Abstract
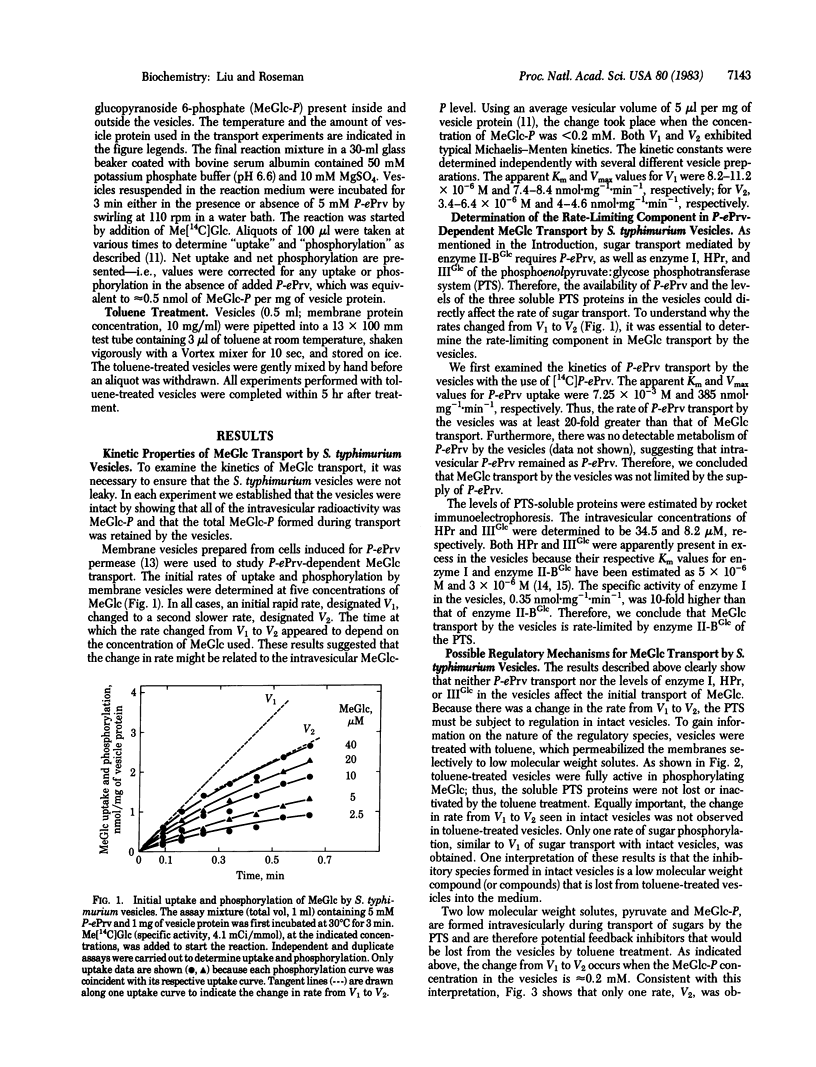
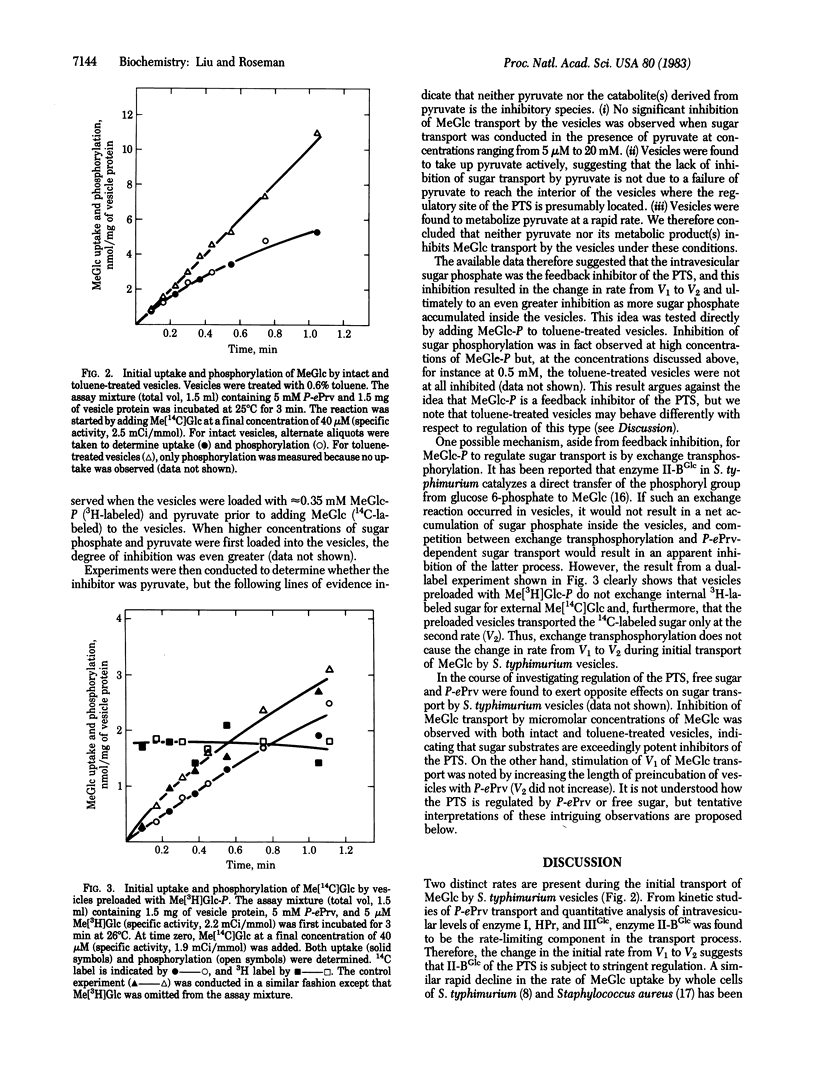
Membrane vesicles from Salmonella typhimurium SB3507 were used to study the kinetics of methyl alpha-D-glucopyranoside (MeGlc) transport by the phosphoenolpyruvate: glycose phosphotransferase system (PTS). During the first minute of phosphoenolpyruvate-dependent MeGlc transport, two distinct rates were observed; an initial rapid rate, V1 (Vmax, 7.4-8.4 nmol X mg-1 X min-1; Km, 8.2-11.2 X 10(-6)M), followed by a second slower rate, V2 (Vmax, 4-4.6 nmol X mg-1 X min-1; Km, 3.4-6.4 X 10(-6) M). The change in rate occurred when the intravesicular MeGlc phosphate concentration was 0.2 mM or less, depending on the external MeGlc concentration. The rate-limiting component in MeGlc transport was found to be enzyme II-BGlc, not phosphoenolpyruvate uptake or the PTS proteins enzyme I, HPr, and IIIGlc. The change from V1 to V2 thus suggests that the PTS is regulated in intact vesicles. However, this regulation was completely relieved by permeabilizing the vesicles with toluene. That is, the toluene-treated vesicles showed only V1 for MeGlc phosphorylation. Evidence was obtained to show that pyruvate and its metabolic products generated by the vesicles exerted no effect on the rate of MeGlc transport. Furthermore, the result from a dual-label experiment excluded exchange transphosphorylation as the mechanism for regulating MeGlc transport by the vesicles. Possible mechanisms for regulation of the PTS are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTTIN G., COHEN G. N., MONOD J., RICKENBERG H. V. La galactoside-perméase d'Escherichia coli. Ann Inst Pasteur (Paris) 1956 Dec;91(6):829–857. [PubMed] [Google Scholar]
