Abstract
Significance: The role of immunonutrition in wound healing has been an area of both interest and controversy for many years. Although deficiencies in certain nutrients have long been known to impair healing, supplementation of specific immune modulating nutrients has not consistently yielded improvements in wound healing. Still, the prospect of optimizing nutrition to assist the immune system in wound repair bears great significance in both medical and surgical fields, as the costs of wound care and repair cannot be ignored.
Recent Advances: Recent studies have rekindled efforts to elucidate the roles of specific immunonutrients, and we now have a better understanding of the conditionally essential role of various nutrients such as arginine, which becomes essential in certain clinical situations such as for the trauma patient or patients at high risk for malnutrition. Immunonutrition in its current formulation usually includes supplementation with arginine, glutamine, omega-3 fatty acids, vitamins, and trace minerals, and its use has often been associated with decreased infectious complications and sometimes with improvements in wound healing.
Critical Issues: A key to understanding the role of immunonutrition in wound healing is recognizing the distinct contributions and importance of the various elements utilized.
Future Directions: Critical areas for future study include identifying the specific populations, timing, and ideal composition of immunomodulating diets in order to optimize the wound healing process.

Adrian Barbul, MD, FACS
Scope and Significance
This review outlines our current knowledge of immunonutrition and its role in wound healing. In addition, the future direction of research and potential areas of innovation will be discussed.
Translational Relevance
The role of immunonutrition in wound healing stems from the physiologic role of specific nutrients. A significant portion of the research that provided a foundation for our current use of immunonutrition was performed in the 1990s as scientists began defining the roles of specific immunological processes in the natural sequence of wound healing. This continues to the present date with studies evaluating the molecular interactions and processes that contribute to the wound healing sequence.
Clinical Relevance
Immunonutrition is already known to decrease wound complications, and there is evidence which suggests that in certain circumstances, it improves the process and efficiency of wound healing. Optimizing nutrition and providing immunonutrients in the correct settings may ultimately provide improved clinical outcomes with regard to decreased wound complications, decreased duration of wound healing, and lower clinical costs associated with wound care.
Background
Overview
Immunonutrition can be defined as the usage of specific nutritional elements in an attempt to modulate the immune system in a way that benefits a certain injury or disease state. In recent years, a number of studies and reviews have evaluated the role of immunomodulating diets (IMDs) or their components in wound healing. Many variations in formulation and route of administration have been used with mixed results. Although most clinicians agree that the presence of an “ideal” nutritional environment, as yet incompletely defined, is beneficial to the process of wound healing, the nature of this environment and how it should be created is still a matter of significant debate. Most of the literature on immunonutrition explores its impact on a wide variety of important clinical outcomes (ventilator times, hospital stays, rates of infection, and mortality), in which the immune response plays a pivotal role. Research on how immunonutrition affects wound healing has often been extrapolated from or interposed with studies looking at these other clinical outcomes.
Methods
We performed a search of the MEDLINE, CINAHL (Cumulate Index to Nursing and Allied Health Literature), and Cochrane Library databases, and reviewed the English language literature of human and animal studies pertaining to immunonutrition and wound healing. MeSH terms used included Immunomodulation, Dietary Supplements, Wound Healing, Glutamine, Arginine, Vitamins, Fatty Acids, Selenium, Zinc, and Antioxidants. Additional keywords included Immunonutrition. We also searched for relevant references using the works cited from existing published articles. No restrictions were made based on date of publication.
The immune system in wound healing
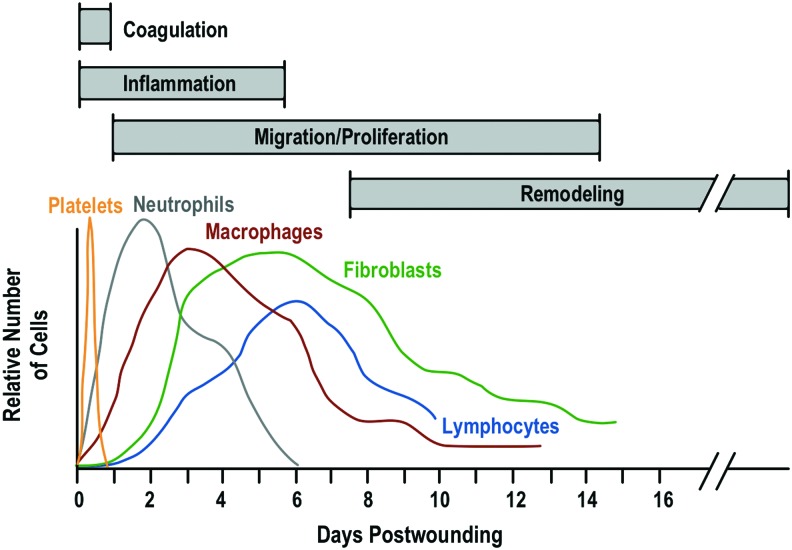
With each successive phase in the normal sequence of wound healing, the immune system orchestrates a wide variety of processes. Different immune cells have been shown to peak in number and activity at different times during the healing cascade, thus helping distinguish the different stages of wound healing (Fig. 1).1
Figure 1.
