Abstract
While some published research indicates a fairly high frequency of Intravenous (IV) medication errors associated with the use of smart infusion pumps, the generalizability of these results are uncertain. Additionally, the lack of a standardized methodology for measuring these errors is an issue. In this study we iteratively developed a web-based data collection tool to capture IV medication errors using a participatory design approach with interdisciplinary experts. Using the developed tool, a prevalence study was then conducted in an academic medical center. The results showed that the tool was easy to use and effectively captured all IV medication errors. Through the prevalence study, violation errors of hospital policy were found that could potentially place patients at risk, but no critical errors known to contribute to patient harm were noted.
Introduction
Several reports have suggested that medication errors occurring in the administration phase of the medication use process may now be the most frequent type of mistake occurring in hospitals1–3, Administration errors clearly are frequent and have considerable potential for injury. Among administration errors, intravenous (IV) medication errors have been identified to be the most dangerous, and can cause considerable patient harm4. The Association for the Advancement of Medical Instrumentation (AAMI) and the Food and Drug Administration (FDA) held the AAMI/FDA Infusion Device Summit in 2010 in part because of 56,000 reported incidents related to IV infusions, and the FDA has increased scrutiny of infusion safety because of these reports. Finding solutions to prevent IV medication errors represents a pressing priority.
Computerized patient infusion devices (called smart pumps or smart infusion pumps) that include features for administration error prevention and data collection represent transformational clinical tools that can greatly decrease the rate of IV medication errors in hospitals5. This technology provides medication error-reduction capabilities via programmed dose limit alerts with audio/visual feedback to staff regarding erroneous orders, improper dose calculations, and/or programming errors. These devices have become popular among acute care facilities with as many as 41% of U.S. hospitals already using smart infusion pumps6. Because of the potential for improvement, “smart” technology is now required in all new pumps being developed in the U.S. However, these devices have not always achieved their potential, and important IV errors still persist. For example, one study by Rothschild et al6 evaluated a very early version of one type of pump and found that although smart intravenous pumps with decision support capabilities had the capacity to intercept many dangerous medication errors and allowed detection of many errors that would have been extremely difficult to find through any other mechanism, smart pumps did not reduce the rate of serious medication errors. In their study the issues related to usage of smart pumps including alert overrides and violation of safety procedures7 prevented realization of the potential medication safety benefits. Further review of lessons learned during this research underscored a number of issues, including the importance of building a “culture of competence and safety among staff.” In addition, the investigators noted the importance of reviewing current practice issues and common errors and assessing the organizations readiness for adoption. They noted that the purchase of smart pump systems cannot be viewed as a “one-time” purchase. Institutions must maintain continuous and ongoing relationships and a dialogue with vendors as the technology upgrades occur8.
A more recent study conducted by Husch, et al8 assessed the frequency of intravenous medication errors and impact of potential smart infusion pump technology9 on the frequency of intravenous medication errors in Northwestern Memorial Hospital in Chicago using a rapid assessment approach. Even though it was done more recently and many of the device-related issues identified in the Rothschild study had been corrected, this research identified major safety issues related to the use of IV pumps including infusions without orders, infusions continuing after a discontinue order is written, wrong infusion rates, wrong dose, incomplete labeling (of bags and lines), incorrect or incomplete documentation, and concentration/weight, or other pump programming issues. In particular, they observed several errors associated with orders, documentation, labeling, and patient identification.
Yet, a significant challenge in evaluating the impact of smart pump technologies is the absence of good quality base line data regarding current error rates that can be used for quality improvement. Also, lack of standardized methodology for measuring these errors is still an issue.
To identify the key issues related to the use of smart pumps across a broad range of hospitals using different types of smart pump devices, we are conducting a multisite study using the general methodology described by Husch et al8, which allowed a rapid assessment, or prevalence study of the frequency and types of IV medication errors. To support standard data collection across all sites, we identified the need to develop an electronic data collection tool (an excel spreadsheet was used in the Husch’s study) to allow us to collect de-identified data spontaneously in multiple sites and aggregate data as a standardized form in an efficient manner.
Our object was to develop an observational tool to collect IV medication error data using a participatory design approach and then validate the tool by collecting data at our academic medical center. This study was conducted as part of our national wide smart pump project to investigate IV medication errors using smart pumps in multiple sites. In this paper, we focus on the iterative data collection tool development process and the findings at one academic medical center (AMC) in Northeastern United States.
