt diabetes in pregnancy (based on the results from the HAPO study and the consensus opinion/criteria for non-pregnant individuals, respectively) are shown in Table 1. These recommendations, the first to provide harmonised, evidence-based criteria for the diagnosis and classification of diabetes in pregnancy, are currently being discussed and accepted worldwide by the relevant authorities, including diabetologists, obstetricians, laboratory professionals and others (8,9). As the acceptance of the proposed criteria has major implications for both clinical and laboratory settings, a concerted action towards necessary changes in practice has to be carefully planned and adjusted to national health-care specificities.
Table 1.
| Gestational diabetes mellitus | |
|
| |
| Venous plasma glucose treshold (mmol/L) | |
| Fasting | ≥ 5.1 |
| 75 g oGTT: 60 min | ≥ 10.0 |
| 75 g oGTT: 120 min | ≥ 8.5 |
| One or more values equal or exceeding diagnostic threshold. | |
|
| |
| Overt diabetes in pregnancy | |
|
| |
| Measure of glycaemia | Diagnostic threshold |
| Fasting plasma glucose (FPG)* | ≥ 7.0 mmol/L |
| HbA1c | ≥ 6.5% (48 mmol/mol) |
| Random plasma glucose* | ≥ 11.1 mmol/L |
| Any of measures of glycaemia equal or exceeding diagnostic threshold. | |
venous plasma
The implementation of the IADPSG recommendations in Croatia
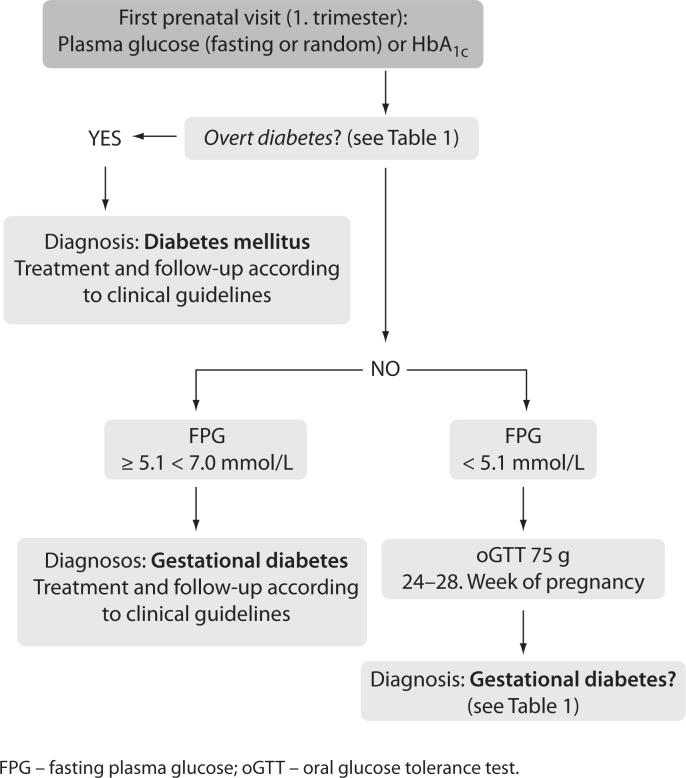
IADPSG criteria have been strongly advocated by the Croatian Perinatology Society, resulting in a new strategy for the detection and diagnosis of hyperglycaemic disorders in pregnancy, including both GDM and overt, previously undiagnosed diabetes in pregnancy (Figure 1) (10). On the other hand, it has been suggested that the current WHO criteria (issued in 1999) should be kept until the end of the ongoing revision process, aiming to issue a new WHO position statement and classification criteria for GDM (11). However, considering the power of evidence of the HAPO study, it is unlikely to expect any significant modification of the IADPSG diagnostic procedure and criteria.
Figure 1.
Strategy for the detection and diagnosis of gestational diabetes and overt diabetes in pregnancy (7,10).
FPG – fasting plasma glucose; oGTT – oral glucose tolerance test.
Laboratory considerations
While the general acceptance of the new criteria still requires a consensus between all relevant subjects, this challenging situation offers a great opportunity to re-evaluate current laboratory practice in the diagnosis of gestational diabetes and prepare laboratories for necessary revisions. To address the respective requirements, in April 2012, the Croatian Chamber of Medical Biochemists appointed a Working Group to provide a standardised procedure for the diagnosis of gestational diabetes, applicable to all laboratories involved in prenatal care, in both primary and specialised health-care facilities.
