Abstract
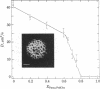
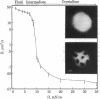
A method is described that eliminates surface flow in monolayers at the air-water interface and makes possible diffusion measurements by fluorescence microphotolysis ("photobleaching"). In contrast to previous studies that did not account for surface flow, lipid probe diffusion has been found to be similar in densely packed monolayers and in related bilayers. Furthermore, it seems that lipid diffusion is based on the same molecular mechanism in monolayers, bilayers, and potentially also cell membranes. In monolayers of L-alpha-dilauroylphosphatidylcholine (Lau2-PtdCho) the translational diffusion coefficient D of the fluorescent lipid probe N-4-nitrobenzo-2-oxa-1,3 diazole egg phosphatidylethanolamine decreased from 110 microns2/s at a surface pressure II = 1 mN/m to 15 microns2/s at II = 38 mN/m (T = 21-22 degrees C). Data could be fitted by the "free volume model." In monolayers of L-alpha-dipalmitoylphosphatidylcholine (Pam2-PtdCho) D decreased by greater than 3 orders of magnitude upon increasing II at constant temperature, thus indicating a fluid-to-crystalline phase transition. In Lau2-PtdCho/Pam2-PtdCho monolayers phase separation has been visualized in the fluorescence microscope and the effect on D measured. These results suggest that monolayers are a promising model system for studying the molecular mobility of lipids and other cell membrane components.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry R. J. Rotational and lateral diffusion of membrane proteins. Biochim Biophys Acta. 1979 Dec 20;559(4):289–327. doi: 10.1016/0304-4157(79)90009-1. [DOI] [PubMed] [Google Scholar]
- Edidin M., Zagyansky Y., Lardner T. J. Measurement of membrane protein lateral diffusion in single cells. Science. 1976 Feb 6;191(4226):466–468. doi: 10.1126/science.1246629. [DOI] [PubMed] [Google Scholar]
- Freire E., Snyder B. Quantitative characterization of the lateral distribution of membrane proteins within the lipid bilayer. Biophys J. 1982 Mar;37(3):617–624. [PMC free article] [PubMed] [Google Scholar]
- Fromherz P. A new technique for investigating lipid protein films. Biochim Biophys Acta. 1971 Feb 2;225(2):382–387. doi: 10.1016/0005-2736(71)90235-5. [DOI] [PubMed] [Google Scholar]
- Galla H. J., Hartmann W., Theilen U., Sackmann E. On two-dimensional passive random walk in lipid bilayers and fluid pathways in biomembranes. J Membr Biol. 1979 Jul 31;48(3):215–236. doi: 10.1007/BF01872892. [DOI] [PubMed] [Google Scholar]
- Hafeman D. G., von Tscharner V., McConnell H. M. Specific antibody-dependent interactions between macrophages and lipid haptens in planar lipid monolayers. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4552–4556. doi: 10.1073/pnas.78.7.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui S. W., Cowden M., Papahadjopoulos D., Parsons D. F. Electron diffraction study of hydrated phospholipid single bilayers. Effects of temperature hydration and surface pressure of the "precursor" monolayer. Biochim Biophys Acta. 1975 Mar 25;382(3):265–275. doi: 10.1016/0005-2736(75)90269-2. [DOI] [PubMed] [Google Scholar]
- Jacobson K., Wu E., Poste G. Measurement of the translational mobility of concanavalin A in glycerol-saline solutions and on the cell surface by fluorescence recovery after photobleaching. Biochim Biophys Acta. 1976 Apr 16;433(1):215–222. doi: 10.1016/0005-2736(76)90189-9. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Wolf D. E. Selectivity of fluorescent lipid analogues for lipid domains. Biochemistry. 1980 Dec 23;19(26):6199–6203. doi: 10.1021/bi00567a039. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Pugh E. N., Jr The control of phosphodiesterase in rod disk membranes: kinetics, possible mechanisms and significance for vision. Vision Res. 1979;19(4):375–380. doi: 10.1016/0042-6989(79)90097-x. [DOI] [PubMed] [Google Scholar]
- Lis L. J., McAlister M., Fuller N., Rand R. P., Parsegian V. A. Measurement of the lateral compressibility of several phospholipid bilayers. Biophys J. 1982 Mar;37(3):667–672. [PMC free article] [PubMed] [Google Scholar]
- Montal M., Darszon A., Schindler H. Functional reassembly of membrane proteins in planar lipid bilayers. Q Rev Biophys. 1981 Feb;14(1):1–79. doi: 10.1017/s0033583500002079. [DOI] [PubMed] [Google Scholar]
- Nagle J. F. Theory of lipid monolayer and bilayer phase transitions: effect of headgroup interactions. J Membr Biol. 1976;27(3):233–250. doi: 10.1007/BF01869138. [DOI] [PubMed] [Google Scholar]
- Pastan I. H., Willingham M. C. Journey to the center of the cell: role of the receptosome. Science. 1981 Oct 30;214(4520):504–509. doi: 10.1126/science.6170111. [DOI] [PubMed] [Google Scholar]