Methods
Operational Definition of Medication Errors for Data Collection
Medication error is defined as an error occurring at any stage in the medication–use process including prescribing, transcribing, dispending, administering, or monitoring10. See Table 1.
Table 1.
Operational definition of medication errors for data collection.
| Error Type | Definition |
|---|---|
| 1. Wrong Dose | The same medication but the dose is different from the prescribed order. |
| 2. Wrong Rate | A different rate is displayed on the pump from that prescribed in the medical record. Also refers to weight based doses calculated incorrectly including using a wrong weight. |
| 3. Wrong Concentration | An amount of a medication in a unit of solution that is different from the prescribed order. |
| 4. Wrong Medication | A different fluid/medication as documented on the IV bag label is being infused compared with the order in the medical record. |
| 5. Known Allergy | Medication is prescribed/administered despite the patient had a known allergy to the drug. |
| 6. Omitted Medication | The medication ordered was not administered to a patient. |
| 7. Delay of Rate or Medication/Fluid Change | An order to change medication or rate not carried out within 4 hours of the written order per institution policy. |
| 8. No Rate Documented on Label | Applies both to items sent from the pharmacy and floor stocked items per institution policy. |
| 9. Incorrect Rate on Label | Rate documented on the medication label is different from that programmed into the pump. Applies both to items sent from the pharmacy and floor stocked items. |
| 10. Patient Identification Error | Patient either has no ID band on wrist or information on the ID band is incorrect. |
| 11. No Documented Order | Fluids/medications are being administered but no order is present in medical record. This includes failure to document a verbal order. |
Development of a Standardized Data Collection Form
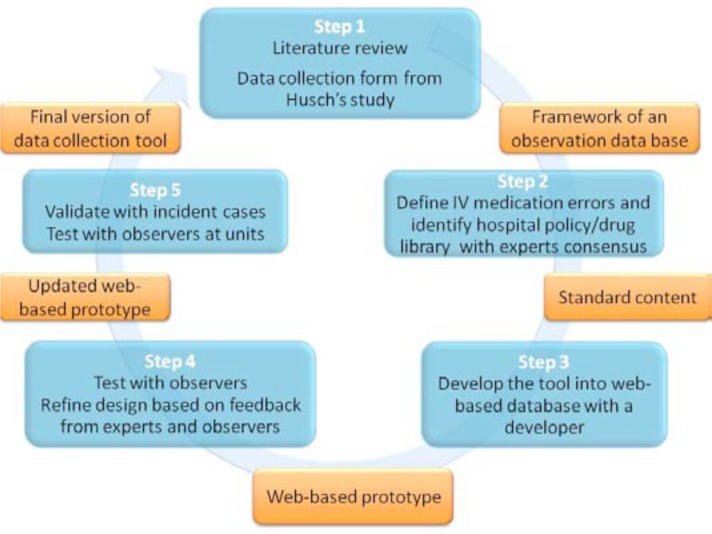
An iterative participatory software development process (see Figure 1) was used to develop a series of prototypes incorporating the expertise of multidisciplinary research team members including physicians, registered nurses, pharmacists and biomedical engineers, and, software developers. The tool supports classification of each error by type (See Table 1) for every medication administered. Multiple errors in a single administration can be recorded.
Figure 1.
An iterative participatory software development process.
The form was designed to classify the severity of each incident as significant, serious, and life threatening. The severity of each error is further classified into one of nine letter designated categories according to the NCC MERP index11 (see table 2) for categorizing medication errors (Table 1). The assigned severity is based on the potential for the error to result in patient harm if it had not been intercepted. This tool is designed to detect both medication errors and system inconsistencies or workarounds. The deviation between minor variations in standard practice and hospital policy may not be considered errors by busy clinicians.
Table 2.
NCC MERP harm index11.
|
Step 1: Designing Framework of an Observation Data Base
Through a literature review of smart pump research the different types of IV medication errors associated with smart pumps were identified. Drawing on previous observational IV medication error studies, a study design and requirements of a data observation tool was considered. Based on an observation sheet from the Husch’s study8, key data elements required to capture all kinds of IV medication errors were identified. Also, some lessons learned from previous Husch’s study8 were included in designing the framework for the observation database.
