Abstract
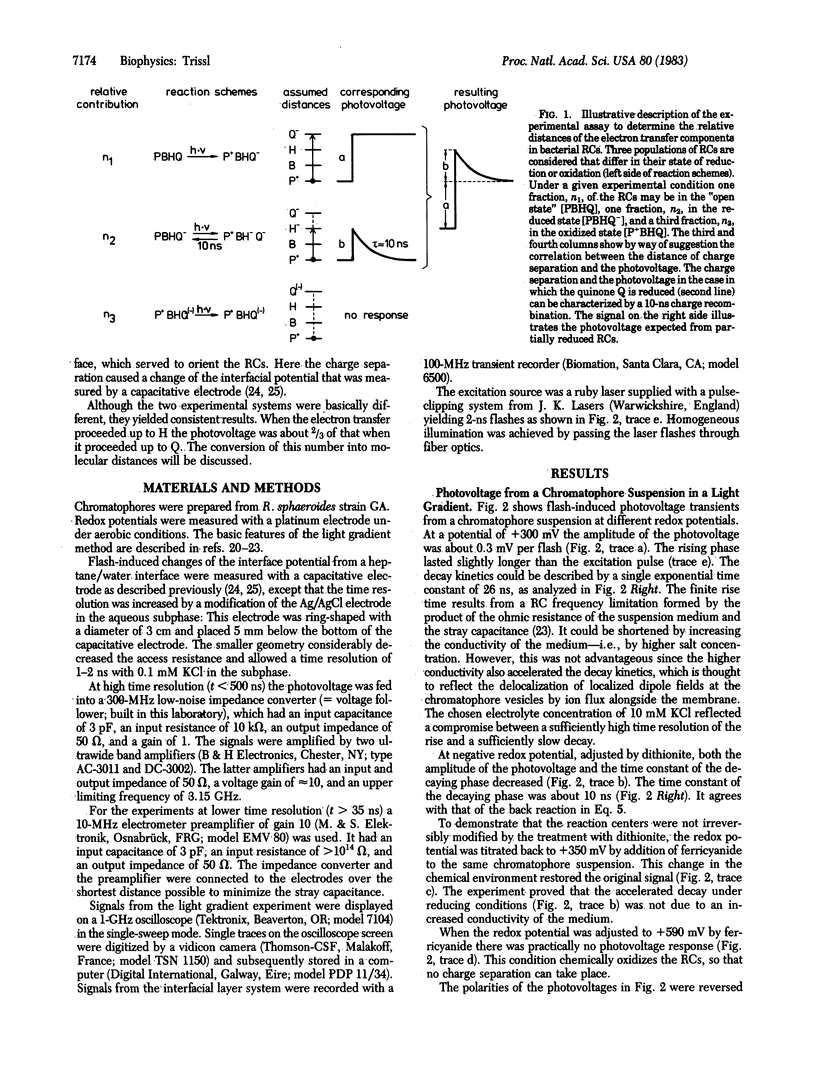
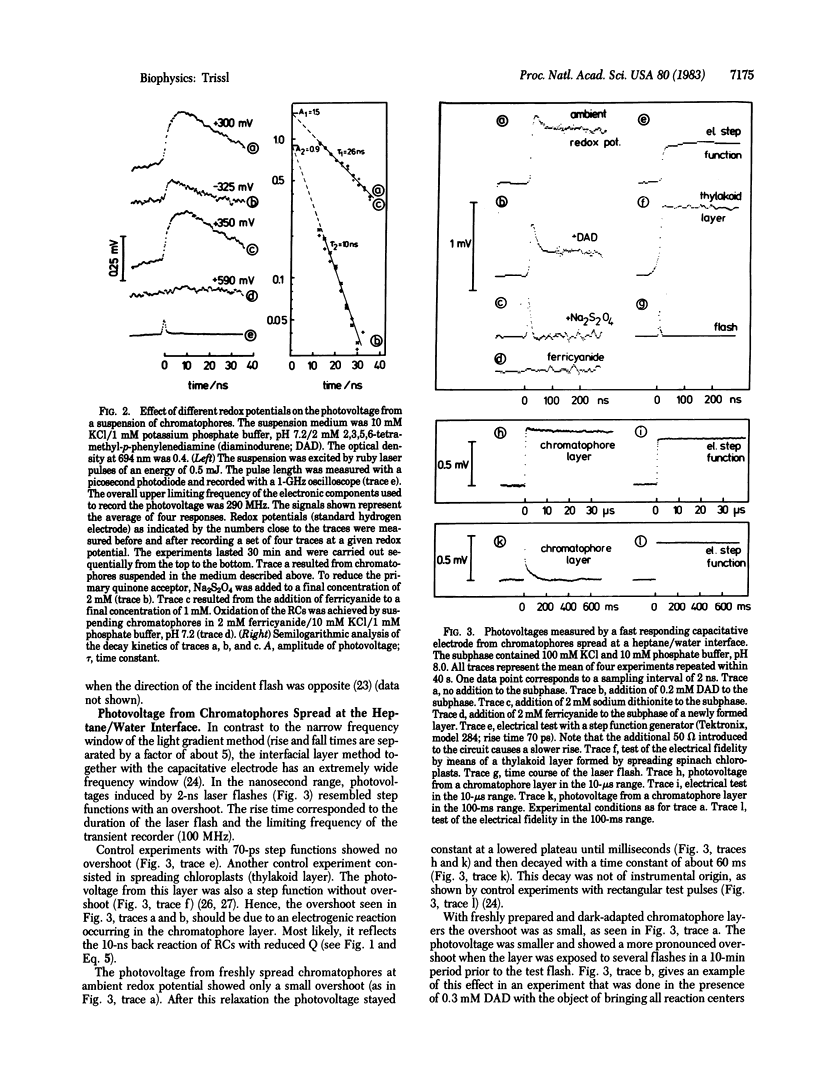
Relative distances between the the primary donor P, the intermediary pheophytin acceptor H, and the iron-quinone acceptor Q of bacterial reaction centers were determined by recording laser flash-induced photovoltages in two experimental systems with nanosecond time resolution. In one system a suspension of chromatophores was subjected to a light gradient and in the other system chromatophores were spread at a heptane/water interface. The 10-ns back reaction occurring in reaction centers with reduced Q could be time resolved. The initial photovoltage amplitude under conditions in which the charge separation proceeded up to the state [P+H-] was about ⅔ of that when it proceeded up to the state [P+HQ-]. If the amplitude of the photovoltage is considered to be proportional to the spatial displacement of charges, this result means that pheophytin lies closer to Q than to P.
