Abstract
Patients that are on many medications are often non-compliant due to the complexity of the medication regimen; consequently, a patient that is non-compliant can have poor medical outcomes. Providers are not always aware of the complexity of their patient’s prescriptions. Methods have been developed to calculate the complexity for a patient’s regimen but there are no widely available automated tools that will do this for a provider. Given that ontologies are known to provide well-principled, sharable, setting-independent and machine-interpretable declarative specification frameworks for modeling and reasoning on biomedical problems, we have explored their use in the context of reducing medication complexity. Previously we proposed an Ontology for modeling drug-related knowledge and a repository for complexity scoring. Here we tested the Ontology with patient data from the University of California San Diego Epic database, and we built a decision aide that computes the complexity and recommends changes to reduce the complexity, if needed.
INTRODUCTION
Anyone that interacts with patients knows the frustration of having a patient that is “non-compliant.” It has been estimated that the rate of non-compliance, or non-adherence, is 30–50% [1]. There have been studies done to determine the causes of non-adherence and they are hard to identify, but some of the prominent causes include polypharmacy and complexity of regiments [1]. Polypharmacy, which is the intake of several medications, can range from as many as three medications to over ten, on a regularly scheduled basis. Complexity can be measured by the number of medicines or tablets taken, the frequency of the medicines taken, any restrictions surrounding the intake of a medicine (like with an empty stomach or after a meal, with water only, etc.), the route of administration of the medicine, and the like. As a patient gets older, the number of medications tends to increase; the estimated compliance rate, in the elderly, ranges from 0% to 59%, depending on the study [1]. This is enough of an issue that the U.S. Department of Health and Human Services has developed an initiative to better care for those with multiple medical problems; one of the issues they often have to deal with is polypharmacy.[2]
Many providers are not always aware of the complexity of the medications taken by their patients; so, when they prescribe a new medication this is not taken into consideration. In one study, done at a Veterans Administration hospital, the residents were given a grid that contained the times of day for administration of the patients’ medications with one column for each day. This pictorial representation of the complexity for the patients impressed the residents with the difficulty of some of the regimens. The results of the study were that the patients’ who were treated by residents that were given a grid, had their number of medications decreased and the number of doses decreased; the other, control patients, had increases in both of these area. [3]
The purpose of this study is to build on our previous research on the development of a drug Ontology[4], further refining and testing the proposed Ontology. Here we report on our experience of building and testing an Ontology-based prototype tool that aims to achieve three goals. The first one is to map Epic prescription medications into the Ontology. The second is to calculate the complexity of the prescriptions and recommend changes to the regiment that could decrease complexity. The third is to evaluate the cost of the current and proposed medication regiment and present this information to the provider.
In our previous AMIA paper[4] we proposed a Web Ontology Language (OWL) Ontology to model drug information, and patient’s drug prescriptions. We also reported on a set of Semantic Web Rule Language (SWRL) rules that we used to implement a method proposed by George et al [5] to compute the complexity of a polypharmacy treatment. The method consists of assigning weights to drug prescriptions based on dosage form and frequency (e.g. a daily dose having a weight of 1 while an every 4 hour dosing having a weight of 6.5), and additional direction (e.g. breaking or crushing a tablet adds 1 to the complexity index.) Previously we defined, in the developed OWL Ontology, hypothetical patient’s polypharmacy prescriptions, which we modeled in the Protégé tool to evaluate the proposed rules. For this study we looked at the problem of mapping University of California San Diego (UCSD) Epic drug prescriptions into the proposed OWL Ontology. The complexity was computed using the already developed SWRL rules.
For the second aim we proposed new SWRL rules to reduce the complexity of drug prescriptions, while suggesting new and safe formulary prescriptions. For example, if a patient is taking Lisinopril 10mg twice daily, the system would recommend that the patient be switched to Lisinopril 20mg once daily, since based on the formulary constraints for Lisinopril, this is an accepted dosing for this medication. Other ways that the system can reduce complexity is by combing drugs. If a combination drug is available in the same dose forms, then the system recommends the change. For example, if the patient is on Lisinopril 10mg daily and Hydrochlorothiazide 12.5mg daily, it can recommend the patient be switched to a combination pill that contains both of these medications.
The third aim was accomplished by reusing the SWRL rules proposed in our previous AMIA paper[4] to compute polypharmacy treatment costs. The cost of the given prescriptions, as well as the new, recommended regimen are computed and presented to the provider. This information is important if the patient is on a fixed income and has a limited income available to pay for their medications; especially if they have no insurance to cover the cost of their medications.