In this process, we considered utilizing a web-service, which allowed us to collect data online and gather data from all participating hospitals into a single online database to facilitate data collection associated with the multi-site study.
Step 2: Create Standard Content and Definitions of Errors
Based on the framework design, each data element and the different types of IV medication errors were defined and operationalized using definitions from the literature and refined among the expert team members. To improve data collection efficiency, a drug library at our hospital was implemented as a dropdown list and other participating hospitals reviewed and added additional medications as needed.
Step 3: Developing into Web-based Database with a Developer
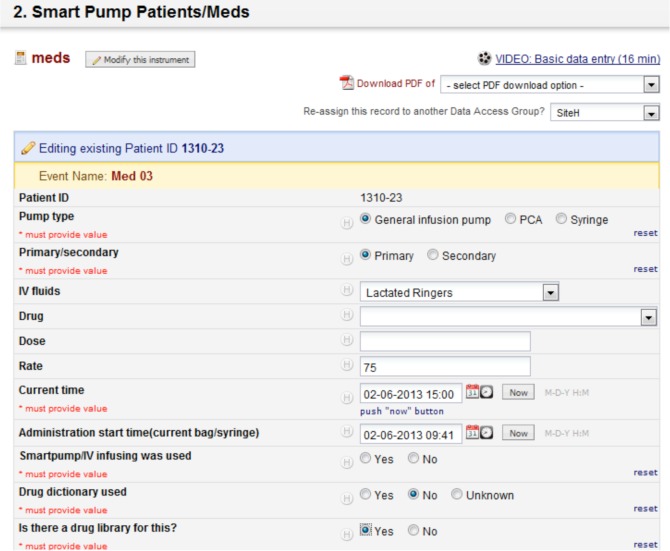
The data collection tool was developed using the REDCap application that allowed users to build and manage online surveys and databases quickly and securely12. This service is HIPAA compliant and has been used for many clinical research studies. Redcap is a web-based application and can be accessed from either PCs or mobile phones. To assist with data collection procedures, a drop down menu for medication name and concentrations/doses was implemented based on the master drug library that was created with the team. Also, each component question was a check box or a radio button to make it easy to collect all of the information needed and to identify medication errors in a proactive manner. All data components and UI designs were reviewed by the team and discussed until they reached a consensus on the final tool. Screen shots of the data collection tool are shown in Figure 2.
Figure 2.
Screen shots of the data collection tool (REDCap).
Step 4: Test and Refine the Tool
Through irretentive processes of refining the proto type tool, trained observers tested the tool in a clinical setting. All feedback from observers was used for further modification of the tool. Also, all modifications were reviewed by the project team and team members were involved in making a decision regarding tool refinements. This iterative, participatory development process continued until the observation tool was finalized.
Step 5: Data Collection Tool Validation
To identify whether this tool could adequately capture medication errors, two observers (registered nurses) went to a clinical unit and conducted observations on test patients. Several data could not be captured and based on that, the tool was modified during the tool validation phase. This process was repeated until data collection on a variety of cases could be recorded in the tool. Once the final version of the data collection tool was developed, observers collected data in clinical units and conducted an inter-rater reliability test. Minor modifications were made until usability met their suggestions.
Measurement of IV Medication Errors
Study Setting/Samples
This study was conducted at Brigham and Women’s Hospital (BWH), a 793-bed tertiary care AMC, in Boston, Massachusetts. Integrated closed loop medication management system including computerized physician order entry (CPOE) pharmacy barcode scanning, and bar-coding/electronic medication administration record (eMAR) has been in place since 2005 at BWH13. Three different units, including one medical ICU, one surgical ICU, and one general surgical unit were recruited for participating in the study. Two registered nurses were trained as observers and collected data on an electric data collection tool for one day per each unit. Inter-rater reliability testing was conducted before staring actual data collection sessions. All general infusion pumps, syringes, and patient controlled analgesia (PCA) except Patient-Controlled Epidural Analgesia (PCEA) on inpatient care units were included in the investigation. This study was approved by Partners Healthcare Human Subjects Committee. All data collected was de-identified in the Redcap application and did not include any protected health information (PHI).