Keywords: photosynthesis, charge separation, electron transfer, light gradient, capacitative electrode
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barouch Y., Clayton R. K. Ubiquinone reduction and proton uptake by chromatophores of Rhodopseudomonas sphaeroides R-26: periodicity of two in consecutive light flashes. Biochim Biophys Acta. 1977 Dec 23;462(3):785–788. doi: 10.1016/0005-2728(77)90120-7. [DOI] [PubMed] [Google Scholar]
- Clayton R. K., Yau H. F. Photochemical electron transport in photosynthetic reaction centers from Rhodopseudomonas spheroides. I. Kinetics of the oxidation and reduction of P-870 as affected by external factors. Biophys J. 1972 Jul;12(7):867–881. doi: 10.1016/S0006-3495(72)86130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogdell R. J., Monger T. G., Parson W. W. Carotenoid triplet states in reaction centers from Rhodopseudomonas sphaeroides and Rhodospirillum rubrum. Biochim Biophys Acta. 1975 Dec 11;408(3):189–199. doi: 10.1016/0005-2728(75)90122-x. [DOI] [PubMed] [Google Scholar]
- Feher G., Isaacson R. A., McElroy J. D., Ackerson L. C., Okamura M. Y. On the question of the primary acceptor in bacterial photosynthesis:manganese substituting for iron in reaction centers of Rhodopseudomonas spheroides R-26. Biochim Biophys Acta. 1974 Oct 18;368(1):135–139. doi: 10.1016/0005-2728(74)90104-2. [DOI] [PubMed] [Google Scholar]
- Fowler C. F., Kok B. Direct observation of a light-induced electric field in chloroplasts. Biochim Biophys Acta. 1974 Aug 23;357(2):308–318. doi: 10.1016/0005-2728(74)90069-3. [DOI] [PubMed] [Google Scholar]
- Godik V. I., Borisov A. Y. Short-lived delayed luminescence of photosynthesizing organisms. II. The ratio between delayed and prompt fluorescence as studied by the modulation method. Biochim Biophys Acta. 1980 Apr 2;590(2):182–193. doi: 10.1016/0005-2728(80)90023-7. [DOI] [PubMed] [Google Scholar]
- Holten D., Hoganson C., Windsor M. W., Schenck G. C., Parson W. W., Migus A., Fork R. L., Shank C. V. Subpicosecond and picosecond studies of electron transfer intermediates in Rhodopseudomonas sphaeroides reaction centers. Biochim Biophys Acta. 1980 Oct 3;592(3):461–477. doi: 10.1016/0005-2728(80)90092-4. [DOI] [PubMed] [Google Scholar]
- Holten D., Windsor M. W., Parson W. W., Thornber J. P. Primary photochemical processes in isolated reaction centers of Rhodopseudomonas viridis. Biochim Biophys Acta. 1978 Jan 11;501(1):112–126. doi: 10.1016/0005-2728(78)90100-7. [DOI] [PubMed] [Google Scholar]
- Jackson J. B., Dutton P. L. The kinetic and redox potentiometric resolution of the carotenoid shifts in Rhodopseudomonas spheroides chromatophores: their relationship to electric field alterations in electron transport and energy coupling. Biochim Biophys Acta. 1973 Oct 19;325(1):102–113. doi: 10.1016/0005-2728(73)90155-2. [DOI] [PubMed] [Google Scholar]
- Kaufmann K. J., Dutton P. L., Netzel T. L., Leigh J. S., Rentzepis P. M. Picosecond kinetics of events leading to reaction center bacteriochlorophyll oxidation. Science. 1975 Jun 27;188(4195):1301–1304. doi: 10.1126/science.188.4195.1301. [DOI] [PubMed] [Google Scholar]
- Kuznetsov A. M., Ulstrup J. The effect of temperature and transmembrane potentials on the rates of electron transfer between membrane-bound biological redox components. Biochim Biophys Acta. 1981 Jun 12;636(1):50–57. doi: 10.1016/0005-2728(81)90074-8. [DOI] [PubMed] [Google Scholar]
- Okamura M. Y., Isaacson R. A., Feher G. Primary acceptor in bacterial photosynthesis: obligatory role of ubiquinone in photoactive reaction centers of Rhodopseudomonas spheroides. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3491–3495. doi: 10.1073/pnas.72.9.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parson W. W., Clayton R. K., Cogdell R. J. Excited states of photosynthetic reaction centers at low recox potentials. Biochim Biophys Acta. 1975 May 15;387(2):265–278. doi: 10.1016/0005-2728(75)90109-7. [DOI] [PubMed] [Google Scholar]