The time course of the different cells appearing in the wound during the healing process. Macrophages and neutrophils are predominant during inflammation, whereas lymphocytes peak somewhat later and fibroblasts are predominant during the proliferative phase. Adapted with permission from Witte and Barbul.1 To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
As platelets aggregate to provide hemostasis to the injured area, they release chemoattractants that draw inflammatory cells toward the area of injury. As neutrophils arrive, they begin clearing bacteria and cellular debris by phagocytosis. Subsequently, the macrophages predominate, both numerically and functionally, and release essential growth factors and chemoattractants that signal fibroblasts to migrate into the injured area. Fibroblasts then carry the wound through the proliferative and remodeling phase, synthesizing collagen and releasing growth factors that facilitate epithelialization, angiogenesis, and granulation.
Each of these cell populations and the complex signaling pathways that regulate these stages of wound healing depend on an intact and operational immune system. T lymphocytes comprise another population of inflammatory/immune cells that routinely invades the wound. Less numerous than macrophages, T-lymphocyte numbers peak at about 1 week post-injury and truly bridge the transition from the inflammatory to the proliferative phase of healing. Though known to be essential to wound healing, the lymphocytes' role in wound healing is not fully defined. Depletion of wound T lymphocytes decreases wound strength and collagen content, while selective depletion of the CD8+ suppressor subset of T lymphocytes enhances wound healing.2,3 Lymphocytes also exert a down-regulating effect on fibroblast collagen synthesis via several secreted lymphokines (interferon gamma, tumor necrosis factor alpha [TNF-α], and interleukin [IL]-1) and by cell-to-cell contact.
“Conditionally essential”
Our growing knowledge of the physiologic role of various nutritional elements has led to the recognition that certain nutrients, which have commonly been classified as nonessential, become essential in certain clinical situations; hence the term, “conditionally essential.” Indeed, most of the studies that have found benefit from immunonutrition have focused mainly on critical care populations, trauma victims, gastrointestinal cancer patients, or other groups at high risk for malnutrition.
A prime example of this is arginine, a non-essential amino acid, which is rapidly depleted during periods of severe stress and is utilized in the synthesis of collagen. It plays several roles which are important to immune function and wound healing that are further outlined next and, as such, becomes essential in these catabolic states even though it is usually abundant and nonessential in normal physiologic states.
Discussion
Immunonutrition, in its current formulation, includes supplementation with arginine and/or glutamine, omega-3 (ω-3) fatty acids, different vitamins, and trace minerals. The role of individual nutrients has been studied with varied degrees of thoroughness. As we review our current understanding of each of the following nutrients' role(s) in the process of wound healing, it becomes apparent that their precise mode of action and/or efficacy are still being elucidated.
Amino acids
Glutamine
Glutamine is the most abundant amino acid in the plasma and is a primary metabolic fuel for rapidly proliferating cells. Although utilized by immunologically active cells and cells that are involved in wound repair, glutamine supplementation has not been shown to benefit wound healing.
Glutamine supplementation decreases infectious complications, which is one of the clinical hallmarks of its use of immunonutrition. Glutamine protects against inflammatory injury by inducing the expression of heat shock proteins, which provide cellular protection in states of inflammation, injury, and stress.4,5 Furthermore, glutamine can modulate and preserve gut function, which is compromised in severe stress. Glutamine, besides being an important metabolic fuel, can also be considered an important nutritional mediator of the inflammatory response. When evaluating glutamine supplementation on wound healing specifically, numerous randomized trials have provided conflicting results. For example, oral supplementation of glutamine has been studied in treating oral mucositis for pediatric patients who received stem cell transplants. One study comparing glutamine supplementation with glycine (control) supplementation found an overall decreased severity of mucositis and a shorter course of analgesia and parenteral nutrition needed.6 Another study using a higher dose of enteral supplementation of glutamine did not show any benefit in terms of incidence or severity of mucositis in a population of pediatric oncology patients receiving chemotherapy.7
Although the benefits of glutamine supplementation have provided ample evidence to justify its inclusion in IMD formulations, there has not been consistent evidence supporting a direct benefit to wound healing. Its role in wound healing is one that is still under considerable debate.