- Peters R., Brünger A., Schulten K. Continuous fluorescence microphotolysis: A sensitive method for study of diffusion processes in single cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):962–966. doi: 10.1073/pnas.78.2.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R., Cherry R. J. Lateral and rotational diffusion of bacteriorhodopsin in lipid bilayers: experimental test of the Saffman-Delbrück equations. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4317–4321. doi: 10.1073/pnas.79.14.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R., Peters J., Tews K. H., Bähr W. A microfluorimetric study of translational diffusion in erythrocyte membranes. Biochim Biophys Acta. 1974 Nov 15;367(3):282–294. doi: 10.1016/0005-2736(74)90085-6. [DOI] [PubMed] [Google Scholar]
- Peters R., Richter H. P. Translational diffusion in the plasma membrane of sea urchin eggs. Dev Biol. 1981 Sep;86(2):285–293. doi: 10.1016/0012-1606(81)90186-x. [DOI] [PubMed] [Google Scholar]
- Peters R. Translational diffusion in the plasma membrane of single cells as studied by fluorescence microphotolysis. Cell Biol Int Rep. 1981 Aug;5(8):733–760. doi: 10.1016/0309-1651(81)90231-9. [DOI] [PubMed] [Google Scholar]
- Phillips M. C., Chapman D. Monolayer characteristics of saturated 1,2,-diacyl phosphatidylcholines (lecithins) and phosphatidylethanolamines at the air-water interface. Biochim Biophys Acta. 1968 Nov 5;163(3):301–313. doi: 10.1016/0005-2736(68)90115-6. [DOI] [PubMed] [Google Scholar]
- Saxton M. J. Lateral diffusion in an archipelago. Effects of impermeable patches on diffusion in a cell membrane. Biophys J. 1982 Aug;39(2):165–173. doi: 10.1016/S0006-3495(82)84504-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separation in phospholipid membranes. Biochemistry. 1973 Jun 5;12(12):2351–2360. doi: 10.1021/bi00736a026. [DOI] [PubMed] [Google Scholar]
- Smith B. A., McConnell H. M. Determination of molecular motion in membranes using periodic pattern photobleaching. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2759–2763. doi: 10.1073/pnas.75.6.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroeve P., Miller I. Lateral diffusion of cholesterol in monolayers. Biochim Biophys Acta. 1975 Aug 20;401(2):157–167. doi: 10.1016/0005-2736(75)90300-4. [DOI] [PubMed] [Google Scholar]
- Teissie J., Tocanne J. F., Baudras A. A fluorescence approach of the determination of translational diffusion coefficients of lipids in phospholipid monolayer at the air-water interface. Eur J Biochem. 1978 Feb 1;83(1):77–85. doi: 10.1111/j.1432-1033.1978.tb12070.x. [DOI] [PubMed] [Google Scholar]
- Träuble H., Sackmann E. Studies of the crystalline-liquid crystalline phase transition of lipid model membranes. 3. Structure of a steroid-lecithin system below and above the lipid-phase transition. J Am Chem Soc. 1972 Jun 28;94(13):4499–4510. doi: 10.1021/ja00768a015. [DOI] [PubMed] [Google Scholar]
- Vaz W. L., Criado M., Madeira V. M., Schoellmann G., Jovin T. M. Size dependence of the translational diffusion of large integral membrane proteins in liquid-crystalline phase lipid bilayers. A study using fluorescence recovery after photobleaching. Biochemistry. 1982 Oct 26;21(22):5608–5612. doi: 10.1021/bi00265a034. [DOI] [PubMed] [Google Scholar]
- Vincent M., de Foresta B., Gallay J., Alfsen A. Nanosecond fluorescence anisotropy decays of n-(9-anthroyloxy) fatty acids in dipalmitoylphosphatidylcholine vesicles with regard to isotropic solvents. Biochemistry. 1982 Feb 16;21(4):708–716. doi: 10.1021/bi00533a019. [DOI] [PubMed] [Google Scholar]
- Weis R. M., Balakrishnan K., Smith B. A., McConnell H. M. Stimulation of fluorescence in a small contact region between rat basophil leukemia cells and planar lipid membrane targets by coherent evanescent radiation. J Biol Chem. 1982 Jun 10;257(11):6440–6445. [PubMed] [Google Scholar]
- Zaccai G., Büldt G., Seelig A., Seelig J. Neutron diffraction studies on phosphatidylcholine model membranes. II. Chain conformation and segmental disorder. J Mol Biol. 1979 Nov 15;134(4):693–706. doi: 10.1016/0022-2836(79)90480-7. [DOI] [PubMed] [Google Scholar]
- von Tscharner V., McConnell H. M. An alternative view of phospholipid phase behavior at the air-water interface. Microscope and film balance studies. Biophys J. 1981 Nov;36(2):409–419. doi: 10.1016/S0006-3495(81)84740-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Tscharner V., McConnell H. M. Physical properties of lipid monolayers on alkylated planar glass surfaces. Biophys J. 1981 Nov;36(2):421–427. doi: 10.1016/S0006-3495(81)84741-8. [DOI] [PMC free article] [PubMed] [Google Scholar]