In this paper we discuss key laboratory-related issues regarding succesful implementation of the IADPSG criteria in Croatia.
1. oGTT
Recommendation: 75 g oGTT should be performed, with blood sampling for plasma glucose measurement immediately before, and at 60 and 120 minutes after a glucose load (7).
Current practice: 75 g oGTT, with basal and 120-min glucose sampling, and WHO classification criteria (12).
Change: Additional sampling at 60 min during a 75 g oGTT should be implemented when diagnosing gestational diabetes.
2. Type of sample
Recommendation: Only venous plasma is a valid sample for glucose analysis, as differences between venous and capillary blood glucose concentrations are variable, and no conversion factor can provide an accurate interconversion. The IADPSG diagnostic criteria are based on venous plasma (7,9).
Current practice: Both venous and capillary plasma/serum samples are used, with WHO-based diagnostic glucose values for respective samples (12).
Change: Switch to venous plasma at all time-points of oGTT.
3. In vitro glycolysis
Recommendation: To minimise the effect of in vitro glycolysis, blood samples for glucose analysis should be cooled (refrigerated or placed in ice) immediately after sampling, and plasma separated from the cells within 30 minutes. If this is not possible, an effective, rapidly-acting glycolysis inhibitor should be used (7,9).
Current practice: Data from a recent survey demonstrated high variability of preanalytical procedures in the management of samples for glucose measurement in Croatian laboratories, with less than half of the laboratories reporting separating plasma within 30 minutes from the time of sampling (13). Moreover, preanalytical differences exist within the same laboratory, depending on the phlebotomy location, i.e. whether it is performed in a laboratory or a family physician’s office. A recently introduced practice of blood sampling in the offices of primary health-care physicians resulted in an unacceptable delay (30–60 min, 1–2 h and >2 h) in the processing of samples for glucose analysis, as reported by 37%, 20% and 9% of the laboratories, respectively (13).
Change: Preanalytical sample handling must be harmonised and performed in accordance with recommendations.
Considering that an average rate of in vitro glycolysis (∼0.6 mmol/L/h) is presumably even more pronounced due to physiological leukocytosis in pregnancy, a rigorous preanalytical control is of utmost importance for the correct classification of patients undergoing diagnostic procedure for gestational diabetes. It should be stressed that the most efficient and cost-effective method for the inhibition of glycolysis is physical separation of plasma from cells. Conventional glycolytic inhibitors (e.g. sodium fluoride, lithium iodoacetate) are not efficient in the first hour from blood sampling, whereas citrate-containing dedicated tubes for glucose sampling, recently reported to be superior in this regard are currently not widely available (9).
4. Analytical issues
Recommendation: Plasma glucose for the di agno sis of gestational diabetes should be measured in a laboratory, by an automated enzymatic method, with an analytical imprecision of ≤ 2.9%, a bias of ≤ 2.2% and a total error of ≤ 6.9% (9).
Current practice: Latest data from the Croatian EQAS revealed excellent compliance of Croatian laboratories (N = 187), with a 100% score regarding methodology (54% and 46% performing the hexokinase and the glucose-oxidase methods, respectively), and an inter-laboratory CV of 2.4% (CroQalm Pilot EQAS report 1/2012, accessible to the participants).
Change: No changes needed.
5. Postanalytical issues
Recommendation: Laboratory reports of oGTT in pregnancy should contain glucose cut-off values according to the IADPSG criteria (7).
Current practice: oGTT laboratory reports contain glucose cut-offs according to WHO criteria, depending on the sample type used (venous/capillary plasma) (12).
Change: Laboratory reports of oGTT in pregnancy should be harmonised to the IADPSG cutoff values. Current WHO cut-off values should be available upon specific clinical request.
6. Supplementary issues
Urine testing for glucose and ketones is not recommended and should not be performed as a part of the GDM diagnostic procedure (7,9).
Hand-held glucose meters are not accurate enough for the screening and diagnosis of diabetes in general, as well as of gestational diabetes, and their use is therefore not recommended for this purpose (9).