Arginine
Arginine has numerous effects on immune function and wound healing. Metabolically, arginine is a precursor to proline, and is thus recruited specifically for collagen synthesis. It is also a precursor for ornithine, which is critical for polyamine synthesis, and for nitric oxide (NO, see below).
Numerous studies in rodents and humans show that supplemental arginine, administered either orally or parenterally, accelerates wound healing mainly by increasing collagen deposition in wounds.8–11 Not only is collagen accumulation increased by arginine supplementation, but peripheral blood lymphocytes show increased mitogenesis and activity as well. Although arginine improves epithelial reconstitution after intestinal injury,12 it has no effect on the re-epithelialization of skin graft donor sites in patients or volunteers, whether given orally or parenterally.13 However, in a randomized study evaluating the effect of different doses of arginine supplementation on stage II–IV pressure ulcers, there was almost a twofold improvement in healing time in the group receiving arginine.14
Arginine is the sole metabolic substrate for the synthesis of NO, which plays a role in wound collagen synthesis. NO is a small, short-lived free radical derived from l-arginine through the actions of three different isoforms of nitric oxide synthase (inducible, endothelial, and neuronal NOS). The expression and activity of inducible NOS is triggered by many of the inflammatory stimuli and cytokines present in the healing wound, leading to high levels of NO that peak during the early inflammatory phase and into the proliferative phase of wound healing. NO can react with oxygen to create reactive oxygen species, and it also regulates gene expression and cellular differentiation. Inhibition of NO synthesis in wounded animals results in weaker wounds and decreased collagen synthesis, while situations of impaired healing such as diabetes and malnutrition are associated with low wound NO levels.15
Arginine availability can affect the immune response in injured states and other disease processes, establishing its role as an immunonutrient. In clinical practice, arginine is included in most tube or parenteral feeding formulas.16 As a single agent, it is the best-studied component of immunonutrition, and the weight of evidence suggests that arginine is beneficial to wound healing.
Omega-3 fatty acids
Essential fatty acids play a major role in immune responses by altering the composition of cell membranes and modulating cell signaling. Arachidonic acid, a ω-6 fatty acid, is arguably the most important eicosanoid precursor to prostaglandins and leukotrienes. On the other hand, ω-3 fatty acids dampen inflammatory responses through their effects on eicosanoid production and specific cytokines.17 As an example, ω-3 fatty acids inactivate the nuclear factor kappa B signal transduction pathway, which mediates TNF-α production from macrophages exposed to lipopolysaccharide.18 Though understood to have anti-inflammatory function, a recent study using an acute blister model showed increased levels of pro-inflammatory cytokines in the blister fluid of volunteers supplemented with fish oil rich in ω-3 fatty acids. In addition, there was marginally longer blister closure time compared with subjects without fish oil supplementation.19
Early studies also suggested that ω-3 fatty acids have a detrimental effect on wound healing. Rats fed diets enriched with ω-3 fatty acids had significantly decreased wound tensile strength 30 days after injury, even though the levels of collagen were similar20; it was postulated that the quality and crosslinking of the collagen fibers were compromised by the ω-3 fatty acid supplementation.
ω-3 fatty acids may prompt faster resolution of inflammation within the wound microenvironment, which should create a situation more conducive to regeneration and re-epithelialization.21 A small recent randomized controlled trial evaluated a formula supplemented with fish oil on patients with pressure ulcers and noted decreased progression of pressure ulcers in those receiving fish oil supplementation.22
In conclusion, although the modulation of the inflammatory response by ω-3 fatty acid supplementation is clear, its overall effect on wound healing is not yet known.
Vitamins
Vitamin C (ascorbic acid)
Vitamin C deficiency results in scurvy, which has numerous cutaneous and wound manifestations due to its critical role in collagen formation and post-translational modification. It is a cofactor in the hydroxylation of proline and lysine residues in procollagen, which is vital for the strength and stability of collagen fibers. In addition, ascorbic acid enhances neutrophil function and acts as an antioxidant.
Although supplementation of non-deficient subjects does not improve wound healing, some studies have shown benefit from vitamin C supplementation after severe stress and/or injury. Mice supplemented with vitamin C had improved full thickness wound contraction time after radiation therapy along with increased collagen deposition and fibroblast numbers.23 In a prospective randomized controlled trial, surgical patients with pressure sores given large doses of ascorbic acid had a significant acceleration in the healing of the pressure sores.24 Overall, vitamin C supplementation has consistently shown benefit to wound healing.