Data Collection Procedure
On assigned inpatient units, using a prospective point prevalence approach, all data from IV mediations at patient bedside were collected on the standardized electronic form (Redcap). Observation nurses compared the infusing medication, dose, and infusion rate on the pump with the prescribed medication, dose, and rate in the medical record. All orders were obtained from electronic medical records and all IV fluids were considered medications. Presence of a correct patient identification (ID) band and name verification was recorded for each patient. Tubing and labeling of the infusing medication according to hospital policies were also assessed.
In order to confirm that an error was present, both observers had to agree that an error was made. If an error was identified that has the potential to cause harm, the staff nurse caring for that particular patient was discreetly informed so that it could be corrected as warranted. Each error was rated by National Coordinating Council for Medication Error Reporting Prevention (NCC MERP) INDEX11. Observers entered all data on the Redcap data collection tool.
Results
Frequency, Type and Potential Severity of Errors
During the data collection period 55 inpatients in 3 units were included in the study. During the data period, 181 medications were observed. Frequency, type and potential harm rating of errors were summarized Table 2. Of the observed medications, 171 (94.5%) had one or more errors associated with their administration Violations of hospital policies regarding labeling and tubing practices were the most frequent error types; 94.5% 44.8% respectively. Excluding these policy violation errors, 66 errors (36.5%) were identified.
Labeling Errors
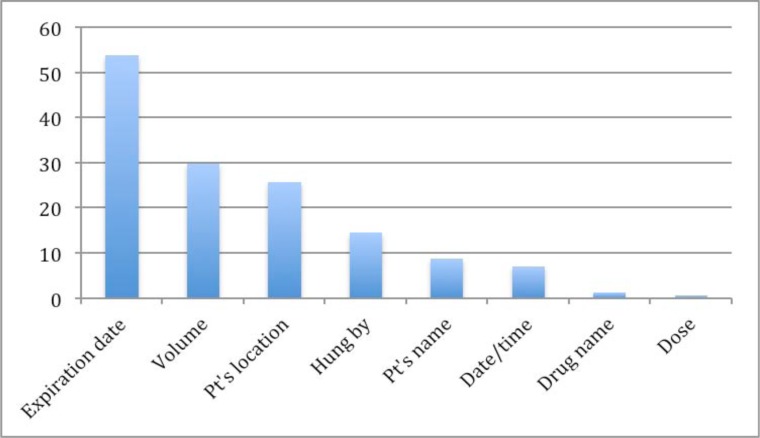
The most common errors were related to our hospital labeling policy. At BWH, “hospital label” is a label attached to each IV medication bag by nurses in the units. Meanwhile, “pharmacy label” is attached by a pharmacist before dispensing medications from the pharmacy department. According to the BWH hospital policy, hospital labels are required for all IV bags including any medications and electrolyte solutions. Fifty-two percent (52%) of IV medications did not have a label. Labels were missing on electrolyte solutions such as normal saline or dextrose solution but also on intravenous drugs including Potassium chloride, Cefepime, Magnesium sulfate, and Cancomycin. In 119 of the hospital labels that were attached, one or more required information categories were missing. With regard to the tubing labeling policy, BWH requires labeling all tubing with a color-coded tubing tag that includes a hand written date. However, either the tubing tag itself or the date was missing on 81 intravenous drugs. Missing information details on hospital labels are shown in Fig 3. The most frequent type of missing information on the hospital label was the expiration date, which was missing on over 25 % of all labels. The volume of medications and patient locations were often omitted. We found one label, which had an incorrect medication name on it.
Figure 3.
Missing information details on hospital labels.
Unauthorized Medication
Sixty-one (61) medications (33.7%) out of 181 did not have medication orders. Of these medications, 34 were normal saline and were hung to keep the vain open (KVO), 27 medications were not infusing and not connected to patients. For these cases, there were no active orders at the time of data collection. There were mostly discontinued medications but still connected to smart pumps (pumps were off). These medications had some contents left in the bags and left at the patient’s bedside. Only one case, lactated ringers was infusing without any active order. Also, a new bag, Ceftazidime was hanging at patient’s bedside but not spiked or connected to a patient. Later observers found that this was a discontinued medication but still left at patient bedside after the order was discontinued.
Pump Handling Errors
Observers found two clamped IV medication bags, which were supposed to be open. Both cases were connected through smart pumps, and all settings were correct. Also, the IV medications were documented as started infusing in the eMAR. Also, two pump setting errors were found; one had an incorrect rate and the other one was incorrect medication.