- Beneski D. A., Misko T. P., Roseman S. Sugar transport by the bacterial phosphotransferase system. Preparation and characterization of membrane vesicles from mutant and wild type Salmonella typhimurium. J Biol Chem. 1982 Dec 10;257(23):14565–14575. [PubMed] [Google Scholar]
- Curtis S. J., Epstein W. Phosphorylation of D-glucose in Escherichia coli mutants defective in glucosephosphotransferase, mannosephosphotransferase, and glucokinase. J Bacteriol. 1975 Jun;122(3):1189–1199. doi: 10.1128/jb.122.3.1189-1199.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORECKER B. L., THOMAS J., MONOD J. Galactose transport in Escherichia coli. I. General properties as studied in a galactokinaseless mutant. J Biol Chem. 1960 Jun;235:1580–1585. [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg H. L. Fine control of sugar uptake by Escherichia coli. Symp Soc Exp Biol. 1973;27:175–193. [PubMed] [Google Scholar]
- Kornberg H. L. The nature and control of carbohydrate uptake by Escherichia coli. FEBS Lett. 1976 Mar 15;63(1):3–9. doi: 10.1016/0014-5793(76)80183-4. [DOI] [PubMed] [Google Scholar]
- Meadow N. D., Roseman S. Sugar transport by the bacterial phosphotransferase system. Isolation and characterization of a glucose-specific phosphocarrier protein (IIIGlc) from Salmonella typhimurium. J Biol Chem. 1982 Dec 10;257(23):14526–14537. [PubMed] [Google Scholar]
- Postma P. W., Roseman S. The bacterial phosphoenolpyruvate: sugar phosphotransferase system. Biochim Biophys Acta. 1976 Dec 14;457(3-4):213–257. doi: 10.1016/0304-4157(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Pouysségur J. M., Faik P., Kornberg H. L. Utilization of gluconate by Escherichia coli. Uptake of D-gluconate by a mutant impaired in gluconate kinase activity and by membrane vesicles derived therefrom. Biochem J. 1974 May;140(2):193–203. doi: 