Vitamin A
Vitamin A deficiency impairs wound healing. Vitamin A has multiple positive effects on wound healing even in non-deficient states. It increases collagen cross-linking and wound breaking strength. Vitamin A increases the inflammatory response in wounds through enhanced lysosomal membrane lability, increased macrophage influx, and activation and stimulation of collagen synthesis. Vitamin A increases the number of monocytes and macrophages at the wound site early in the inflammatory phase, facilitating epithelial cell differentiation.25 Importantly, it reverses corticosteroid-induced inhibition of cutaneous wound healing.26 Vitamin A supplementation facilitates wound healing.
Trace elements
Zinc
Zinc is a cofactor in a number of intracellular enzymatic reactions pertaining to wound healing. It is also an antioxidant and confers resistance against epithelial apoptosis; it even has significant antibacterial properties. Its use as a topical agent in the form of zinc oxide is widespread, such as in the Unna boot bandages used in venous stasis ulcerations. Oral or systemic supplementation of zinc does not improve wound healing except possibly in individuals with low serum levels.27 A recent Cochrane update determined that there is no benefit of oral zinc supplementation in patients with leg ulcerations.28 Thus, although zinc deficiency impairs wound healing and topical zinc has been shown to be of benefit for wound healing, zinc supplementation does not appear to improve wound healing.
Selenium
Selenium has strong antioxidant roles. There is some suggestion that it may expedite wound healing in burn patients. A prospective, randomized controlled trial investigated the effect of large intravenous doses of trace elements (copper, selenium, and zinc) on patients with major burns and found that cutaneous concentrations of those trace elements were increased, antioxidant status (as measured by normalization of plasma glutathione peroxidase level) was improved, and wound healing was benefited as measured by decreased graft requirement.29 The antioxidant properties of selenium benefit wound healing.
Iron
Iron serves as a cofactor in collagen synthesis. There are no studies which suggest that iron supplementation alone benefits wound healing in the absence of a severe deficiency in the host. There are some suggestions in the critical care literature, however, that iron supplementation moderates the immune response in inflammatory states and especially in the prolongation of inflammation seen in iron deficiency.30 At this time, there is no evidence that iron supplementation benefits wound healing.
Antioxidants
Many of the individual nutrients discussed earlier and others (vitamins A, C, E, zinc, selenium, and β-carotene) have roles either as antioxidants or as cofactors to antioxidants. Oxidative stresses, while vital to innate immune responses, can cause significant cellular damage, which can be detrimental to wound healing.
In a randomized double-blinded study, the role of supplementation with vitamin E, vitamin C, and zinc in 32 children was evaluated; the supplementation decreased the time of wound healing, which correlated with decreased oxidative stress.31 In a controlled trial of 20 trauma patients with wounds that were failing to heal after trauma or surgery, the provision of antioxidant micronutrients and glutamine for 2 weeks resulted in significantly reduced wound closure time.32
It appears that antioxidants have the capacity to expedite wound healing in certain clinical situations, but further studies are needed to elucidate the role of oxidative stress in wound healing.
Immunomodulating formulas
In recent years, the use of IMDs has increased, primarily in the critical care setting. The components vary among different formulations, but most contain a complement of vitamins, trace elements, and key amino acids, including arginine (Table 1).
Table 1.
Key components of commonly used immunomodulating formulas
| Impact | Immun-Aid | Perative | |
|---|---|---|---|
| Calories (kcal/L) | 1,000 | 1,000 | 1,300 |
| Protein (g/L) | 56 | 80 | 66.7 |
| Carbohydrate (g/L) | 130 | 120 | 180.3 |
| Fat (g/L) | 28 | 22 | 37.3 |
| ω-3 fatty acids (g/L) | 2 | 1.1 | 1.5 |
| Arginine (g/L) | 12.5 | 14 | 8 |
| Glutamine (g/L) | — | 12 | — |
| Vitamin C (mg/L) | 80 | 60 | 260 |
| Iron (mg/L) | 12 | 9 | 16 |
| Zinc (mg/L) | 15 | 26 | 20 |
| Selenium (mcg/L) | 100 | 100 | 61 |
| Copper (mg/L) | 1.7 | 2 | 1.8 |
Impact (Nestlé S.A., Vevey, Switzerland; www.nestle-nutrition.com); Immun-Aid (B Braun, Irvine, CA); Perative (Abbott Laboratories, Columbus, OH; www.abbottnutrition.com).