Patient Identification Errors
There were no errors with regard to adherence of using patient ID bands or identification errors.
Potential harms of errors
All errors we found fell into NCC MERP category “A” and “C”. Labeling and tubing errors were mostly rated as “C”. Some example error cases with potential harms are summarized in Table 3. Based on collected data, efficacy in error prevention was projected. Of these 66 errors, which are applicable for any hospital (excluding hospital specific policy violation errors), likelihood of preventability with smart pump technology was assessed. Any errors were rated unlikely to be prevented by in-use smart pump technology. The ratings were based on the assumption that the nurses would have used the smart pump technology to its fullest capacity.
Table 3.
Examples of errors with potential harms.
| Error case # | NCC MERP | Type of error | Medication and dose infusing via IV pump | Medical record order | Likelihood of preventability with smart pump technology |
|---|---|---|---|---|---|
| 1 | C | Clamp closed | Cefepime 2g/50mL, 16.7m/hr(observed at 9:49am) | Cefepime 2g/50mL, 16.7m/hr administered at 9:02am | No |
| 2 | C | Clamp closed | Phytonadione(vit K) 10mg/100mL, 50mL/hr(observed at 9:49am | Phytonadione(vit K) 10mg/100mL, 50mL/hr administered at 9:02am | No |
| 3 | C | Right meds programmed in correct channel /pump | 1/2Normal Saline 125mL/h, pump programmed as Normal Saline | 1/2Normal Saline 125mL/h | Yes(with closed loop smart pump) |
| 4 | C | Rate deviation | Normal Saline 5mL/h | Normal Saline 3mL/h | Yes(with closed loop smart pump) |
| 5 | C | Incorrect info on label | Heparin 25,000 unit / 250 mL, 1250units/hr 12.5mL/hr, drug name on a hospital label prepared by nurses was wrong(pharmacy label was correct) | Heparin 25,000 unit / 250 mL, 1250units/hr 12.5mL/hr | No |
| 6 | C | Unauthorized medication | Normal Saline 5mL/h | No order | No |
Discussion
In this prevalence study, we found that over 90% of IV medications had some type of error. The two most common errors; Labeling complete according to policy (94.5%) and Tubing tagged according to policy (44.8%), were both related to deviations from hospital policies. The definition of an administration error was expanded from programming errors to deviations from policies. The previous study recognized that this expanded definition would identify events that had the capacity to contribute to patient harm8. Our team agreed that it was important for us to include these policy violations to assess the potential harm of medication errors using smart pumps.
Labeling Errors
Missing hospital labels attached by nurses for medication (not electrolyte solutions) may increase the risk of administering wrong IV medications for a patient. Since nurses need to scan barcodes before hanging a medication in a smart pump, they will catch any error if they attempt to hang the wrong medications. However, there might be a chance to make mistakes due to not being able to identify whether the medication is for a specific patient visibly. Meanwhile, missing information on hospital labels would be a different situation. Expiration date on the hospital label was omitted over 50% of the time. Although the hospital policy requires including the expiration date, including this information might not be critical since all bags were processed through electronic Medication Administration Records (eMAR) systems and nurses can get information through eMAR when needed. Some medication labels (pharmacy labels) prepared by the pharmacy department do not require nurses to write the expiration date on their labels. Therefore, nurses may consider including the expiration date and other information on the label a low priority. Since volume and the drug’s name are already on the manufacturer’s packages or pharmacy labels, nurses may believe that adding information on hospital labels is duplicative. By the same token, the patient’s location and name tend to be omitted. Nurses need to obtain medications through auto-dispensing cabinets; the medications picked up through a cabinet were verified with patient information. Also, information about the time and the person who hang the meds are in the eMAR system as well. While these types of labeling practices made a lot of sense when manual and paper medication administration processes were in place, they may have less relevance when CPOE, eMAR, closed loop bar-coding and IV smart infusion pumps are in place. We found tubing tags were missing as well as labeling on medications. Tubing tags will not be able to replace any systems, even though integrated clinical systems are in place, we might still need to use these labels. The labeling policies and practices might need to be addressed through evaluation of the policy revision if needed, and then nursing education in the hospital. Therefore, a hospital may need to update its labeling policy to suit current nursing practices with various safety systems. Our study results may inform a new policy related to IV medication labels.
