Abstract
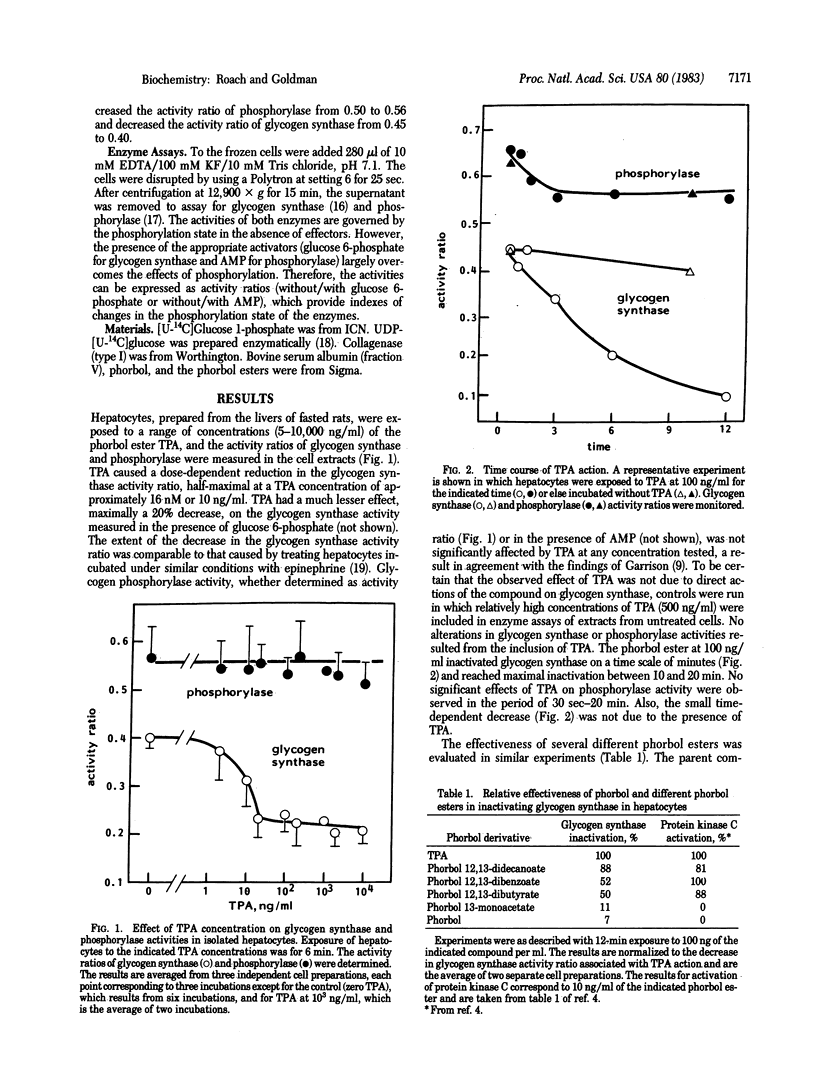
Glycogen synthase (UDPglucose:glycogen 4-alpha-D-glucosyltransferase, EC 2.4.1.11), in isolated rat hepatocytes, has been identified as a novel intracellular target for tumor-promoting phorbol esters such as phorbol 12-tetradecanoate 13-acetate (TPA). Exposure of hepatocytes to TPA resulted in a 50% decrease in the activity ratio of glycogen synthase without/with glucose 6-phosphate. The inactivation was dose dependent and was half-maximal at a TPA concentration of approximately 16 nM (10 ng/ml). Phorbol and phorbol 13-monoacetate, ineffective tumor promoters, had little influence on glycogen synthase activity. Other biologically active diesters, phorbol 12,13-didecanoate, phorbol 12,13-dibutyrate, and phorbol 12,13-dibenzoate, caused significant inactivation of glycogen synthase. Glycogen phosphorylase (1,4-alpha-D-glucan:orthophosphate alpha-D-glucosyltransferase, EC 2.4.1.1) activity, however, was unaffected by TPA or any of the tumor-promoting phorbol esters mentioned above. It is concluded that phorbol diesters can interact in the regulatory pathway for glycogen synthase, but the lack of effect on phosphorylase argues that distinct mechanisms can operate for the control of glycogen synthase and phosphorylase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad Z., DePaoli-Roach A. A., Roach P. J. Purification and characterization of a rabbit liver calmodulin-dependent protein kinase able to phosphorylate glycogen synthase. J Biol Chem. 1982 Jul 25;257(14):8348–8355. [PubMed] [Google Scholar]
- Blumberg P. M. In vitro studies on the mode of action of the phorbol esters, potent tumor promoters, part 2. Crit Rev Toxicol. 1981 Jun;8(3):199–234. doi: 10.3109/10408448109109658. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Diamond L., O'Brien T. G., Baird W. M. Tumor promoters and the mechanism of tumor promotion. Adv Cancer Res. 1980;32:1–74. doi: 10.1016/s0065-230x(08)60360-7. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Mechanisms involved in alpha-adrenergic effects of catecholamines on liver metabolism. J Cyclic Nucleotide Res. 1979;5(4):277–287. [PubMed] [Google Scholar]
- Geelen M. J., Beynen A. C., Christiansen R. Z., Lepreau-Jose M. J., Gibson D. M. Short-term effects of insulin and glucagon on lipid synthesis in isolated rat hepatocytes. Covariance of acetyl-CoA carboxylase activity and the rate of 3H2O incorporation into fatty acids. FEBS Lett. 1978 Nov 15;95(2):326–330. doi: 10.1016/0014-5793(78)81022-9. [DOI] [PubMed] [Google Scholar]
- Gilboe D. P., Larson K. L., Nuttall F. Q. Radioactive method for the assay of glycogen phosphorylases. Anal Biochem. 1972 May;47(1):20–27. doi: 10.1016/0003-2697(72)90274-6. [DOI] [PubMed] [Google Scholar]