Wound complications and infections are decreased with the use of IMDs. In one metaanalysis of 22 studies, infectious complications were decreased with an odds ratio of 0.49.33 These findings confirm previous analyses on the use of IMDs in critically ill patients and patients with cancer, which also found decreased infectious complications in supplemented patients.34,35 However, few randomized trials have looked primarily at the effect of IMDs on wound healing. One of the few such trials studied 66 patients with gastric cancer who were randomized to receive early postoperative enteral immunonutrition (with arginine, ω-3 fatty acids, and RNA supplementation), and measured hydroxyproline deposition (as an index of deposited collagen) in a subcutaneously implanted catheter along with wound complication rates. Patients provided with the IMD had higher local hydroxyproline levels along with a significantly decreased incidence of wound complication compared with a control formula.36
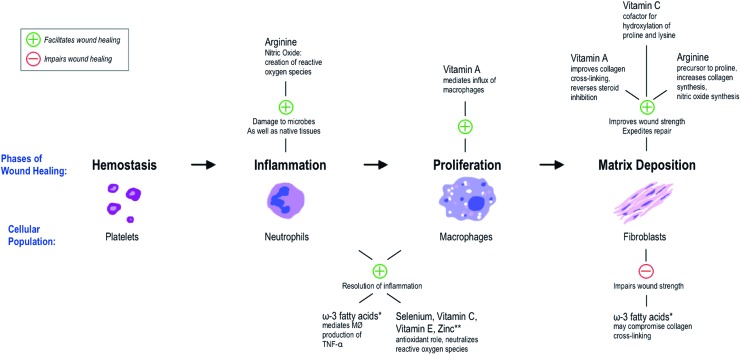
Although the mechanism by which IMDs affect wound healing is not entirely clear, a basic schema is offered to summarize the role of various immunonutrients on the process of wound healing (Fig. 2).
Figure 2.
Schema for the immunological role of various immunonutrients in wound healing. *Some studies suggest that the anti-inflammatory effects of ω-3 fatty acids are beneficial to wound healing, rather than detrimental, as shown (discussed further in text). **Other antioxidants and antioxidant cofactors play similar roles; these have been tested in trials as having an antioxidant effect mediating wound healing. MΦ, macrophage; TNF-α, tumor necrosis factor alpha. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Ideal patient population
Trauma patients have been the most commonly studied group to receive IMDs. The nature of their illness and recovery more often than any other group of patients represents an immediate challenge: They are generally in good health and nutritional status immediately before their traumatic event, but their injuries often result in malnutrition, septic complications, and prolonged recovery.37
Immunonutrition has also been studied in patients undergoing extirpative operations for upper gastrointestinal cancers and oropharyngeal cancers, both conditions notable for a high incidence of malnutrition and postoperative wound infections. IMDs have been found to be most effective in these populations at high risk for malnutrition and septic complications, which lends further support to the principle that certain nutrients are conditionally essential in populations under stress following trauma.
Ideal timing of immunonutritional intervention
The ideal timing and duration of utilizing immunomodulating feeding is not yet established. A randomized trial compared outcomes in patients undergoing surgery for gastrointestinal malignancies who received standard diets or 7 days of preoperative immunomodulating formula against another group receiving both preoperative and postoperative immunomodulating formula (perioperative group). A decreased incidence of complications was noted in the groups receiving immunomodulating formulas, with the best outcomes in the perioperative group.38
Similarly, another study examined patients undergoing elective major abdominal surgery for upper gastrointestinal malignancies, randomizing them to IMD starting 5 days before, 2 days before, or only after surgery. Each group was continued on the diet for 7 days after the surgery. C-reactive protein and IL-6 levels on postoperative day 3 were lower, and there were fewer infectious and noninfectious complications in the 5 day group.39
Hospital costs for patients receiving 5 days of preoperative immunonutrition were lower compared with conventional treatment in a clinical trial for patients with gastrointestinal cancer given that perioperative complications were higher in the conventional treatment group.40
Future Directions
A more recent development is a new focus on the effects of high doses of key nutrients as opposed to providing broader and higher volumes of supplementation, an effort called “pharmaconutrition.”41
A prospective, randomized clinical trial showed that early enteral supplementation with a regimen high in key immunonutrients was associated with a faster resolution of sequential organ failure assessment (SOFA) scores in critically ill patients.42 This subtle change in the field bears significance, as there are now a number of clinical trials that are close to completion, such as the REDOXS trial (Glutamine and antioxidant supplementation)43 and the GLINT trial (Glutamine supplementation alone)44, which are aimed at showing specific clinical outcome differences in the manipulation of levels of specific immunonutrients rather than an entire formula. Although this shift still focuses on mortality rather than wound healing specifically, it will be interesting to see what these new studies will reveal regarding the specific role of individual immunonutrients on wound healing or wound complications.
Take-Home Messages.