Unauthorized Medication Errors
We found many normal saline IVs running at a KVO rate did not have an order. According to hospital policy, normal saline is required to be ordered by a physician. However, we found that nurses commonly use normal saline to hang with a secondary bag without securing an order. This might not be a critical risk, however any medications should be going through the eMAR system to check whether the right medications are being hung before administration. A solution for these issues would be if normal saline for KVO is ordered as a set order along with secondly IV medications in eMAR, then administration processes would be safer and seamless. One case without any order was a nurse started infusion before a pharmacy approved an order therefore it was intended workaround. However, any workaround that is outside the closed loop system is unable to gain system benefits from the closed loop system. Other medications, which were rated as unauthorized medication, were either completed secondary bags hanging for a while or discontinued medications, which were also supposed to be taken down by nurses. This may have a capacity to cause errors or confusion if IV bags remain at the patient’s bedside for a long time. It will be important to familiarize the staff with how to take down IV bags once they are completed or discontinued through nursing education.
Pump Handling Errors
Compared with other errors, these pump handling error cases can be critical. In error case 1 and 2 in Table 3, the clamps were closed and medication was not being administered when observers came in the patient room. Observers intercepted this error and told the nurse, however, it was still after one hour of scheduled administering time. Therefore, these cases were not recorded as errors and we would not know what happened if observers did not intercept. Current smart pumps can detect primary flows but not secondary IV bag flow. Since this needs to be changed physically as a pump’s functionality, this could be a future improvement for smart pumps to prevent these kinds of errors.
In error case 3 and 4(in Table 3), both were human programming errors. A nurse chose a normal saline setting instead of half normal saline in a pump. Also, for normal saline infusion, the rate was set as 5ml instead of 3mL in the patient order. Both cases were not a critical error and there was no harm to the patients. However, these error cases imply that a nurse can choose the wrong drug library or type in the wrong dose for high-risk drugs. These “slip of the finger” type of errors can occur anytime by using a smart pump. We did not find any errors introduced by use of new technologies, which is a concern associated with using smart pump technologies14. Selecting a wrong item from a lengthy list or pushing a key to change a number of digits are common errors associated with smart pumps. In order to achieve maximum protection to these programming errors, smart pumps that seamlessly connected with order entry systems and/or eMAR will be able to achieve a meaningful improvement of reducing errors. These closed loop smart pumps would be a next generation advanced mart pumps to intercept errors which cannot be prevented errors, which cannot be prevented, by independent smart pumps from clinical systems. When all medication data including dose, concentration and rate can be retrieved from physician order on electronic patient records in real time, this will eliminate any typing and selecting errors of smart pump settings. Our results did not show many errors related to unintentional errors, however, any institution should encourage implementation of the closed loop system to intercept critical errors. In our institution, we have not used a closed loop smart pump yet but we consider implementing in the near future.
Compared with the findings reported from a previous study of smart infusion pumps in our institution (Rothschild’s study6), a number of medication errors were significantly reduced. The previous study was conducted in 2002, when our hospital just started implementing the smart pump. Their study results did not show a significant effect of smart pumps on decreasing errors, but the study identified issues related to the use of smart pumps. Their results showed that even though a drug library was available for a drug, about 25% of IV medications were bypassed intentionally or accidentally and typed in manually. Also, override alerts including the use of inappropriate boluses, contributed to not significant effects of the smart pumps on reduce presentable errors and adverse drug events. Our study found that 8% of medications were bypassed the pump library. All of these were only for electrolyte solutions and no potential harm was identified. Meanwhile, in the previous study Rothschild reported that 24.7% of medications, including heparin, intravenous anesthetics, and vasopressors bypassed the drug library. Based on our findings, it is safe to say that the compliance rate of using the drug library has dramatically improved in the past ten years.
Significant reductions in the medication error rate could be due to soft and hard limit functions. The early version of the smart pump did not provide a strict hard limit. This functionality is in place now and has prevented miscalculation dose errors or typing dose errors.
In a manner of speaking, it was no surprise that we had very low rate of errors since our institution has fully implemented the integrated closed loop clinical system. It is preventing errors in four phases of medication use: ordering, dispensing, transcription and administration phases14. However, there is still a gap in between existing bar-code eMAR and smart infusion pumps—in other words; smart infusion pump is not yet within the integrated closed loop system. Compared to previous studies at our institution, this may be one of the reasons why medication errors have been dramatically reduced. This study’s results also support the notion that integrated systems that are successfully implemented and utilized to get the full benefits of the safety system reduce the rate of errors. Including smart pumps into this closed loop would complete the safety loop to eliminate further errors.