BACKGROUND
Considering the difficulty in getting patients to comply with their prescribed medications, it is not an issue often discussed by providers. To the best of our knowledge there are no widely available, automated tools, to advise a prescriber on how to decrease the complexity of a patients prescribed medications. The process of medical decision-making that providers use to accomplish this task, can be thought of as a domain of knowledge with specified relations that can often be presented as a clinical algorithm[6] or a clinical pathway.[7] An Ontology is an explicit formal set of specifications of the terms in a domain and the relations among them[8]; or more simply, it is a way to “model a domain of knowledge.”[9] This makes an Ontology an ideal method to model the process used to help decrease the complexity of a patients prescribed medications. The Protégé Ontology editing[10] tool was used to model the Ontology; it uses OWL to represent the Ontology.[11] It is the most widely used Ontology editor[12] that allows the sharing of ontologies amongst users.[11] SWRL was used as the specification language for our decision support rules since it is based on OWL and includes high-level abstract syntax for Horn-like rules (given an antecedent is true then the consequent must be true)[4, 13]. The implementation was built as a Java program that accesses the OWL Ontology through queries expressed in SWRL.
Within the studies that focused on safe and effective polypharmacy prescription, we can mention Elliot’s work where pharmacists manually evaluated patients’ prescriptions prior to discharging them from the hospital to determine if the complexity could be decreased. The pharmacists were tasked to present this information to the patient and the provider prior to discharge. Of the 221 patients in the study, only 84.4% had their medication regimen reviewed and only 31% of those patients had the changes implemented. The main reason cited for not reviewing or not implementing changes was “lack of time.”[14]
Similar to Elliott, et.al[14], in Muir, et.al.[3] pharmacists evaluated the medication regimen for inpatients, at the time of admission, based on the resident’s computerized admission note. The regimen was then presented in a grid format to the admitting resident the morning after admission. The grid contained the times of day for administration and the columns listed the days of administration. The residents were not given any instruction or training on medication complexity reduction. The study evaluated the differences in the number of medications and number of doses between admission and discharge from the hospital, between the intervention and control group. The intervention group, which was made up of 568 patients, had the number of medications decreased by almost one and the number of doses decreased by almost 2.5 per patient. While the control group, made up of 661 patients, had medications increased by over 1.5 per patient and the doses increased by over 3.5 per patient.[3] There was no computerized intervention in this study.
In Calabrese, et.al.[15] pharmacists reviewed the prescribing of fifteen different medications administered by the study pharmacy; the goal was to change prescriptions for medications that were prescribed more than once daily, to once daily, if medically applicable. If a patient had a prescription that was flagged by the pharmacies intervention application software, as a medication that could have the frequency reduced, then a clinical pharmacist generated three items: an authorization form for the provider to review, a pre-printed prescription for the suggested alternative dosage regimen, and a personalized letter from the clinician to the patient explaining the change in the dose regimen. This paperwork was presented to the provider for approval or denial; once completed, the paperwork was returned to the pharmacy for processing and tracking. Of the interventions sent to providers (927), 49% were approved, but 32% were not returned to the pharmacy during the study period.[15] No reason was given for providers not returning the paperwork, but lack of time was inferred as a main reason. The changes that were approved and implemented saved $390,662 annually or $1.67 per member per year for the health system evaluated since often the different doses of a medication have the same price.[15]
The reported studies [3] [14] [15] involved human evaluation and recommendations. This can be very costly since the prescriptions have to be evaluated by a trained individual, initially. Then the changes have to be approved by the provider, and finally implemented by a pharmacist. Here we explore the use of a decision support system (DSS) that could suggest changes to the polypharmacy prescriptions to reduce cost and overall treatment’s complexity. Studies have shown that if a provider is aware of the complexity they will try to decrease it, even if they are not provided recommended changes.[3] The proposed decision aide is an enhancement of a previously proposed DSS[4] which calculated the complexity and cost of a patient’s prescriptions but did not recommend changes. To add this capability, we built on the previous approach reusing: a) the existing Ontology framework which already supported the modeling of drugs’ cost, formulary constraints, and patient’s polypharmacy prescription, and b) the SWRL rules that computed cost and complexity and checked satisfaction of formulary constraints.
METHODS
This research study was focused on achieving three aims:
Aim 1: Mapping the Epic prescriptions into the OWL Ontology
From the Epic patient data we were provided we selected twenty patients. The general guideline used was that the patient had to be on at least three medications and no more than one medication that was used for another medical pathology other than hypertension (HTN) or hypercholesterolemia (HLP). The data provided from Epic included old and new prescriptions. (See Table 1 for example.) If there was more than one prescription written for a medication, the newest prescription was chosen. There were some medications listed as null in the dose and unit categories; it was assumed that these were voided or expired prescriptions. Also, if there was one prescription that was more than a year older than the other prescriptions in the group for that particular patient, it was considered expired and no longer active since the common practice in pharmacies is to not refill prescriptions more than a year old.
