Abstract
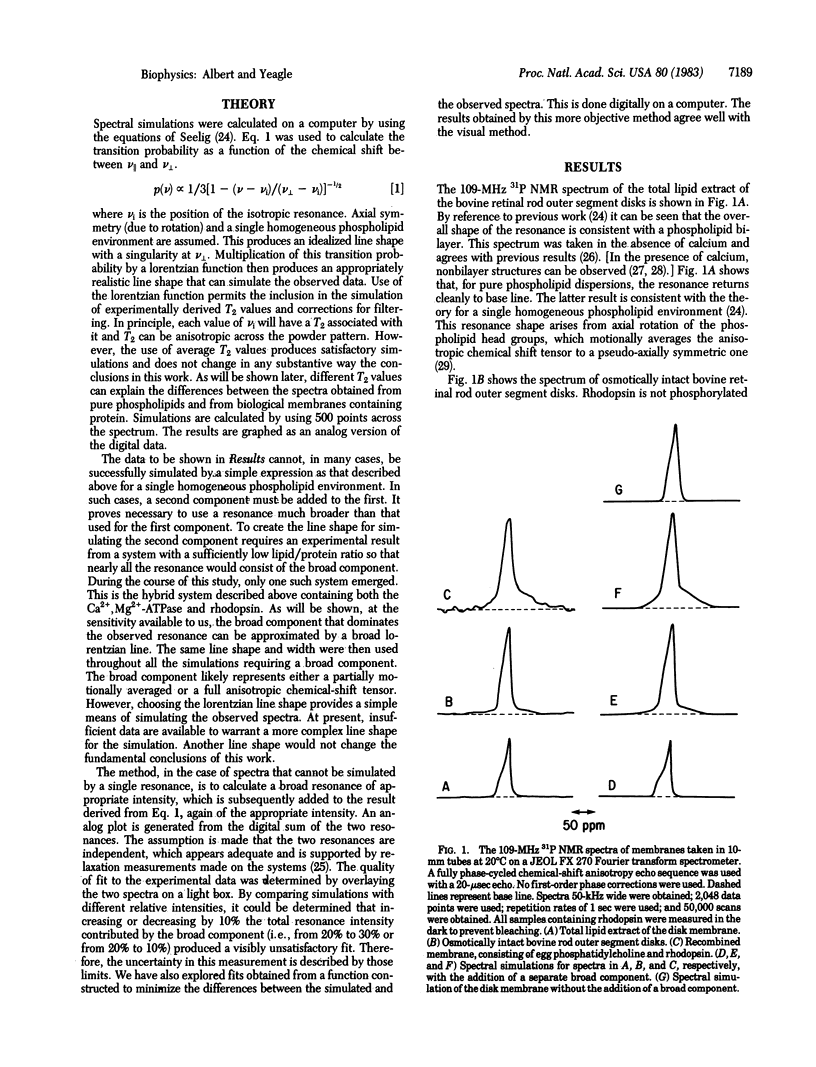
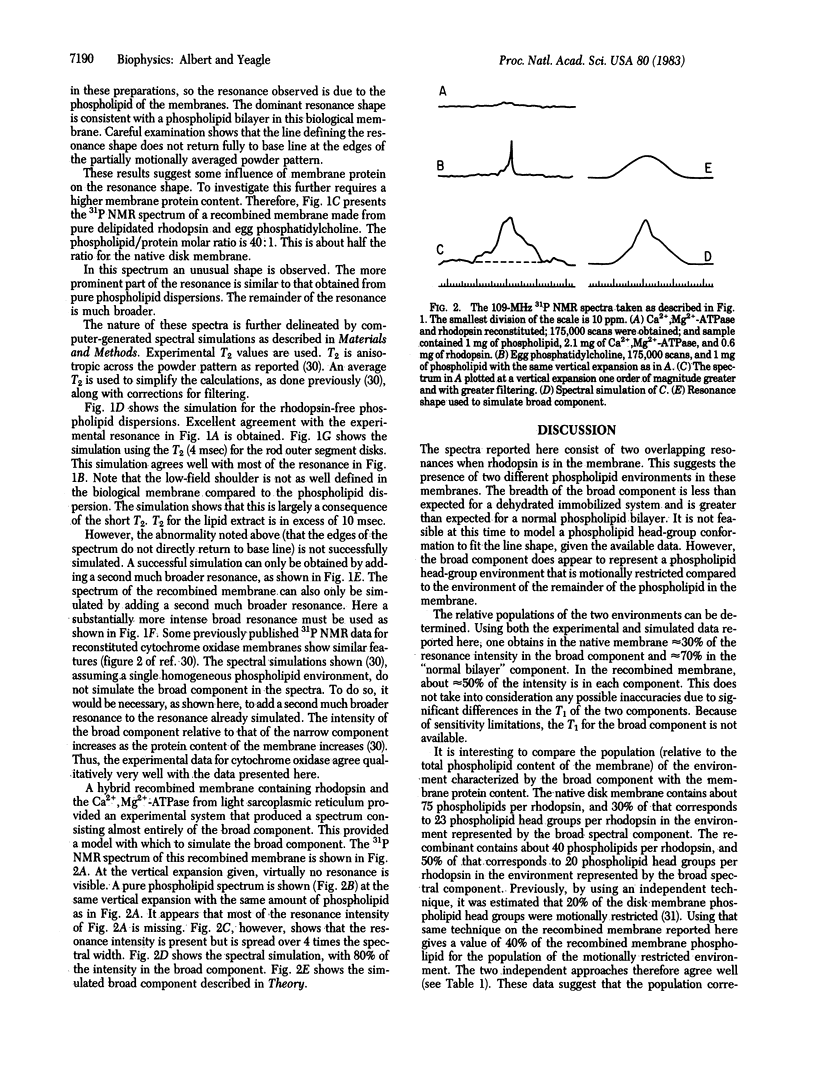
Phospholipid behavior in bovine retinal rod outer segment disk membranes and in phosphatidylcholine membranes containing the photopigment rhodopsin is explored. 31P NMR spectra of these systems show two distinguishable resonances. One resembles closely the 31P NMR resonance normally obtained from phospholipid bilayers. The other resonance is much broader. Thus, there appear to be two phospholipid head-group domains in this retinal membrane. Each environment confers different properties on the head groups. Phosphatidylcholine membranes containing the disk photopigment also show two phospholipid domains. Therefore, the environment in the retinal rod outer segment disk membranes characterized by the broad resonance may arise from the influence of the integral membrane protein rhodopsin on the membrane phospholipid bilayer.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert D. M., Todes-Taylor N., Wagoner M., Nordlund J. J., Lerner A. B. Vitiligo or halo nevi occurring in two patients with choroidal melanoma. Arch Dermatol. 1982 Jan;118(1):34–36. [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Borggreven J. M., Daemen F. J., Bonting S. L. Biochemical aspects of the visual process. VI. The lipid composition of native and hexane-extracted cattle rod outer segments. Biochim Biophys Acta. 1970 Mar 10;202(2):374–381. [PubMed] [Google Scholar]
- Chan S. I., Bocian D. F., Petersen N. O. Nuclear magnetic resonance studies of the phospholipid bilayer membrane. Mol Biol Biochem Biophys. 1981;31:1–50. doi: 10.1007/978-3-642-81537-9_1. [DOI] [PubMed] [Google Scholar]
- Crain R. C., Marinetti G. V., O'Brien D. F. Topology of amino phospholipids in bovine retinal rod outer segment disk membranes. Biochemistry. 1978 Oct 3;17(20):4186–4192. doi: 10.1021/bi00613a012. [DOI] [PubMed] [Google Scholar]
- De Grip W. J., Drenthe E. H., Van Echteld C. J., De Kruijff B., Verkleij A. J. A possible role of rhodopsin in maintaining bilayer structure in the photoreceptor membrane. Biochim Biophys Acta. 1979 Dec 12;558(3):330–337. doi: 10.1016/0005-2736(79)90269-4. [DOI] [PubMed] [Google Scholar]
