Abstract
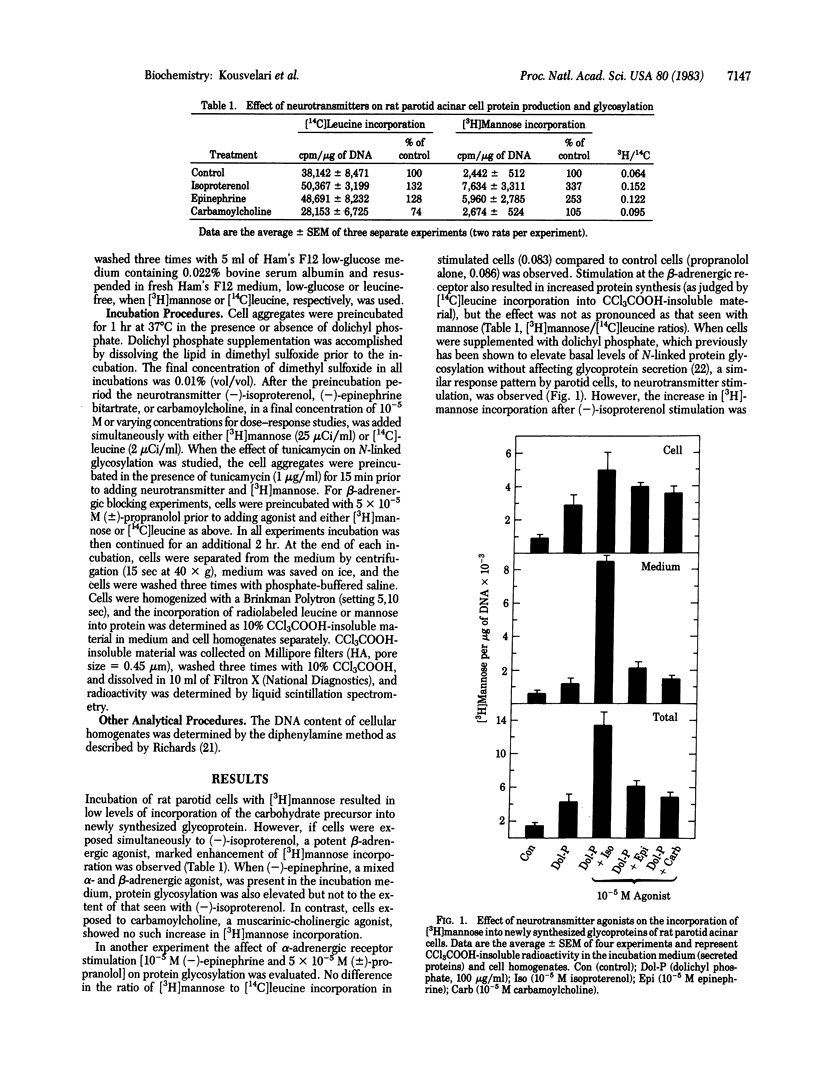
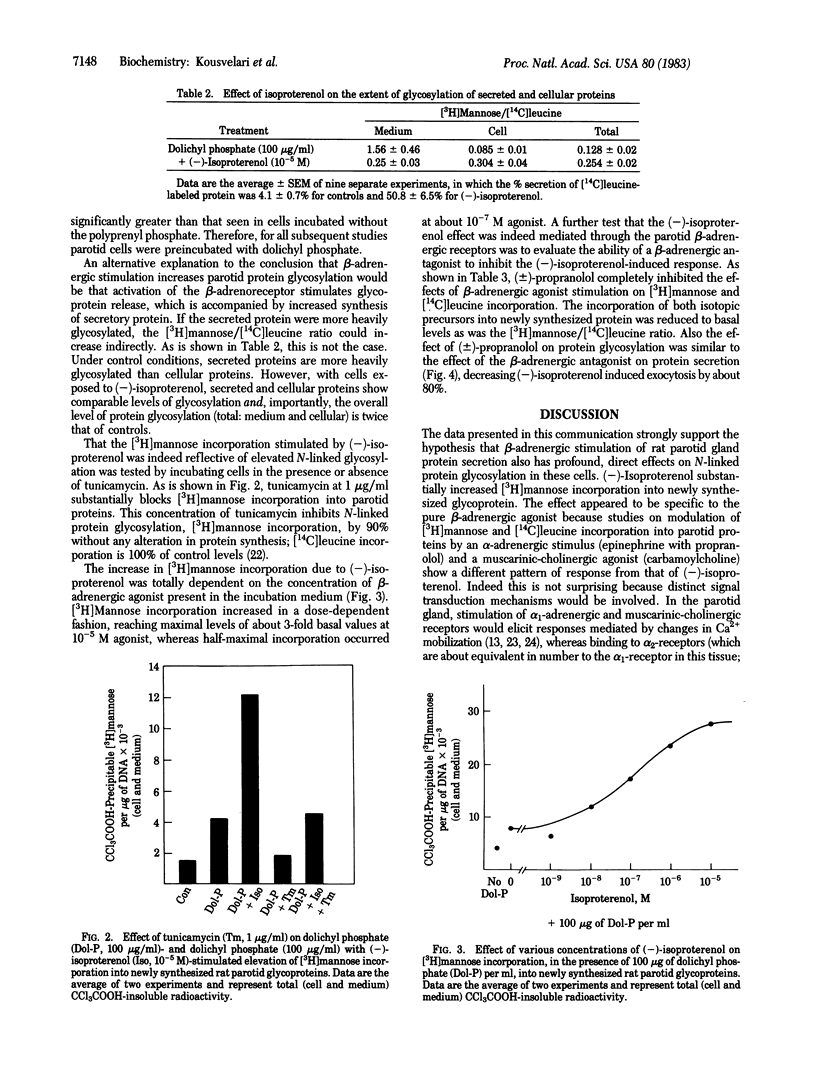
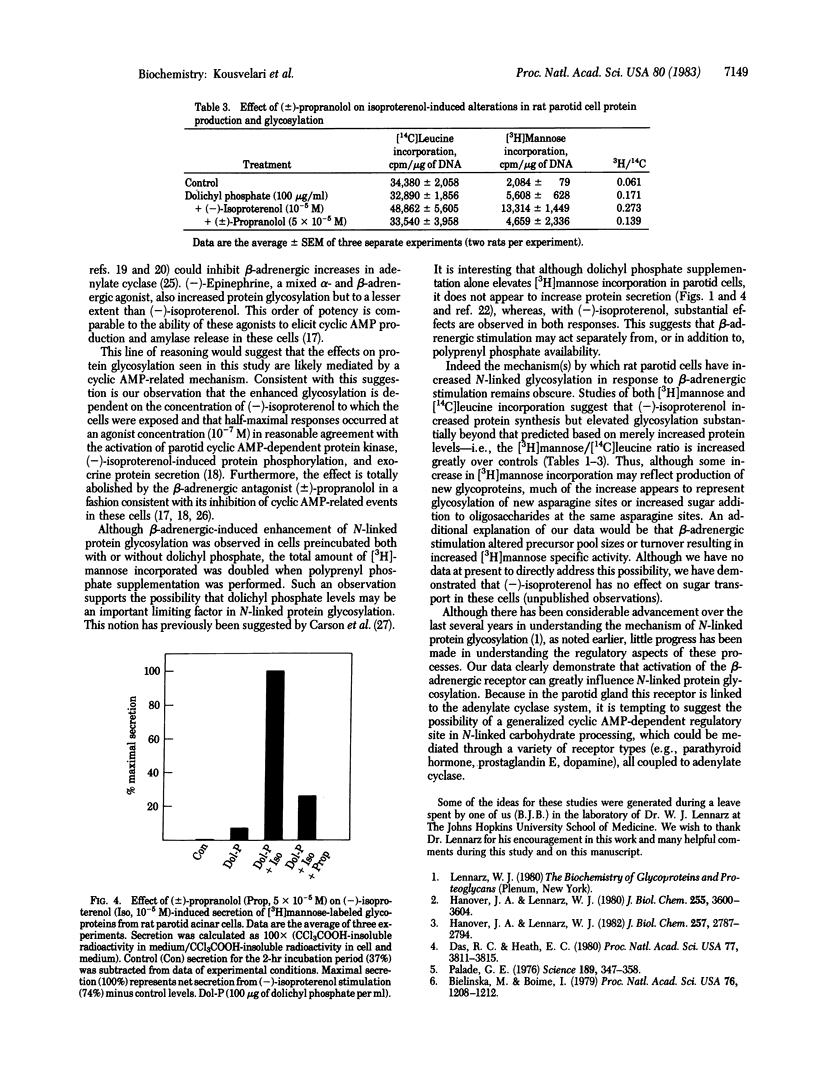
We have investigated the relationship between beta-adrenergic receptor stimulation and protein glycosylation and secretion in rat parotid gland cells in vitro. The potent beta-adrenergic agonist (-)-isoproterenol increases [3H]mannose incorporation into newly synthesized glycoproteins. This effect is enhanced if cells are first preincubated with dolichyl phosphate and is not observed after muscarinic-cholinergic or alpha-adrenergic stimulation of cells. The increase in [3H]mannose incorporation is abolished by incubation of cells with tunicamycin, suggesting that the glycosylation events being studied involved asparagine-linked oligosaccharides. The extent of increase in glycosylation is dependent on the concentration of (-)-isoproterenol to which cells are exposed. (+/-)-Propanolol totally abolishes the (-)-isoproterenol-induced increase in [3H]mannose incorporation, in a manner similar to its effects on exocrine secretion. Our findings suggest that beta-adrenergic receptor activation has a profound influence on N-linked protein glycosylation in rat parotid cells in addition to eliciting exocrine protein release.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baum B. J., Freiberg J. M., Ito H., Roth G. S., Filburn C. R. beta-Adrenergic regulation of protein phosphorylation and its relationship to exocrine secretion in dispersed rat parotid gland acinar cells. J Biol Chem. 1981 Sep 25;256(18):9731–9736. [PubMed] [Google Scholar]
- Bielinska M., Boime I. Glycosylation of human chorionic gonadotropin in mRNA-dependent cell-free extracts: post-translational processing of an asparagine-linked mannose-rich oligosaccharide. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1208–1212. doi: 10.1073/pnas.76.3.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher F. R., Putney J. W., Jr Regulation of parotid gland function by cyclic nucleotides and calcium. Adv Cyclic Nucleotide Res. 1980;13:215–249. [PubMed] [Google Scholar]