Table 1:
The prescription data provided by Epic for patient #10. The prescriptions in bold are the ones picked to model into the Ontology.
| contact_date | name | rx_dose | rx_unit | rx_frequency |
|---|---|---|---|---|
| 9/3/08 | ATORVASTATIN CALCIUM 10 MG OR TABS | NULL | NULL | 1 TABLET DAILY |
| 12/2/09 | ATORVASTATIN CALCIUM 10 MG OR TABS | 10 | mg | Take 1 Tab by mouth daily. |
| 7/11/11 | ATORVASTATIN CALCIUM 10 MG OR TABS | 10 | mg | Take 1 tablet by mouth daily. |
| 12/2/09 | FUROSEMIDE 20 MG OR TABS | 20 | mg | Take 1 Tab by mouth daily. |
| 6/15/11 | FUROSEMIDE 20 MG OR TABS | 20 | mg | Take 1 tablet by mouth 2 times daily. |
| 6/20/11 | FUROSEMIDE 20 MG OR TABS | 20 | mg | Take 1 tablet by mouth 2 times daily. |
| 7/11/11 | FUROSEMIDE 20 MG OR TABS | 20 | mg | Take 1 tablet by mouth 2 times daily. |
| 9/3/08 | HYDROCHLOROTHIAZIDE 25 MG OR TABS | NULL | NULL | 1 TABLET DAILY |
| 6/20/11 | HYDROCHLOROTHIAZIDE 25 MG OR TABS | 25 | mg | Take 1 tablet by mouth daily. |
| 7/11/11 | HYDROCHLOROTHIAZIDE 25 MG OR TABS | 25 | mg | Take 1 tablet by mouth daily. |
| 9/3/08 | LISINOPRIL-HYDROCHLOROTHIAZIDE 20-12.5 MG OR TABS | NULL | NULL | 1 TABLET DAILY |
| 6/20/11 | LISINOPRIL-HYDROCHLOROTHIAZIDE 20-12.5 MG OR TABS | 1 | tablet | Take 1 tablet by mouth daily. |
| 7/11/11 | LISINOPRIL-HYDROCHLOROTHIAZIDE 20-12.5 MG OR TABS | 1 | tablet | Take 1 tablet by mouth daily. |
| 12/2/09 | METOPROLOL TARTRATE 25 MG OR TABS | 25 | mg | Take 1 Tab by mouth 3 times daily. |
| 6/15/11 | METOPROLOL TARTRATE 25 MG OR TABS | 25 | mg | Take 1 tablet by mouth every 6 hours. |
| 7/11/11 | METOPROLOL TARTRATE 25 MG OR TABS | 25 | mg | Take 1 tablet by mouth every 6 hours. |
| 9/3/08 | METOPROLOL TARTRATE 50 MG OR TABS | NULL | NULL | 1 TID |
| 6/15/11 | VERAPAMIL HCL 180 MG OR TB24 | 90 | mg | Take 1 Tab by mouth 3 times daily. |
| 9/3/08 | VERAPAMIL HCL 180 MG OR TBCR | NULL | NULL | 1 tablet every 12 hours |
| 6/20/11 | VERAPAMIL HCL 180 MG OR TBCR | 90 | mg | Take 90 mg by mouth 2 times daily. Or as directed by cardiologist. |
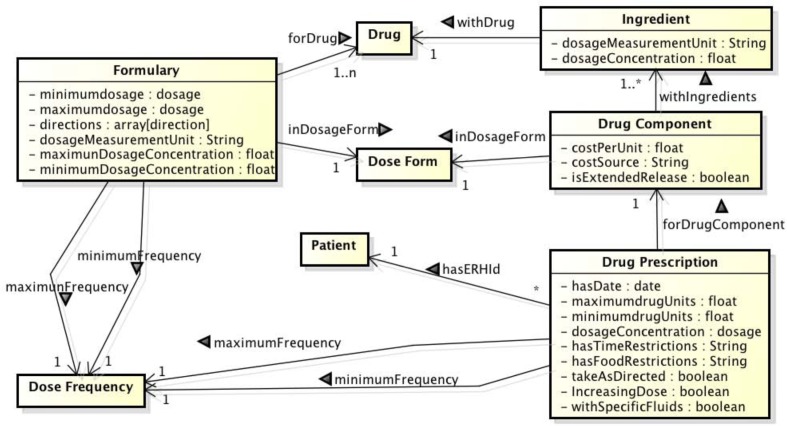
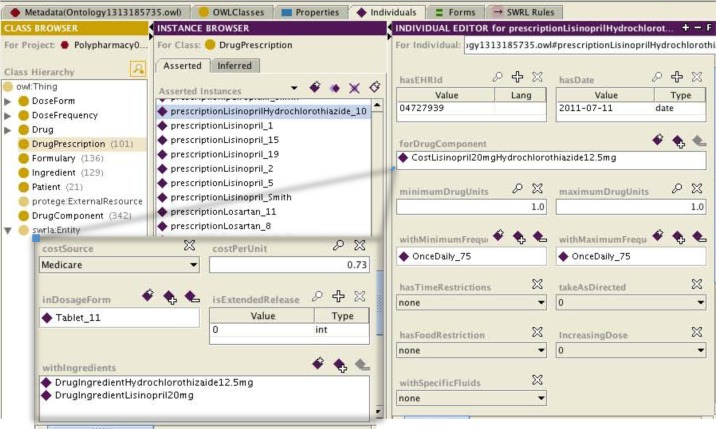
Starting with the OWL Ontology from our previous project, we looked at the problem of how to map Epic prescriptions into the Ontology (Fig 1). The OWL concept that was used to enter the patient’s prescriptions was “Drug Prescription.” In this section the medications that each patient was prescribed were entered individually. The information entered included the date of the prescription (contact_date ➔ hasDate), the drug component (name, rx_dose, rx_unit ➔ forDrugComponent), the minimum and maximum drug units (rx_frequency ➔ minimumDrugUnits, maximumDrugUnits), and the intake directions (rx_frequency ➔ hasTimeRestrictions, takeAsDirected, hasFoodRestriction, IncreasingDose, withSpecificFluids). They were linked to the patient via the unique patient identifier (hasEHRId) given in the Epic data. While mapping Epic data into the Ontology was a manual process, this process could be automatic. To automatically map the name of the medication, as in the “name” field in Epic, will require the use of simple natural language processing rules to separate it from the dosage and dosage form. For instance if the Epic drug name is METOPROLOL TARTRATE 25 MG OR TABS, then the drug name of Metoprolol Tartrate should be separated from the dosage 25 mg and the dosage form oral Tablets. It will be slightly