- Deese A. J., Dratz E. A., Brown M. F. Retinal rod outer segment lipids form bilayers in the presence and absence of rhodopsin: a 31P NMR study. FEBS Lett. 1981 Feb 9;124(1):93–99. doi: 10.1016/0014-5793(81)80061-0. [DOI] [PubMed] [Google Scholar]
- Eletr S., Inesi G. Phospholipid orientation in sarcoplasmic membranes: spin-label ESR and proton MNR studies. Biochim Biophys Acta. 1972 Sep 1;282(1):174–179. doi: 10.1016/0005-2736(72)90321-5. [DOI] [PubMed] [Google Scholar]
- Fernandez J. L., Rosemblatt M., Hidalgo C. Highly purified sarcoplasmic reticulum vesicles are devoid of Ca2+-independent ('basal') ATPase activity. Biochim Biophys Acta. 1980 Jul;599(2):552–568. doi: 10.1016/0005-2736(80)90199-6. [DOI] [PubMed] [Google Scholar]
- Fung B. K., Hurley J. B., Stryer L. Flow of information in the light-triggered cyclic nucleotide cascade of vision. Proc Natl Acad Sci U S A. 1981 Jan;78(1):152–156. doi: 10.1073/pnas.78.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler S. J., Klein M. P. Orientation and dynamics of phospholipid head groups in bilayers and membranes determined from 31P nuclear magnetic resonance chemical shielding tensors. Biochemistry. 1977 Feb 8;16(3):519–526. doi: 10.1021/bi00622a028. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Litman B. J. Lipid model membranes. Characterization of mixed phospholipid vesicles. Biochemistry. 1973 Jun 19;12(13):2545–2554. doi: 10.1021/bi00737a028. [DOI] [PubMed] [Google Scholar]
- Litman B. J. Purification of rhodopsin by concanavalin A affinity chromatography. Methods Enzymol. 1982;81:150–153. doi: 10.1016/s0076-6879(82)81025-2. [DOI] [PubMed] [Google Scholar]
- Montal M., Darszon A., Trissl H. W. Transmembrane channel formation in rhodopsin-containing bilayer membranes. Nature. 1977 May 19;267(5608):221–225. doi: 10.1038/267221a0. [DOI] [PubMed] [Google Scholar]
- Nielsen N. C., Fleischer S., McConnell D. G. Lipid composition of bovine retinal outer segment fragments. Biochim Biophys Acta. 1970 Jul 7;211(1):10–19. doi: 10.1016/0005-2736(70)90118-5. [DOI] [PubMed] [Google Scholar]