• Certain immunonutrients that are usually abundant and “non-essential” become conditionally essential in specific clinical situations.
• Glutamine decreases infectious complications but has not been shown to benefit wound healing directly.
• Arginine improves collagen accumulation and improves healing time.
• ω-3 fatty acids have been shown to both impair and benefit wound healing in different studies; its role in wound healing is still being elucidated.
• Supplementation of Vitamin A, C, and selenium benefit wound healing, partly through antioxidant mechanisms
• IMDs have been found to be most effective in populations at high risk for malnutrition and sepsis, such as trauma patients and patients with upper gastrointestinal malignancies.
• Perioperative immunonutrition has shown promising results in decreasing infectious complications, but wound healing has not yet been specifically evaluated.
Summary
Many advances have been made in the field of immunonutrition. The widespread adoption of immunomodulating diets is a testament to the clinical value of our progress in this field. With continued research, we can expect to see new discoveries and therapies that will further improve our ability to optimize wound healing.
Abbreviations and Acronyms
- IL-6
interleukin-6
- IMD
immunomodulating diet
- MΦ
macrophage
- NO
nitric oxide
- NOS
nitric oxide synthase
- TNF-α
tumor necrosis factor alpha
About The Authors
Oliver Chow, MD, is a third-year general surgery resident at New Jersey Medical School (UMDNJ) in Newark, NJ. Adrian Barbul, MD, FACS, is a board-certified surgeon, internationally recognized for his research and contributions to the fields of wound repair, healing, and gastrointestinal healing. In the course of his career, he has been a professor of surgery and plastic surgery at Johns Hopkins University School of Medicine in Boston, the Surgeon-in-Chief at the Sinai Hospital of Baltimore and the program director for its surgical residency, the Chair and Surgeon-in-Chief of the Department of Surgery at Hackensack University Medical Center, and the President of the Wound Healing Society. He has published more than 170 scientific articles, serves on the editorial board for more than 20 journals, and has mentored dozens of fellows and residents.
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
References
- 1.Witte MB. and Barbul A: General principles of wound healing. Surg Clin North Am 1997; 77:509. [DOI] [PubMed] [Google Scholar]
- 2.Peterson JM, Barbul A, Breslin RJ, Wasserkrug HL, and Efron G: Significance of T-lymphocytes in wound healing. Surgery 1987; 102:300. [PubMed] [Google Scholar]
- 3.Efron JE, Frankel HL, Lazarou SA, Wasserkrug HL, and Barbul A: Wound healing and T-lymphocytes. J Surg Res 1990; 48:460. [DOI] [PubMed] [Google Scholar]
- 4.Wischemeyer PE, Kahana M, Wolfson R, Ren H, Musch MM, and Chang EB: Glutamine induces heat shock protein and protects against endotoxin shock in the rat. J Appl Physiol 2001; 90:2403. [DOI] [PubMed] [Google Scholar]
- 5.Wischemeyer PE: Glutamine and heat shock protein expression. Nutrition 2002; 18:225. [DOI] [PubMed] [Google Scholar]
- 6.Aquino VM, Harvey AR, Garvin JH, Godder KT, Nieder ML, Adams RH, Jackson GB, and Sandler ES: A double-blind randomized placebo-controlled study of oral glutamine in the prevention of mucositis in children undergoing hematopoietic stem cell transplantation: a pediatric blood and marrow transplant consortium study. Bone Marrow Transplant 2005; 36:611. [DOI] [PubMed] [Google Scholar]
- 7.Ward E, Smith M, Henderson M, Reid U, Lewis I, Kinsey S, Allgar V, Bowers D, and Picton SV: The effect of high-dose enteral glutamine on the incidence and severity of mucositis in paediatric oncology patients. Eur J Clin Nutr 2009; 63:134. [DOI] [PubMed] [Google Scholar]
- 8.Barbul A, Lazarou S, Efron DT, Wasserkrug HL, and Efron G: Arginine enhances wound healing in humans. Surgery 1990; 108:331. [PubMed] [Google Scholar]
- 9.Barbul A, Sisto DA, Wasserkrug HL, Yoshimura NN, and Efron G: Metabolic and immune effects of arginine in post-injury hyperalimentation. J Trauma 1981; 21:970. [DOI] [PubMed] [Google Scholar]