more difficult to map the combination drug names since the mapping needs to be able to distinguish between each separate medication name and the dosages for each component. Fig. 2 exemplifies how we mapped the prescription LISINOPRIL-HYDROCHLOROTHIAZIDE 20-12.5 MG OR TABS from patient #10 in the Protégé specification of our drug Ontology. The patient identifier (04727939) was used to populate the “hasEHRId” field. The date of the prescription (7/11/2011) was used to populate the “hasDate” field. In the “forDrugComponent” field the entry was selected from a list of instances from class “DrugComponent.” The specification of the instance CostLisinopril20mgHydrochlorothiazide12.5mg from “DrugComponent” indicates the cost source, cost per unit, dosage form, if it is an extended release (1= extended release, 0= not extended release) and drug ingredients (in this case Lisinopril 20 mg and Hydrochlorothiazide 12.5mg) as instances of class “Ingredients”. The number of tablets taken for each dose is entered into the “minimumDrugUnits” and “maximumDrugUnits” fields. In this case 1 tablet is the minimum and maximum drug unit. The frequency is entered into the “withMinimumFrequency” and “withMaximumFrequency” fields; in this case the maximum and minimum frequency is once daily. This prescription has no additional intake directions, as indicated by the fields “hasTimeRestrictions”, “takeAsDirected”, “hasFoodRestrictions”, “increasingDose”, and “withSpecificFluids”
Figure 1:
Class diagram depicting the main classes and relationship in the proposed Drug Ontology.
Figure 2:
Snapshot of Protégé illustrating the drug Ontology. On the left the class hierarchy is depicted and on the right an instance of how the patients prescription is entered. The entry shown is for patient #10 and it is a combination tablet containing Lisinopril and Hydrochlorothiazide that is taken once daily. The DrugComponent concept is shown as an inset.
Aim 2: Reducing the complexity of drug prescriptions, while suggesting new and safe formulary prescriptions
The rules we implemented as SWRL specifications come from several different articles on reducing complexity to help increase adherence and reduce cost. The implemented rules were:
Medications that can be taken once a day are preferred, as long as the increased cost of once-daily formulation does not pose a barrier to adherence.[14–16]
Decrease the number of dose units (e.g. tablets) if a larger dose is available.[14]
Combine to individual medications into a combination product, if available.[14, 17]
Based on George et. al.[5] each prescription dosage form (i.e. tablet, liquid, topical cream), dosing frequency (i.e. once daily, every 4 hours, on alternate days), and additional directions (i.e. multiple units at one time, relationship to food, taken with a specific fluid) is assigned a weight. Some dosage form weighting examples include: an oral tablet = 1; a liquid = 2; a powder/granules = 2. Examples of dosage frequency weighting: once daily = 1; once daily as needed = 0.5; every 6 hours = 4, twice daily= 2. Additional direction weighting includes items such as breaking or crushing tablet = 1; multiple units at one time = 1; take with food = 1. Taking items from Table 2, for patient #10, the weight for Atorvastatin would be: tablet (=1) + once daily (=1) = 2. A higher weight would go to Metoprolol Tartarate: tablet (=1) + every 6 hours (=4) + with food (=1)= 6.
