Abstract
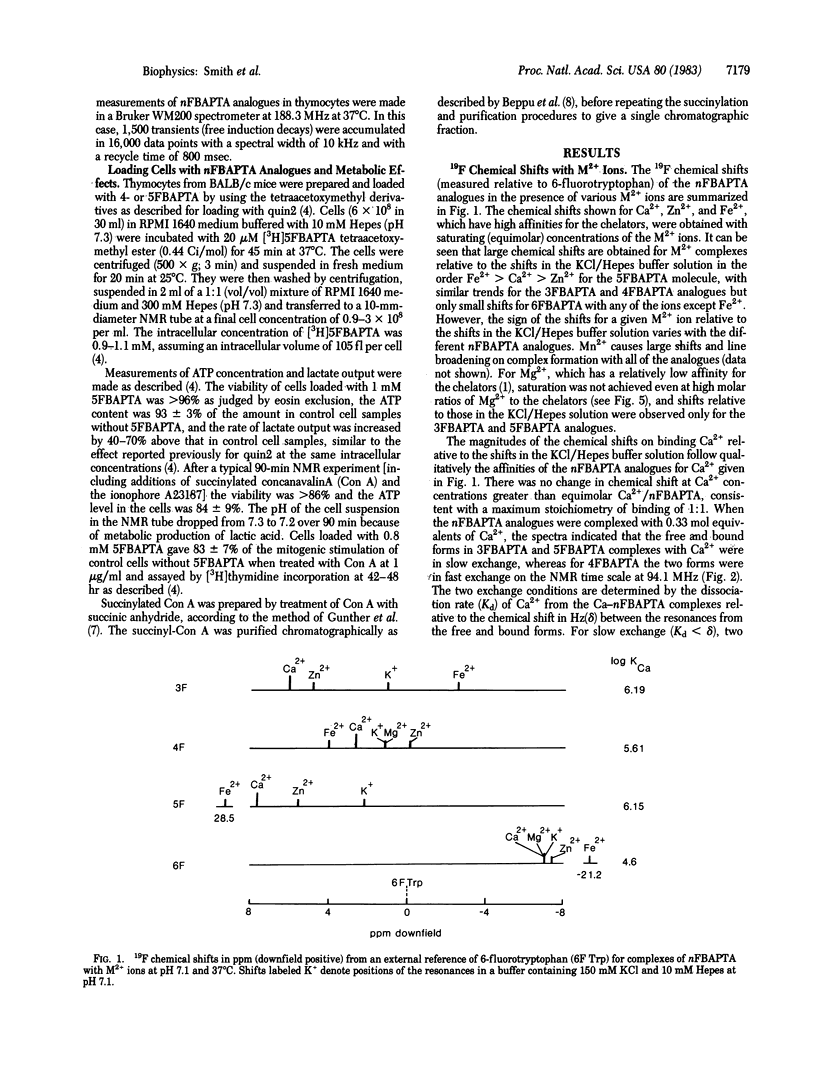
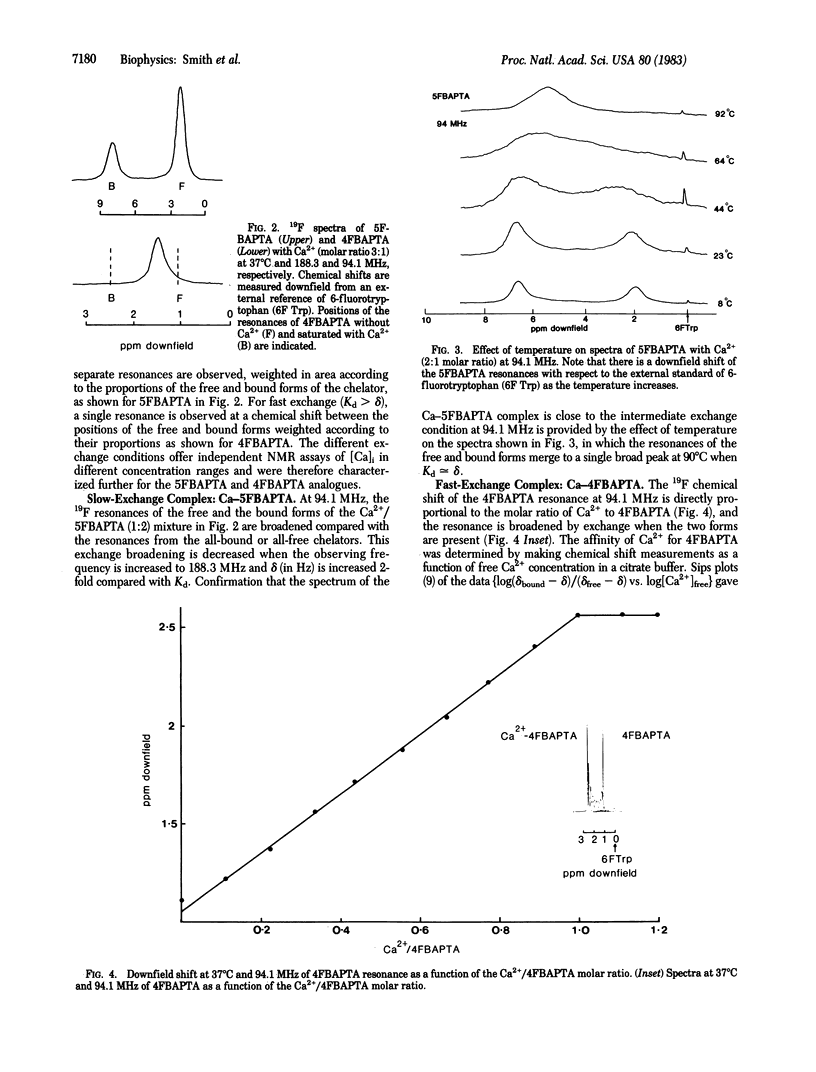
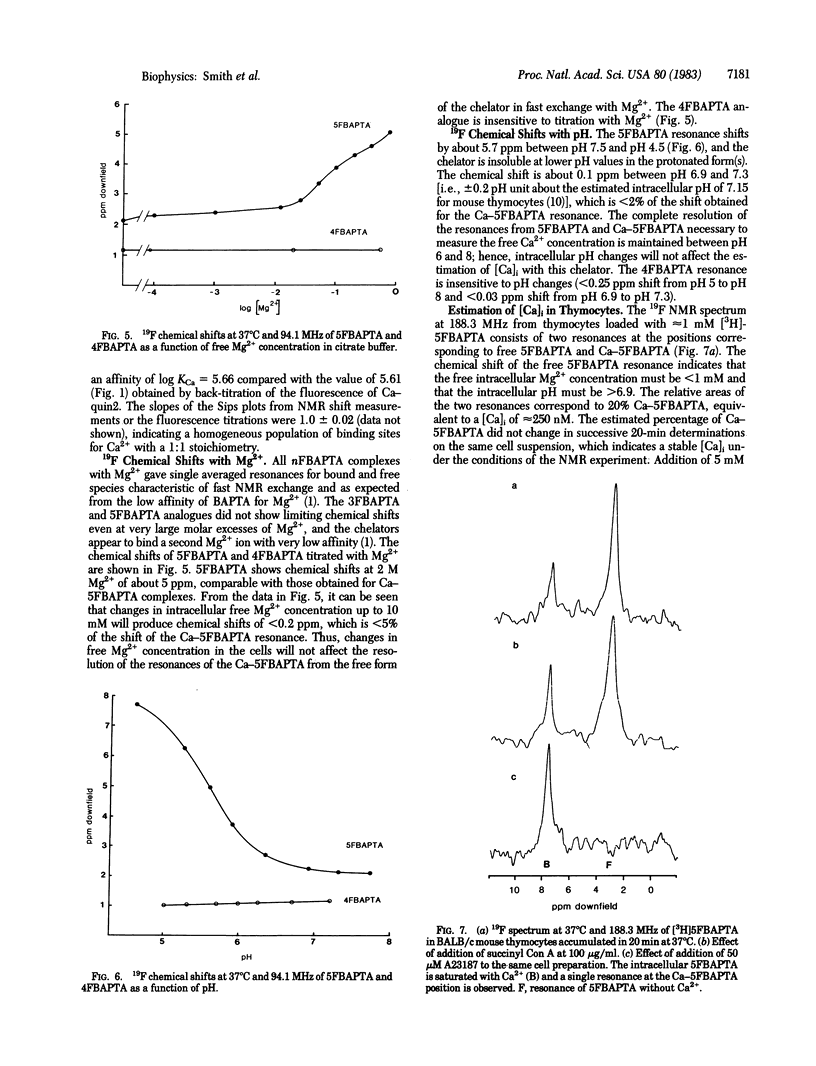
Symmetrically substituted difluoro derivatives of 1,2-bis(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid (nFBAPTA) show large 19F NMR chemical shifts on chelating divalent cations. The complexes of Ca2+ with 4FBAPTA and 5FBAPTA show fast and slow exchange behavior, respectively, and the chemical shift or the areas of the resonances from the free and complexed forms can be used to determine the free Ca2+ concentration. The measurement of the free Ca2+ concentration by either ligand is unaffected by free Mg2+ concentrations less than 10 mM, by pH 6-8, or by contaminating divalent ions of high affinity (Zn2+, Fe2+, Mn2+). The tetraacetoxymethyl ester derivative of 5FBAPTA was used to load mouse thymocytes with 5FBAPTA to intracellular concentrations of 1 mM, and the 19F spectrum indicated a free intracellular Ca2+ concentration [( Ca]i) of 250 nM. The [Ca]i was increased to 350 nM by addition of succinylated concanavalin A at mitogenic concentrations, and the addition of A23187 saturated the intracellular