- 10.Debats IB, Wolfs TG, Gotoh T, Cleutjens JP, Peutz-Kootstra CJ, and Van der Hulst RR: Role of arginine in superficial wound healing in man. Nitric Oxide 2009; 21:175. [DOI] [PubMed] [Google Scholar]
- 11.Kirk SJ, Hurson M, Regan MC, Holt DR, Wasserkrug HL, and Barbul A: Arginine stimulates wound healing and immune function in elderly human beings. Surgery 1993; 114:155. [PubMed] [Google Scholar]
- 12.Singh K, Coburn LA, Barry DP, Boucher J, Chaturvedi R, and Wilson KT: L-arginine uptake by cationic amino acid transporter 2 is essential for colonic epithelial cell restitution. Am J Physiol Gastrointest Liver Physiol 2012; 302:G1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Debats IB, Koeneman MM, Booi DI, Bekers O, and van der Hulst RR: Intravenous arginine and human skin graft donor site healing: a randomized controlled trial. Burns 2011; 37:420. [DOI] [PubMed] [Google Scholar]
- 14.Leigh B, Desneves K, Rafferty J, Pearce L, King S, Woodward MC, Brown D, Martin R, and Crowe TC: The effect of different doses of an arginine-containing supplement on the healing of pressure ulcers. J Wound Care 2012; 21:150. [DOI] [PubMed] [Google Scholar]
- 15.Schäffer MR, Tantry U, Thornton FJ, and Barbul A: Inhibition of nitric oxide synthesis in wounds: pharmacology and effect on accumulation of collagen in wounds in mice. Eur J Surg 1999; 165:262. [DOI] [PubMed] [Google Scholar]
- 16.Ueno C, Fukatsu K, Maeshima Y, Moriya T, Omata J, Saitoh D, and Mochizuki H: Arginine-enriched total parenteral nutrition improves survival in peritonitis by normalizing NFkappaB activation in peritoneal resident and exudative leukocytes. Ann Surg 2010; 251:959. [DOI] [PubMed] [Google Scholar]
- 17.Alexander JW: Immunonutrition: the role of omega-3 fatty acids. Nutrition 1998; 14:627. [DOI] [PubMed] [Google Scholar]
- 18.Novak TE, Babcock TA, Jho DH, Helton WS, and Espat NJ: NF-kappa B inhibition by omega-3 fatty acids modulates LPS-stimulated macrophage TNF-alpha transcription. Am J Physiol Lung Cell Mol Physiol 2003; 284:L84. [DOI] [PubMed] [Google Scholar]
- 19.McDaniel JC, Belury M, Ahijevych K, and Blakely W: Omega-3 fatty acids effect on wound healing. Wound Rep Reg 2008; 16:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albina JE, Gladden P, and Walsh WR: Detrimental effects of an omega-3 fatty acid-enriched diet on wound healing. JPEN 1993; 17:519. [DOI] [PubMed] [Google Scholar]
- 21.McDaniel JC, Massey K, and Nicolaou A: Fish oil supplementation alters levels of lipid mediators of inflammation in microenvironment of acute human wounds. Wound Rep Reg 2011; 19:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Theilla M, Schwartz B, Cohen J, Shaprio H, Anbar R, and Singer P: Impact of a nutritional formula enriched in fish oil and micronutrients on pressure ulcers in critical care patients. Am J Crit Care 2012; 21:102. [DOI] [PubMed] [Google Scholar]
- 23.Jagetia GC, Rajanikant GK, and Mallikarjun Rao KVN: Ascorbic acid increases healing of excision wounds of mice whole body exposed to different doses of γ-radiation. Burns 2007; 33:484. [DOI] [PubMed] [Google Scholar]
- 24.Taylor TV, Rimmer S, Day B, Butcher J, and Dymock IW: Ascorbic acid supplementation in the treatment of pressure-sores. Lancet 1974; 2:544. [DOI] [PubMed] [Google Scholar]
- 25.Levenson SM, Gruber CA, Rettura G, Gruber DK, Demetriou AA, and Seifter E: Supplemental vitamin A prevents the acute radiation-induced defect in wound healing. Ann Surg 1984; 200:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunt TK, Ehrlich HP, Garcia JA, and Dunphy JE: Effect of vitamin A on reversing the inhibitory effect of cortisone on healing of open wounds in animals and man. Ann Surg 1969; 170:633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilkinson EA. and Hawke CI: Does oral zinc aid the healing of chronic leg ulcers? A systematic literature review. Arch Dermatol 1998; 134:1556. [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson EA: Oral zinc for arterial and venous leg ulcers. Cochrane Database Syst Rev 2012; 8:CD001273. [DOI] [PubMed] [Google Scholar]