Table 2:
As an example, patient #10 is on the above medications, with a complexity score and cost for each medication. Complexity= dosage form+ drug units+ frequency+ additional directions. Total complexity is 19; total monthly cost $206.40
| Drug | Dosage form | Dosage concentration | Drug units | Frequency | Additional directions | Complexity | Cost |
|---|---|---|---|---|---|---|---|
| Atorvastatin Calcium | Tablet | 10 mg | 1 | Once daily | None | 1+0+1+0=2 | 114.9 |
| Furosemide | Tablet | 20 mg | 1 | Twice daily | None | 1+0+2+0=3 | 8.40 |
| Hydrochlorothiazide | Tablet | 25 mg | 1 | Once daily | None | 1+0+1+0=2 | 3.9 |
| Lisinopril Hydrochlorothiazide | Tablet | 20 mg 12.5mg | 1 | Once daily | None | 1+0+1+0=2 | 21.90 |
| Metoprolol Tartrate | Tablet | 25 mg | 1 | Every 6 hours | With food | 1+0+4+1=6 | 51.60 |
| Verapamil HCL | Tablet | 90 mg | 0.5 | Twice daily | None | 1+1+2+0=4 | 5.70 |
To implement polypharmacy complexity, first, we defined the following SWRL rule to obtain, for each prescribed drug (denoted as drugClass) concentration (?concentration), the frequency of intake (?freq), prescribed units (?units), measurement unit (?measurement), food restrictions (?food) and dosage form (?form):
DrugPrescription(?presc) ^ hasEHRId(?presc, patient) ^
forDrugComponent(?presc, ?drugcomponent) ^ withIngredients(?drugcomponent, ?ingr) ^
withDrug(?ingr,?d) ^ “+ drugClass+”(?d) ^ dosageConcentration(?ingr, ?concentration) ^
withMinimumFrequency(?presc, ?freq) ^ withMaximumFrequency(?presc, ?freq) ^
minimumDrugUnits(?presc, ?units) ^ maximumDrugUnits(?presc, ?units) ^
dosageMeasurementUnit(?ingr, ?measurement) ^ hasFoodRestriction(?presc, ?food)^
inDosageForm(?dc, ?form) -> sqwrl:select(?concentration, ?freq, ?units, ?measurement, ?food, ?form)
Second, we defined algorithms for the following:
Reduce medications frequency [14–16]. We implemented the algorithm as a set of simple if-then-else rules. For instance if the intake of a drug is twice daily we tried to reduce it to once daily. Also, if the frequency is every 6 hours we tried to reduce it to twice daily, and so on. Then, to suggest the new prescription drug concentration and units we proposed a new concentration that doubled the old concentration. The suggested new drug unit was always 1. Thus, for the example of Lisinopril 10mg 1 unit twice daily the proposed new prescription was Lisinopril 20 mg 1 unit with frequency once daily, because 20 mg is the double of 10 mg.
Decrease number of dose units[14]. The precondition for decreasing dose units is that the drug prescription has more than 1 unit. In that case, the suggested new drug prescription has as a new concentration that is the product of the old concentration and the old units. The proposed new drug unit is always 1. For instance, if the prescription was Lisinopril 10mg, 2 tablets once daily, it could be replaced by a new concentration 20mg= 10mg x 2. This algorithm was also implemented as a set of if-then-rules.
Third, we defined SWRL rules for suggesting drug combinations. If a patient is on two medications that are available in a tablet with both medications – a combination product – then the patient should be switched [14, 17]. For example if the patient is on Lisinopril 20mg once daily and Hydrochlorothiazide 12.5mg once daily, then they can be switched to a combination tablet with the same doses of each medication.
By enacting the SWRL rule listed below twice, we can select two drug prescriptions from the set of the patient’s drug prescriptions. The selected prescriptions should not correspond to a combined drug (more than one ingredient). For each of the selected prescriptions the SWRL rule returns the prescribed drug (?drug1 and ?drug2), prescribed units (?unit1 and ?unit2), concentration (?concentration1 and ?concentration2), measurement unit (?measurement1 and ?measurement2), and frequency of intake (?freq1 and ?freq2). The precondition for enacting this SWRL rules is that both drugs were prescribed with the same frequency. For instance, Amlodipine 5mg once daily and Atorvastatin 40mg once daily have the same frequency.