Following the research results evaluating an early version of a smart pump in our institution, the hospital leadership has actively worked on improving patient safety. Behind these smart pump improvements, there has been a lot of effort in the past ten years to improve patient safety and nursing practice. As one example of improvement activities, our drug dictionary is reviewed by interdisciplinary committee members routinely and maintained up-to-date, evidence based practice. Also, based on incident reports pertaining to any medications errors using smart pumps, smart pump logs are assessed in the biomedical engineering department and either physical or mechanical pump issues, or human errors investigated. Reviewing reports by the patient safety committee makes changes to hospital policies or provide training around nursing practice routinely. Moreover, hospital leadership works with a smart pump vender to improve their products. These activities related to smart pumps in the past decade can contribute to safer practice than before.
Moreover, when comparing our results with the Husch study8, the error rates of our study were relatively small. The most significant difference was our study did not find any patient identification errors. The compliance of attaching patient ID band was 100%. This may be improved by now in that site which was an early adopter of the new smart pump technology at that time. In addition, rate deviation (9%) errors and incorrect medication errors (3%) were detected and these errors were rated as D–F in the NCC MARP harm index. In contrast, errors found in our study were rated from A to C. The severity of harms was greatly reduced.
The limitation of our study was that it was conducted as a point prevalence study and was not a long-term observation. It is not always true that the collected data reflects the practice on a single day. Since we will conduct the same observations in ten institutions, we chose a point prevalence study so that we can compare with multiple sites’ data as well as pre-post intervention data. Also, our sample size (N=55) was smaller than the sample used in both Rothschild’s (N=380) and Husch’s (N=486) studies. However, we did find various types of errors and identified trends of medication errors around use of smart pumps at our site before conducting larger sample size study.
As a next step of this study, we will conduct interviews/focus groups with nurses to identify why the error occurs or any specific reasons if the error was intentional. It is important to distinguish common errors and the issues associated with using smart pumps.
Also, the standard practice may be different from the hospital policy and this deviation can be clinically insignificant (i.e., rate of normal saline for keep vein open). Through further data analysis and qualitative data through interviews, we will investigate why this inconsistency occurred either due to misunderstanding of current policy or pump setting errors.
Our goal of this study will be to conduct a national-wide data collection in nine additional hospitals. Our vision of the research is to generalize the results and intervention plans. Once completed, we will have data from ten sites that may be applicable to other institutions since our sample includes not only AMCs but also community hospitals. By successfully capturing smart pump medication errors on our data collection tool at a range of facilities, we can further verify the feasibility of the data collection tool for using in multiple sites.
Conclusion
An electronic data collection tool was irrelatively developed using a participatory design process with interdisciplinary team members. The tool was validated in a clinical setting and was used to capture medication errors at an academic medical hospital. Although there were not many high-risk medication errors, violations of hospital policy for tubing tags and labeling on IV were identified. Information from this study can be used to help to improve safety of administration process, identify areas where improvements in policy and practice are needed. We found that the number and the severity of medication errors have dramatically decreased from rates reported in previous studies. Collecting the same data using the electronic data collection form will allow us to compare these findings across a broad range of hospitals.
Table 2.
Frequency, type and potential harm rating of errors.
| Type of error | # of errors | Frequency per medication observations(n = 181)* | NCC MERP severity rating | ||
|---|---|---|---|---|---|
| C | B | A | |||
| Label not completed according to policy | 171 | 94.5 | 171 | ||
| Tubing not tagged according to policy | 81 | 44.8 | 81 | 13 | |
| Unauthorized medication | 61 | 33.7 | 35 | 26 | |
| Clamp closed | 2 | 1.1 | 2 | ||
| Incorrect meds programmed in incorrect channel /pump | 1 | 0.6 | 1 | ||
| Rate deviation | 1 | 0.6 | 1 | ||
| Incorrect info on label | 1 | 0.6 | 1 | ||
| Incorrect medication | 0 | 0 | |||
| Delay of rate or medication change Total | 0 | 0 | |||
| Patient identification error | 0 | 0 | |||
|
| |||||
| Total | 318 | ||||
Percentages in this column do not add to 100 because some medications had multiple errors.