- Hems D. A., Whitton P. D. Control of hepatic glycogenolysis. Physiol Rev. 1980 Jan;60(1):1–50. doi: 10.1152/physrev.1980.60.1.1. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Sawamura M., Hoshijima M., Fujikura T., Nishizuka Y. Synergistic functions of protein phosphorylation and calcium mobilization in platelet activation. J Biol Chem. 1983 Jun 10;258(11):6701–6704. [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Minakuchi R., Inohara S., Nishizuka Y. Calcium-activated, phospholipid-dependent protein kinase from rat brain. Subcellular distribution, purification, and properties. J Biol Chem. 1982 Nov 25;257(22):13341–13348. [PubMed] [Google Scholar]
- Kishimoto A., Mori T., Takai Y., Nishizuka Y. Comparison of calcium-activated, cyclic nucleotide-independent protein kinase and adenosine 3':5'-monophosphate-dependent protein kinase as regards the ability to stimulate glycogen breakdown in vitro. J Biochem. 1978 Jul;84(1):47–53. doi: 10.1093/oxfordjournals.jbchem.a132118. [DOI] [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne M. E., Soderling T. R. Calmodulin-dependent glycogen synthase kinase. J Biol Chem. 1980 Sep 10;255(17):8054–8056. [PubMed] [Google Scholar]
- Roach P. J., DePaoli-Roach A. A., Larner J. Ca2+-stimulated phosphorylation of muscle glycogen synthase by phosphorylase b kinase. J Cyclic Nucleotide Res. 1978 Aug;4(4):245–257. [PubMed] [Google Scholar]
- Roach P. J. Glycogen synthase and glycogen synthase kinases. Curr Top Cell Regul. 1981;20:45–105. doi: 10.1016/b978-0-12-152820-1.50006-7. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Strickland W. G., Blackmore P. F., Exton J. H. The role of calcium in alpha-adrenergic inactivation of glycogen synthase in rat hepatocytes and its inhibition by insulin. Diabetes. 1980 Aug;29(8):617–622. doi: 10.2337/diab.29.8.617. [DOI] [PubMed] [Google Scholar]
- Strickland W. G., Imazu M., Chrisman T. D., Exton J. H. Regulation of rat liver glycogen synthase. Roles of Ca2+, phosphorylase kinase, and phosphorylase a. J Biol Chem. 1983 May 10;258(9):5490–5497. [PubMed] [Google Scholar]
- Tan A. W. A simplified method for the preparation of pure UDP[14C] glucose. Biochim Biophys Acta. 1979 Feb 1;582(3):543–547. doi: 10.1016/0304-4165(79)90146-6. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Schlender K. K., Larner J. A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal Biochem. 1968 Oct 24;25(1):486–499. doi: 10.1016/0003-2697(68)90127-9. [DOI] [PubMed] [Google Scholar]
- Yamanishi J., Takai Y., Kaibuchi K., Sano K., Castagna M., Nishizuka Y. Synergistic functions of phorbol ester and calcium in serotonin release from human platelets. Biochem Biophys Res Commun. 1983 Apr 29;112(2):778–786. doi: 10.1016/0006-291x(83)91529-2. [DOI] [PubMed] [Google Scholar]