DrugPrescription(?presc) ^ hasEHRId(?presc, patient) ^ forDrugComponent(?presc, ?drugcomp) ^
dosageConcentration(?d, ?concentration) ^ withMinimumFrequency(?presc, ?freq) ^
withIngredients(?drugcomp,?ingr) ^ sqwrl:makeSet(?set, ?d) ^ sqwrl:size(?n, ?set) ^ swrlb:equal(?n, 1) ^
withDrug(?ingr,?drug1) ^ “+drugClass+ “(?drug) ^ withMaximumFrequency(?presc, ?freq) ^
minimumDrugUnits(?presc, ?units) ^maximumDrugUnits(?presc, ?units) ^
dosageMeasurementUnit(?d1, ?measurement) ^ inDosageForm(?drugccomp, ?form) ->
sqwrl:selectDistinct( ?drug, ?units, ?concentration, ?measurement, ?freq)
By applying the SWRL rule listed below we can obtain, from the set of available drug components, a combined drug (?drugcomponent) that contains as ingredients both drugs (?drug1 and ?drug2) in the required concentrations (for ?drug1 the concentration should be the product of ?unit1 and ?concentration1, and for ingredient ?drug2 the concentration should be the product of ?unit2 and ?concentration2). For instance, if our input is ?drug1=Lisinopril, ?drug2= Hydrochlorothiazide, ?unit1=mg, ?unit2=mg, ?concentration1=20, ?concentration2=12.5 we can retrieve from the set of drug combinations the drug component listed in row 4 of Table 3.
Table 3:
New polypharmacy, as suggested by our DSS. New complexity is 15; new cost is $164.40.
| Drug | Dosage form | Dosage concentration | Drug units | Frequency | Additional directions | Complexity | Cost |
|---|---|---|---|---|---|---|---|
| Atorvastatin Calcium | Tablet | 10 mg | 1 | Once daily | None | 1+0+1+0=2 | 114.9 |
| Furosemide | Tablet | 40 mg | 1 | Once daily | None | 1+0+1+0=2 | 4.20 |
| Hydrochlorothiazide | Tablet | 25 mg | 1 | Once daily | None | 1+0+1+0=2 | 3.90 |
| Lisinopril Hydrochlorothiazide |
Tablet | 20 mg 12.5mg |
1 | Once daily | None | 1+0+1+0=2 | 21.90 |
| Metoprolol Tartrate | Tablet | 50 mg | 1 | Twice daily | With food | 1+0+2+1=4 | 13.80 |
| Verapamil HCL | Tablet | 90 mg | 1 | Once daily | None | 1+1+1+0=3 | 5.70 |
DrugComponent(?drugcomponent) ^ withIngredients(?dcomponent, ?ingr3) ^ withDrug(?ingr3,drug1) ^
withIngredients(?dcomponent, ?ingr4) ^ sqwrl:makeSet(?set, ?ingr3) ^ sqwrl:size(?n, ?set) ^
swrlb:greaterThan(?n, 1) ^ dosageConcentration(?ingr3, ?concentration3) ^ dosageConcentration(?ingr4, ?concentration4) ^ withDrug(?ingr4,drug2) ^ swrlb:multiply(?result1, units1, concentration1) ^
swrlb:multiply(?result2, units2, concentration2) ^ swrlb:equal(?result1, ?concentration3) ^
swrlb:equal(?result2, ?concentration4) ^ swrlb:add(?totalconcentration, ?result1, ?result2) ->
sqwrl:selectDistinct(?dcomponent, ?totalconcentration)
Finally, we reused the rules proposed in the previous AMIA paper[4] for: 1) suggesting the drug prescription’s intake directions (for instance take the drug before bedtime, or take with food); and 2) checking that the prescription does not violate drug formulary constraints related to prescription units, dosage concentration, frequency and intake directions (for example Lisinopril’s formulary constraint is that the recommended minimum daily dosage is 10mg, and the recommended maximum dosage is 40mg).
In the case of patient #10, once the rules are applied to the polypharmacy prescription from Table 2 a new polypharmacy prescription, depicted in Table 3, is suggested to the clinician. The new treatment has a new complexity of 15. Therefore, compared with the original polypharmacy treatment prescribed to patient #10 there is a reduction in complexity of 4 units.
Aim 3: Evaluating the cost of the current and proposed medication regiment and present this information to the provider
The SWRL rules proposed in the previous AMIA paper[4] were used to compute the polypharmacy treatment costs. The cost information was obtained from Epocrates, a free access tool with wide spread use as a mobile drug reference among US physicians. (www.epocrates.com/who) If we use, as an example, row 5 from Table 3, the monthly cost of the drug Metoprolol Tartrate, with a concentration of 50mg per tablet is $13.80. The cost is based on considering the intake of 60 pills (1 pill twice daily) per month, where the cost of each pill is $0.23. So the cost of the simplified polypharmacy treatment for patient #10 is $164.40. Therefore the simplified polypharmacy treatment suggested by the DSS would help to save $42 dollars per month on the treatment of patient #10.