Conclusions
As regards laboratory practice, changes in Croatian laboratories include not only additional glucose-sampling during oGTT (60 min), but above all, a careful re-consideration and harmonisation of pre-analytical sample handling in order to control in vitro glycolysis. Considering that the first step of screening for hyperglycaemia in pregnancy involves fasting plasma glucose in the first trimester of gestation and diagnosis of either GDM or overt diabetes according to the IADPSG criteria (Figure 1), it is essential to ensure accurate glucose results in all laboratories involved in pregnancy health care. Correct classification in the first place will not only improve pregnancy outcomes, but also reduce unnecessary oGTT, as only women with fasting plasma glucose below 5.1 mmol/L are eligible for testing at 24–28 weeks of gestation.
Redefinition of gestational diabetes, based on the IADPSG-criteria, has major implications for both clinical and laboratory practice. For the first time, a harmonised strategy is available for screening and diagnosis of hyperglycaemic disorders in pregnancy in terms of both timing and procedure of glycaemic testing. The use of the new diagnostic glucose values will definitely increase the prevalence of gestational diabetes, but appropriate clinical interventions will also improve the outcomes of hyperglycaemia-associated pregnancies. Laboratories have therefore a unique role and responsibility to contribute to this important process.
Footnotes
Potential conflict of interest
None declared.
References
- 1.American Diabetes Association Clinical practice recommendations 2001: gestational diabetes mellitus. Diabetes Care. 2001;24:S77–9. [PubMed] [Google Scholar]
- 2.Agarwal MM, Dhatt GS, Zayed R, Bali N. Gestational diabetes: relevance of diagnostic criteria and preventive strategies for Type 2 diabetes mellitus. Arch Gynecol Obstet. 2007;276:237–43. doi: 10.1007/s00404-007-0334-4. http://dx.doi.org/10.1007/s00404-007-0334-4. [DOI] [PubMed] [Google Scholar]
- 3.IDF Clinical Guidelines Taskforce . Global Guideline on Pregnancy and Diabetes. Brussels: International Diabetes Federation; 2009. [Google Scholar]
- 4.Metzger BE, Buchanan TA, Coustan DR, de Leiva A, Dunger DB, Hadden DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(Suppl 2):S251–60. doi: 10.2337/dc07-s225. http://dx.doi.org/10.2337/dc07-s225. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . Definition, Diagnosis and Classification of Diabetes Mellitus and its complications, WHO/NCD/NCS/99.2. WHO; Geneva: 1999. [Google Scholar]
- 6.The HAPO Study Cooperative Research Group Hyperglycemia and Adverse Pregnancy Outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. http://dx.doi.org/10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 7.Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–82. doi: 10.2337/dc09-1848. http://dx.doi.org/10.2337/dc10-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ADA Standards of medical care in diabetes-2012. Diabetes Care. 2012;35(Suppl 1):S11–63. doi: 10.2337/dc12-s011. http://dx.doi.org/10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Kirkman MS, et al. Guidelines and Recommendations for Laboratory Analysis in the Diagnosis and Management of Diabetes Mellitus. Clin Chem. 2011;57:e1–e47. doi: 10.1373/clinchem.2010.161596. http://dx.doi.org/10.1373/clinchem.2010.161596. [DOI] [PubMed] [Google Scholar]
- 10.Đelmiš J, Ivanišević M, Juras J, Herman M. [Dijagnoza hiperglikemije u trudnoći] Gynaecol Perinatol. 2010;19:86–89. (in Croatian). [Google Scholar]
- 11.Roglić G. Defining and measuring gestational diabetes mellitus. IDF World Diabetes Congress 2011 Abstract book:A107.
- 12.Stavljenić Rukavina A, Čvorišćec D. [Harmonizacija laboratorijskih nalaza u području opće, specijalne i visokodiferentne medicinske biokemije] Hrvatska komora medicinskih biokemičara. Zagreb: Medicinska Naklada; 2007. (in Croatian). [Google Scholar]
- 13.Vučić Lovrenčić M, Božičević S, Radišić Biljak V, Mesić R, Poljičanin T, Metelko Ž. Implications of variable preanalytical procedures for the diagnosis of diabetes mellitus in Croatia. Biochem Med. 2012;22:A92–A93. [Google Scholar]