- Carson D. D., Earles B. J., Lennarz W. J. Enhancement of protein glycosylation in tissue slices by dolichylphosphate. J Biol Chem. 1981 Nov 25;256(22):11552–11557. [PubMed] [Google Scholar]
- Das R. C., Heath E. C. Dolichyldiphosphoryloligosaccharide--protein oligosaccharyltransferase; solubilization, purification, and properties. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3811–3815. doi: 10.1073/pnas.77.7.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanover J. A., Lennarz W. J. N-Linked glycoprotein assembly. Evidence that oligosaccharide attachment occurs within the lumen of the endoplasmic reticulum. J Biol Chem. 1980 Apr 25;255(8):3600–3604. [PubMed] [Google Scholar]
- Hanover J. A., Lennarz W. J. Transmembrane assembly of N-linked glycoproteins. Studies on the topology of saccharide synthesis. J Biol Chem. 1982 Mar 25;257(6):2787–2794. [PubMed] [Google Scholar]
- Hickman S., Kornfeld S. Effect of tunicamycin on IgM, IgA, and IgG secretion by mouse plasmacytoma cells. J Immunol. 1978 Sep;121(3):990–996. [PubMed] [Google Scholar]
- Ito H., Baum B. J., Roth G. S. Beta-adrenergic regulation of rat parotid gland exocrine protein secretion during aging. Mech Ageing Dev. 1981 Feb;15(2):177–188. doi: 10.1016/0047-6374(81)90073-7. [DOI] [PubMed] [Google Scholar]
- Ito H., Baum B. J., Uchida T., Hoopes M. T., Bodner L., Roth G. S. Modulation of rat parotid cell alpha-adrenergic responsiveness at a step subsequent to receptor activation. J Biol Chem. 1982 Aug 25;257(16):9532–9538. [PubMed] [Google Scholar]
- Ito H., Hoopes M. T., Baum B. J., Roth G. S. K+ release from rat parotid cells: an alpha 1-adrenergic mediated event. Biochem Pharmacol. 1982 Feb 15;31(4):567–573. doi: 10.1016/0006-2952(82)90161-7. [DOI] [PubMed] [Google Scholar]
- Mandel I. D. Sialochemistry in diseases and clinical situations affecting salivary glands. Crit Rev Clin Lab Sci. 1980;12(4):321–366. doi: 10.3109/10408368009108733. [DOI] [PubMed] [Google Scholar]