In our DSS the cost information is presented to the provider, for both the current prescriptions and the proposed or alternate prescriptions. Often the cost of a tablet is about the same, regardless of the dose.[15] If cost is an issue then this will play a part in the decision process of the provider, especially if it helps to decrease the complexity for the patient, as well. The SWRL rules can also propose combination drugs that help decrease the complexity, but they are often more expensive, since many are still not available as generics. If cost is not an issue, then the change to a combination drug may help increase compliance thus decreasing the risk of cardiovascular disease and stroke.[18]
RESULTS
Aim 1: Mapping the Epic prescriptions into the OWL Ontology
The George et. al. approach [5] for computing polypharmacy complexity was evaluated with 134 medication regimens. In our first AMIA paper[4] we further tested the method using theoretical prototypical patient cases. In this paper we wanted to explore the possiblity of automatically computing the complexity of polypharmacy treatments defined in the Epic system. For this project we looked at how to map the Epic medications of 20 patients, for a total of 84 prescriptions, into the proposed drug Ontology to compute their complexity. As we explained in Aim 1 of the Method Section we discovered simple rules that could be used to automatically map the data from Epic into the drug Ontology that supports the polypharmacy complexity computing method.
An issue that arose was for a patient that was prescribed Terazosin 2mg, 2 tablets in the morning and 3 at bedtime. Given the constraints of how the data was entered into the Ontology, it had to be entered as two different prescriptions; Terazosin 2mg, 2 tablets in the morning and Terazosin 2mg, 3 tablets at bedtime. This is not that common when prescribing medications taken on a regular basis but it is an issue that increases complexity.
Aim 2: Reducing the complexity of drug prescriptions, while suggesting new and safe formulary prescriptions
Initially when the DSS was run, there were only a few recommended changes; a few patients were on medications that were taken more than once daily that could be taken on a daily basis. But, many were already on combination drugs since Epic suggests combination drugs to the providers when they enter prescriptions for their patients. To evaluate the SWRL rule that suggest drug combinations, a few of the previously entered prescriptions for the patients on combination drugs were changed to two individual drugs and when the DSS was run it recommended that the patient be switched to the combination drug, as expected.
When the combination drugs were being entered into the Ontology it was noticed that the combination drugs do not always cover all the possible combinations when using individual medications. For example, Amlodipine is available in three doses – 2.5mg, 5mg, and 10mg. Benazepril is available in four doses – 5mg, 10mg, 20mg, and 40mg. It would then be expected that there would be 12 different combinations if all combinations were available but there are only six combinations available. This was not an issue with the 20 patient profiles that were used. For some of the combination drugs, the prescribing information from the manufacturer gives directions on how to switch a patient to the combination drugs; this information would have to be modeled in the Ontology and SWRL rules developed to implement this process.
Aim 3: Evaluating the cost of the current and proposed medication regiment and present this information to the provider
This aim was accomplished using the data that was already in the Ontology, from our previous work. The existing rules were used to compute the cost. The cost was calculated for the patients existing prescription, and for the proposed prescriptions, if changes were proposed. The issue encountered was that prices were not available for all medications within the chosen data source. If the data is available from the pharmacy database this could be automatically mapped into the Ontology.
DISCUSSION
Our DSS, as proposed here, is an important tool in attempting to decrease the number of medications taken by patients [14] which ultimately can lead to a decrease in the cost of medical care.[15] Of the four articles that recommended rules to decrease the complexity of a patients’ polypharmacy, there were a total of seven rules.[14–17]
We were able to implement three of these rules. The rules that were not implemented, but we would like to implement in the future, include:
If the patient is taking other medications, consider recommending that all be taken at the same time of day.[14, 16]
Change the dose form, such as from Verapamil three times daily to once daily extended-release Verapamil.[14]
Change strength to help decrease complexity, such as Aspirin 300mg half a tablet daily to Aspirin 100mg daily,[14] as long as clinical guidelines are still followed.
Medication with special requirements, such as bedtime dosing, avoiding meals, should be used only if alternatives are unavailable or atypical circumstances exist. For example, Simvastatin is recommended to be dosed at bedtime while Atorvastatin can be taken at any time of day.[16]
In addition to implementing the above rules, another area for future work would be to automatically map the Epic prescriptions into the proposed Ontology following the methodology proposed in the Methods section. Furthermore, we could look at the problem of automatically populating drug cost and formulary constraints and alerts from a database such as Epocrates, or from the pharmacy cost and alert database, which many automated prescription systems already have.