10.1042/bj1400193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rephaeli A. W., Saier M. H., Jr Kinetic analyses of the sugar phosphate:sugar transphosphorylation reaction catalyzed by the glucose enzyme II complex of the bacterial phosphotransferase system. J Biol Chem. 1978 Nov 10;253(21):7595–7597. [PubMed] [Google Scholar]
- Saier M. H., Jr, Wentzel D. L., Feucht B. U., Judice J. J. A transport system for phosphoenolpyruvate, 2-phosphoglycerate, and 3-phosphoglycerate in Salmonella typhimurium. J Biol Chem. 1975 Jul 10;250(13):5089–5096. [PubMed] [Google Scholar]
- Simoni R. D., Roseman S., Saier M. H., Jr Sugar transport. Properties of mutant bacteria defective in proteins of the phosphoenolpyruvate: sugar phosphotransferase system. J Biol Chem. 1976 Nov 10;251(21):6584–6597. [PubMed] [Google Scholar]
- Simoni R. D., Roseman S. Sugar transport. VII. Lactose transport in Staphylococcus aureus. J Biol Chem. 1973 Feb 10;248(3):966–974. [PubMed] [Google Scholar]
- Stock J. B., Waygood E. B., Meadow N. D., Postma P. W., Roseman S. Sugar transport by the bacterial phosphotransferase system. The glucose receptors of the Salmonella typhimurium phosphotransferase system. J Biol Chem. 1982 Dec 10;257(23):14543–14552. [PubMed] [Google Scholar]
- Waygood E. B., Meadow N. D., Roseman S. Modified assay procedures for the phosphotransferase system in enteric bacteria. Anal Biochem. 1979 May;95(1):293–304. doi: 10.1016/0003-2697(79)90219-7. [DOI] [PubMed] [Google Scholar]
- Weigel N., Kukuruzinska M. A., Nakazawa A., Waygood E. B., Roseman S. Sugar transport by the bacterial phosphotransferase system. Phosphoryl transfer reactions catalyzed by enzyme I of Salmonella typhimurium. J Biol Chem. 1982 Dec 10;257(23):14477–14491. [PubMed] [Google Scholar]