- Papermaster D. S., Dreyer W. J. Rhodopsin content in the outer segment membranes of bovine and frog retinal rods. Biochemistry. 1974 May 21;13(11):2438–2444. doi: 10.1021/bi00708a031. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Bitensky M. W. Light-regulated enzymes of vertebrate retinal rods. Adv Cyclic Nucleotide Res. 1979;11:265–301. [PubMed] [Google Scholar]
- Rajan S., Kang S. Y., Gutowsky H. S., Oldfield E. Phosphorus nuclear magnetic resonance study of membrane structure. Interactions of lipids with protein, polypeptide, and cholesterol. J Biol Chem. 1981 Feb 10;256(3):1160–1166. [PubMed] [Google Scholar]
- Seelig J. 31P nuclear magnetic resonance and the head group structure of phospholipids in membranes. Biochim Biophys Acta. 1978 Jul 31;515(2):105–140. doi: 10.1016/0304-4157(78)90001-1. [DOI] [PubMed] [Google Scholar]
- Smith H. G., Jr, Stubbs G. W., Litman B. J. The isolation and purification of osmotically intact discs from retinal rod outer segments. Exp Eye Res. 1975 Mar;20(3):211–217. doi: 10.1016/0014-4835(75)90134-7. [DOI] [PubMed] [Google Scholar]
- WALD G., BROWN P. K., GIBBONS I. R. The problem of visual excitation. J Opt Soc Am. 1963 Jan;53:20–35. doi: 10.1364/josa.53.000020. [DOI] [PubMed] [Google Scholar]
- Warren G. B., Toon P. A., Birdsall N. J., Lee A. G., Metcalfe J. C. Reconstitution of a calcium pump using defined membrane components. Proc Natl Acad Sci U S A. 1974 Mar;71(3):622–626. doi: 10.1073/pnas.71.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts A., Volotovski I. D., Marsh D. Rhodopsin-lipid associations in bovine rod outer segment membranes. Identification of immobilized lipid by spin-labels. Biochemistry. 1979 Oct 30;18(22):5006–5013. doi: 10.1021/bi00589a031. [DOI] [PubMed] [Google Scholar]
- Yeagle P. L. 31P nuclear magnetic resonance studies of the phospholipid-protein interface in cell membranes. Biophys J. 1982 Jan;37(1):227–239. doi: 10.1016/S0006-3495(82)84672-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Breugel P. J., Geurts P. H., Daemen F. J., Bonting S. L. Biochemical aspects of the visual process. XXXVIII. Effects of lateral aggregation on rhodopsin in phospholipase C-treated photoreceptor membranes. Biochim Biophys Acta. 1978 May 4;509(1):136–147. doi: 10.1016/0005-2736(78)90014-7. [DOI] [PubMed] [Google Scholar]