- Oron Y., Kellogg J., Larner J. Stable cholinergic-muscarinic inhibition of rat parotid adenylate cyclase. FEBS Lett. 1978 Oct 15;94(2):331–334. doi: 10.1016/0014-5793(78)80969-7. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Biphasic modulation of potassium release in rat parotid gland by carbachol and phenylephrine. J Pharmacol Exp Ther. 1976 Aug;198(2):375–384. [PubMed] [Google Scholar]
- Putney J. W., Jr Muscarinic, alpha-adrenergic and peptide receptors regulate the same calcium influx sites in the parotid gland. J Physiol. 1977 Jun;268(1):139–149. doi: 10.1113/jphysiol.1977.sp011851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards G. M. Modifications of the diphenylamine reaction giving increased sensitivity and simplicity in the estimation of DNA. Anal Biochem. 1974 Feb;57(2):369–376. doi: 10.1016/0003-2697(74)90091-8. [DOI] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strous G. J., Lodish H. F. Intracellular transport of secretory and membrane proteins in hepatoma cells infected by vesicular stomatitis virus. Cell. 1980 Dec;22(3):709–717. doi: 10.1016/0092-8674(80)90547-4. [DOI] [PubMed] [Google Scholar]
- Struck D. K., Siuta P. B., Lane M. D., Lennarz W. J. Effect of tunicamycin on the secretion of serum proteins by primary cultures of rat and chick hepatocytes. Studies on transferrin, very low density lipoprotein, and serum albumin. J Biol Chem. 1978 Aug 10;253(15):5332–5337. [PubMed] [Google Scholar]
- Tabas I., Kornfeld S. Purification and characterization of a rat liver Golgi alpha-mannosidase capable of processing asparagine-linked oligosaccharides. J Biol Chem. 1979 Nov 25;254(22):11655–11663. [PubMed] [Google Scholar]
- Tulsiani D. R., Hubbard S. C., Robbins P. W., Touster O. alpha-D-Mannosidases of rat liver Golgi membranes. Mannosidase II is the GlcNAcMAN5-cleaving enzyme in glycoprotein biosynthesis and mannosidases Ia and IB are the enzymes converting Man9 precursors to Man5 intermediates. J Biol Chem. 1982 Apr 10;257(7):3660–3668. [PubMed] [Google Scholar]