Acknowledgments
We acknowledge our national smart pump project team members, Marla M. Husch, RPh, Ray Maddox, PharmD, Deb Saine, MS, RPh, FASHP, Deborah Ariosto, PhD, RN, Diane Carroll, RN, Elizabeth Wade (Fang), PharmD, Moreen Donahue, DNP, RN, NEA-BC, FAAN, Nicole McDonald, PharmD for coordinating the study and develop the data collection tool. This study was conducted as part of nationalwide smart pumpstudy supported by Association for the Advancement of Medical Instrumentation (AAMI) and Carefusion Foundation in 2012–2015.
References
- 1.Runy L. The cause and effect of medication errors. Hosp Health Netw. 2004 Apr;:30. [PubMed] [Google Scholar]
- 2.Anon . A national database for hospital medication error reporting. Rockville, MD: United States Pharmacopeia; 2000. Summary of the 1999 information submitted to MEDMARX. [Google Scholar]
- 3.Hicks RW, Cousins DD, Williams RL. Summary of information submitted to MEDMARX in the year 2002: the quest for quality. Rockville, MD: United States Pharmacopeia; 2003. [Google Scholar]
- 4.Taxis K, Barber N. Ethnographic study of incidence and severity of intravenous drug errors. BMJ. 2003;326:684–7. doi: 10.1136/bmj.326.7391.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fields M, Peterman J. Intravenous medication safety system averts high-risk medication errors and provides actionable data. Nurs Adm Q. 2005;29(1):78Y87. doi: 10.1097/00006216-200501000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Ritter T. Perspectives from ECRI: Infusion pump error reduction. J Clin Eng. 2005 Apr-Jun;30(2):81–2. [Google Scholar]
- 7.Rothschild J, Keohane C, Cook E, Orav E, Burdick E. A controlled trial of smart infusion pumps to improve medication safety in critically ill patients. Crit Care Med. 2005;33(3):533Y540. doi: 10.1097/01.ccm.0000155912.73313.cd. [DOI] [PubMed] [Google Scholar]
- 8.Keohane CA, Hayes J, Saniuk C, Rothschild JM, Bates DW. Intravenous medication safety and smart infusion systems: lessons learned and future opportunities. J Infus Nurs. 2005 Sep-Oct;28(5):321–8. doi: 10.1097/00129804-200509000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Husch M, Sullivan C, Rooney D, Barnard C, Fotis M, Clarke J, Noskin G. Insights from the sharp end of intravenous medication errors: implications for infusion pump technology. Qual Saf Health Care. 2005 Apr;14(2):80–6. doi: 10.1136/qshc.2004.011957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bates DW, Boyle DL, Vander Vliet MB, et al. Relationship between medication errors and adverse drug events. J Gen Intern Med. 1995;10(4):199–205. doi: 10.1007/BF02600255. [DOI] [PubMed] [Google Scholar]
- 11.National Coordinating Council for Medication Error Reporting Prevention (NCC MERP). NCC MERP index for categorizing medication errors. Available at http://www.nccmerp.org (accessed Nov 9, 2011) [DOI] [PubMed]
- 12.Harris Paul A, Taylor Robert, Thielke Robert, Payne Jonathon, Gonzalez Nathaniel, Conde Jose G. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poon EG, Keohane CA, Yoon CS, Ditmore M, Bane A, Levtzion-Korach O, Moniz T, Rothschild JM, Kachalia AB, Hayes J, Churchill WW, Lipsitz S, Whittemore AD, Bates DW, Gandhi TK. Effect of bar-code technology on the safety of medication administration. N Engl J Med. 2010 May 6;362(18):1698–707. doi: 10.1056/NEJMsa0907115. [DOI] [PubMed] [Google Scholar]
- 14.Leape LL, Bates DW, Cullen DJ, et al. Systems analysis of adverse drug events: ADE Prevention Study Group. JAMA. 1995;274:35–43. [PubMed] [Google Scholar]
- 15.Ash JS. Unintended consequences of information technology. J Am Med Inform Assoc. 2004;11:104–12. doi: 10.1197/jamia.M1471. [DOI] [PMC free article] [PubMed] [Google Scholar]