chelator with Ca2+ from the external medium. The method provides a measurement of [Ca]i and other divalent cation concentrations with direct identification of the ionic species chelated.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beppu M., Terao T., Osawa T. Covalently cross-linked monovalent, divalent, and tetravalent derivatives of concanavalin A. J Biochem. 1979 May;85(5):1275–1287. [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Gunther G. R., Wang J. L., Yahara I., Cunningham B. A., Edelman G. M. Concanavalin A derivatives with altered biological activities. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1012–1016. doi: 10.1073/pnas.70.4.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesketh T. R., Smith G. A., Moore J. P., Taylor M. V., Metcalfe J. C. Free cytoplasmic calcium concentration and the mitogenic stimulation of lymphocytes. J Biol Chem. 1983 Apr 25;258(8):4876–4882. [PubMed] [Google Scholar]
- Pozzan T., Arslan P., Tsien R. Y., Rink T. J. Anti-immunoglobulin, cytoplasmic free calcium, and capping in B lymphocytes. J Cell Biol. 1982 Aug;94(2):335–340. doi: 10.1083/jcb.94.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzan T., Corps A. N., Montecucco C., Hesketh T. R., Metcalfe J. C. Cap formation by various ligands on lymphocytes shows the same dependence on high cellular ATP levels. Biochim Biophys Acta. 1980 Nov 18;602(3):558–566. doi: 10.1016/0005-2736(80)90334-x. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Tsien R. Y., Warner A. E. Free calcium in Xenopus embryos measured with ion-selective microelectrodes. Nature. 1980 Feb 14;283(5748):658–660. doi: 10.1038/283658a0. [DOI] [PubMed] [Google Scholar]
- Rogers J., Hesketh T. R., Smith G. A., Metcalfe J. C. Intracellular pH of stimulated thymocytes measured with a new fluorescent indicator. J Biol Chem. 1983 May 25;258(10):5994–5997. [PubMed] [Google Scholar]
- Steinhardt R., Zucker R., Schatten G. Intracellular calcium release at fertilization in the sea urchin egg. Dev Biol. 1977 Jul 1;58(1):185–196. doi: 10.1016/0012-1606(77)90084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. T-cell mitogens cause early changes in cytoplasmic free Ca2+ and membrane potential in lymphocytes. Nature. 1982 Jan 7;295(5844):68–71. doi: 10.1038/295068a0. [DOI] [PubMed] [Google Scholar]