- Rockley M. G., Windsor M. W., Cogdell R. J., Parson W. W. Picosecond detection of an intermediate in the photochemical reaction of bacterial photosynthesis. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2251–2255. doi: 10.1073/pnas.72.6.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuvalov V. A., Klevanik A. V., Sharkov AV J. u., Matveetz A., Krukov P. G. Picosecond detection of BChl-800 as an intermediate electron carrier between selectively-excited p870 and bacteriopheophytin in Rhodospirillum rubrum relaction centers. FEBS Lett. 1978 Jul 1;91(1):135–139. doi: 10.1016/0014-5793(78)80034-9. [DOI] [PubMed] [Google Scholar]
- Shuvalov V. A., Klimov V. V. The primary photoreactions in the complex cytochrome-P-890-P-760 (bacteriopheophytin760) of Chromatium minutissimum at low redox potentials. Biochim Biophys Acta. 1976 Sep 13;440(3):587–599. doi: 10.1016/0005-2728(76)90044-x. [DOI] [PubMed] [Google Scholar]
- Shuvalov V. A., Parson W. W. Energies and kinetics of radical pairs involving bacteriochlorophyll and bacteriopheophytin in bacterial reaction centers. Proc Natl Acad Sci U S A. 1981 Feb;78(2):957–961. doi: 10.1073/pnas.78.2.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C., Parson W. W., Mauzerall D. C., Clayton R. K. Pigment content and molar extinction coefficients of photochemical reaction centers from Rhodopseudomonas spheroides. Biochim Biophys Acta. 1973 Jun 28;305(3):597–609. doi: 10.1016/0005-2728(73)90079-0. [DOI] [PubMed] [Google Scholar]
- Tiede D. M., Leigh J. S., Dutton P. L. Structural organization of the Chromatium vinosum reaction center associated c-cytochromes. Biochim Biophys Acta. 1978 Sep 7;503(3):524–544. doi: 10.1016/0005-2728(78)90151-2. [DOI] [PubMed] [Google Scholar]
- Trissl H. W. Electrical responses to light: fast photovoltages of rhodopsin-containing membrane systems and their correlations with the spectral intermediates. Methods Enzymol. 1982;81:431–439. doi: 10.1016/s0076-6879(82)81060-4. [DOI] [PubMed] [Google Scholar]
- Trissl H. W., Gräber P. II. Electrical measurements in the nanosecond range of the charge separation from chloroplasts spread at a heptane-water interface. Application of a novel capacitive electrode. Biochim Biophys Acta. 1980;595(1):96–108. doi: 10.1016/0005-2736(80)90251-5. [DOI] [PubMed] [Google Scholar]
- Trissl H. W. I. Novel capacitative electrode with a wide frequency range for measurements of flash-induced changes of interface potential at the oil-water interface. Mechanical construction and electrical characteristics of the electrode. Biochim Biophys Acta. 1980;595(1):82–95. doi: 10.1016/0005-2736(80)90250-3. [DOI] [PubMed] [Google Scholar]
- Vermeglio A., Martinet T., Clayton R. K. Mode of inhibition of electron transport by orthophenanthroline in chromatophores and reaction centers of Rhodopseudomonas sphaeroides. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1809–1813. doi: 10.1073/pnas.77.4.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeglio A. Secondary electron transfer in reaction centers of Rhodopseudomonas sphaeroides. Out-of-phase periodicity of two for the formation of ubisemiquinone and fully reduced ubiquinone. Biochim Biophys Acta. 1977 Mar 11;459(3):516–524. doi: 10.1016/0005-2728(77)90050-0. [DOI] [PubMed] [Google Scholar]
- Warshel A., Schlosser D. W. Electrostatic control of the efficiency of light-induced electron transfer across membranes. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5564–5568. doi: 10.1073/pnas.78.9.5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt H. T., Zickler A. Electrical evidence for the field indicating absorption change in bioenergetic membranes. FEBS Lett. 1973 Dec 1;37(2):307–310. doi: 10.1016/0014-5793(73)80484-3. [DOI] [PubMed] [Google Scholar]
- Zankel K. L., Reed D. W., Clayton R. K. Fluorescence and photochemical quenching in photosynthetic reaction centers. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1243–1249. doi: 10.1073/pnas.61.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]