We would also like to combine our previous DSS[4], which used clinical practice guidelines, to evaluate a patient’s care and make prescription recommendations, if the current prescriptions do not follow the guidelines. This could be further implemented by evaluating recent labs and vital signs, to ensure appropriate control of the medical diagnosis assigned to the patient, and if they are not well controlled, then making recommendations that minimize cost and/or complexity, and will attempt to bring the patient’s medical problems under control.
References
- 1.Banning M. A review of interventions used to improve adherence to medication in older people. Int J Nurs Stud. 2009 Nov;46(11):1505–15. doi: 10.1016/j.ijnurstu.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Services USDoHaH, editor. Multiple chronic conditions - a strategic framework: Optimum health and quality of life for inividuals with multiple chronic conditions. Washington, DC: Dec, 2010. [Google Scholar]
- 3.Muir AJ, Sanders LL, Wilkinson WE, Schmader K. Reducing medication regimen complexity: A controlled trial. J Gen Intern Med. 2001 Feb;16(2):77–82. doi: 10.1046/j.1525-1497.2001.016002077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grando M, Farrish S, Boyd C, Boxwala A. Ontological approach for safe and effective polypharmacy prescription. AMIA Conference; Chicago, IL. 2012. pp. 291–300. [PMC free article] [PubMed] [Google Scholar]
- 5.George J, Phun YT, Bailey MJ, Kong DC, Stewart K. Development and validation of the medication regimen complexity index. Ann Pharmacother. 2004 Sep;38(9):1369–76. doi: 10.1345/aph.1D479. [DOI] [PubMed] [Google Scholar]
- 6.Shortliffe E, Buchanan B, Feigenbaum E. Knowledge engineering for medical decision making: A review of computer-based clinical decision aids. Proceedings of the IEEE. 1979 1979 Sep;67(9):1207–24. [Google Scholar]
- 7.Ye Y, Jiang Z, Diao X, Yang D, Du G. An ontology-based hierarchical semantic modeling approach to clinical pathway workflows. Computers in biology and medicine. 2009 Aug;39(8):722–32. doi: 10.1016/j.compbiomed.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Noy N, McGuinness D. Ontology development 101: A guide to creating your first ontology. Stanford Knowledge Systems Laboratory Technical Report KSL-01-05 and Stanford Medical Informatics Technical Report SMI-2001-0880: March 2001.
- 9.Gruber T. Ontology. In: Liu L, Ozsu MT, editors. Encyclopedia of Database Systems. Springer-Verlag; 2009. [Google Scholar]
- 10.Noy N, Tudorache T, Nyulas C, Musen M. The ontology life cycle: Integrated tools for editing, publishing, peer review, and evolution of ontologies. AMIA Annual Symposium proceedings / AMIA Symposium AMIA Symposium; 2010; 2010. pp. 552–6. [PMC free article] [PubMed] [Google Scholar]
- 11.Bright TJ, Yoko Furuya E, Kuperman GJ, Cimino JJ, Bakken S. Journal of biomedical informatics. Vol. 45. Elsevier Inc; 2012. Development and evaluation of an ontology for guiding appropriate antibiotic prescribing; pp. 120–8. United States: 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bodenreider O, Stevens R. Brief bioinform. Vol. 7. England: 2006. Bio-ontologies: Current trends and future directions; pp. 256–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horrocks I, Patel-Schneider P, Boley H, Tabet S, Grosof B, Dean M. Swrl: A semantic web rule language combining owl and ruleml. [updated 21 May 2004; cited 2013 Feb 25]. Available from: http://www.w3.org/Submission/SWRL/Overview.html.
- 14.Elliott RA. Reducing medication regimen complexity for older patients prior to discharge from hospital: Feasibility and barriers. J Clin Pharm Ther. 2012 Dec;37(6):637–42. doi: 10.1111/j.1365-2710.2012.01356.x. [DOI] [PubMed] [Google Scholar]
- 15.Calabrese DC, Baldinger SL. Dose-optimization intervention yields significant drug cost savings. J Managed Care Pharm. 2002;8(2):146–51. [Google Scholar]
- 16.Domino FJ. Improving adherence to treatment for hypertension. Am Fam Physician. 2005 Jun;71(11):2089–90. [PubMed] [Google Scholar]
- 17.Williams B, Shaw A, Durrant R, Crinson I, Pagliari C, de Lusignan S. Patient perspectives on multiple medications versus combined pills: A qualitative study. QJM. 2005 Dec;98(12):885–93. doi: 10.1093/qjmed/hci139. [DOI] [PubMed] [Google Scholar]
- 18.Bautista LE, Vera-Cala LM, Ferrante D, et al. A ‘polypill’ aimed at preventing cardiovascular disease could prove highly cost-effective for use in latin america. Health Aff (Millwood) 2013 Jan;32(1):155–64. doi: 10.1377/hlthaff.2011.0948. [DOI] [PubMed] [Google Scholar]