- 29.Berger MM, Baines M, Raffoul W, Benathan M, Chiolero RL, Reeves C, Revelly JP, Cayeux MC, Sénéchaud I, and Shenkin A: Trace element supplementation after major burns modulates antioxidant status and clinical course by way of increased tissue trace element concentrations. Am J Clin Nutr 2007; 85:1293. [DOI] [PubMed] [Google Scholar]
- 30.Muñoz M, Romero A, Morales M, Campos A, Garcia-Erce JA, and Ramirez G: Iron metabolism, inflammation and anemia in critically ill patients. A cross-sectional study. Nutr Hosp 2005; 20:115. [PubMed] [Google Scholar]
- 31.Barbosa E, Faintuch J, Machado Moreira EA, Goncalves da Silva VR, Lopes Pereima MJ, Martins Fagundes RL, and Filho DW: Supplementation of vitamin E, vitamin C, and zinc attenuates oxidative stress in burned children: a randomized, double-blind, placebo-controlled pilot study.J Burn Care Res 2009; 30:859. [DOI] [PubMed] [Google Scholar]
- 32.Blass SC, Goose H, Tolba RH, Stoffel-Wagner B, Kabir K, Burger C, Stehle P, and Ellinger S: Time to wound closure in trauma patients with disorders in wound healing is shortened by supplements containing antioxidant micronutrients and glutamine: a PRCT. Clin Nutr 2012; 31:469. [DOI] [PubMed] [Google Scholar]
- 33.Marik PE. and Zaloga GP: Immunonutrition in high-risk surgical patients: a systematic review and analysis of the literature. JPEN J Parenter Enteral Nutr 2010; 34:378. [DOI] [PubMed] [Google Scholar]
- 34.Heys SD, Walker LG, Smith I, and Eremin O: Enteral nutritional supplementation with key nutrients in patients with critical illness and cancer: a meta-analysis of randomized controlled clinical trials. Ann Surg 1999; 229:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heyland DK, Novak F, Drover JW, Jain M, Su X, and Suchner U: Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA 2001; 286:944. [DOI] [PubMed] [Google Scholar]
- 36.Farreras N, Artigas V, Cardona D, Rius X, Trias M, and González JA: Effect of early postoperative enteral immunonutrition on wound healing in patients undergoing surgery for gastric cancer. Clin Nutr 2005; 24:55. [DOI] [PubMed] [Google Scholar]
- 37.McCowen KC. and Bistrian BR: Immunonutrition: problematic or problem solving? Am J Clin Nutr 2003; 77:764. [DOI] [PubMed] [Google Scholar]
- 38.Braga M, Gianotti L, Nespoli L, Radaelli G, and Di Carlo V: Nutritional approach in malnourished surgical patients: a prospective randomized study. Arch Surg 2002; 137:174. [DOI] [PubMed] [Google Scholar]
- 39.Giger U, Büchler M, Farhadi J, Berger D, Hüsler J, Schneider H, Krähenbühl S, and Krähenbühl L: Preoperative immunonutrition suppresses perioperative inflammatory response in patients with major abdominal surgery–a randomized controlled pilot study. Ann Surg Oncol 2007; 14:2798. [DOI] [PubMed] [Google Scholar]
- 40.Braga M. and Gianotti L: Preoperative immunonutrition: cost-benefit analysis. JPEN 2005; 29:S57. [DOI] [PubMed] [Google Scholar]
- 41.Jones NE. and Heyland DK: Pharmaconutrition: a new emerging paradigm. Curr Opin Gastroenterol 2008; 24:215. [DOI] [PubMed] [Google Scholar]
- 42.Beale RJ, Sherry T, Lei K, Campbell-Stephen L, McCook J, Smith J, Venetz W, Alteheld B, Stehle P, and Schneider H: Early enteral supplementation with key pharmaconutrients improves sequential organ failure assessment score in critically ill patients with sepsis: outcome of a randomized, controlled, double-blind trial. Crit Care Med 2008; 36:131. [DOI] [PubMed] [Google Scholar]
- 43.Heyland DK, Dhaliwal R, Day AG, Muscedere J, Drover J, Suchner U, Cook D, and Canadian Critical Care Trials Group: Reducing deaths due to oxidative stress (The REDOXS Study): rationale and study design for a randomized trial of glutamine and antioxidant supplementation in critically-ill patients. Proc Nutr Soc 2006; 65:250. [DOI] [PubMed] [Google Scholar]
- 44.Al Balushi RM, Paratz JD, Cohen J, Banks M, Dulhunty J, Roberts JA, and Lipman J: Effect of intravenous glutamine supplementation in trauma patients receiving enteral nutrition study protocol (GLINT Study): a prospective, blinded, randomised, placebo-controlled clinical trial. BMJ Open 2011; 1:2011. [DOI] [PMC free article] [PubMed] [Google Scholar]