Abstract
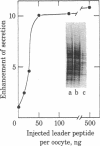
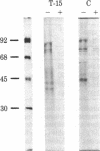
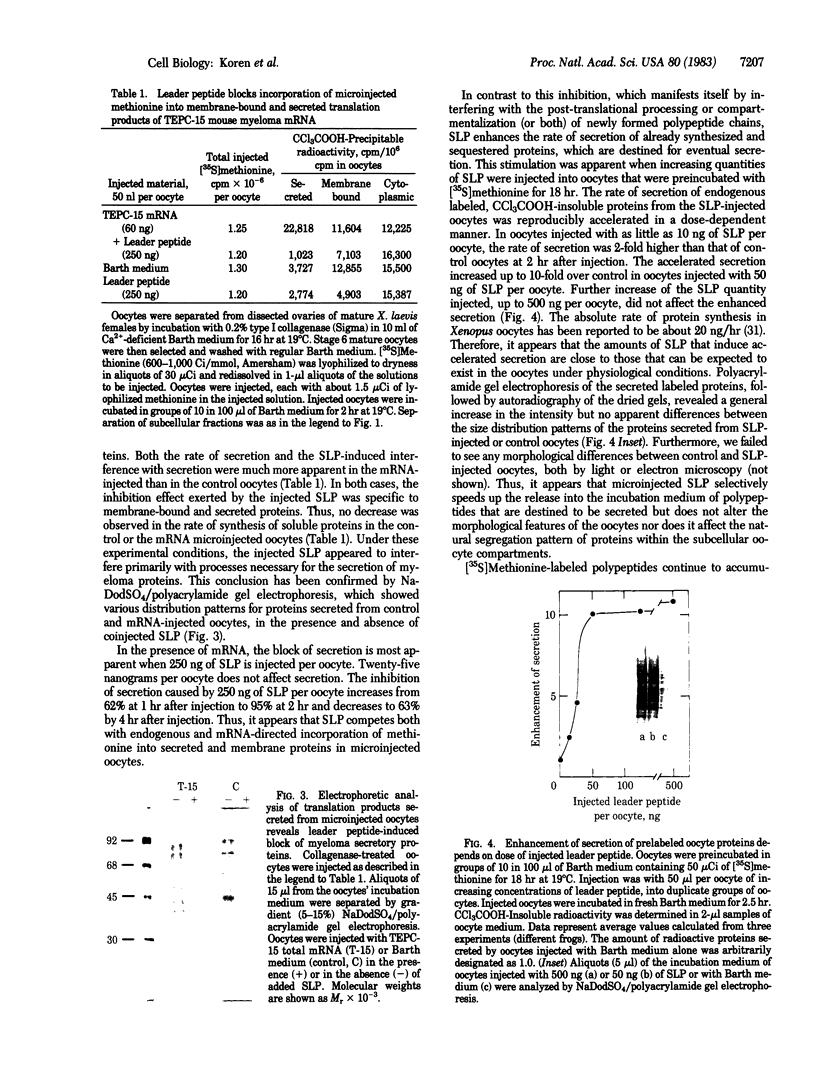
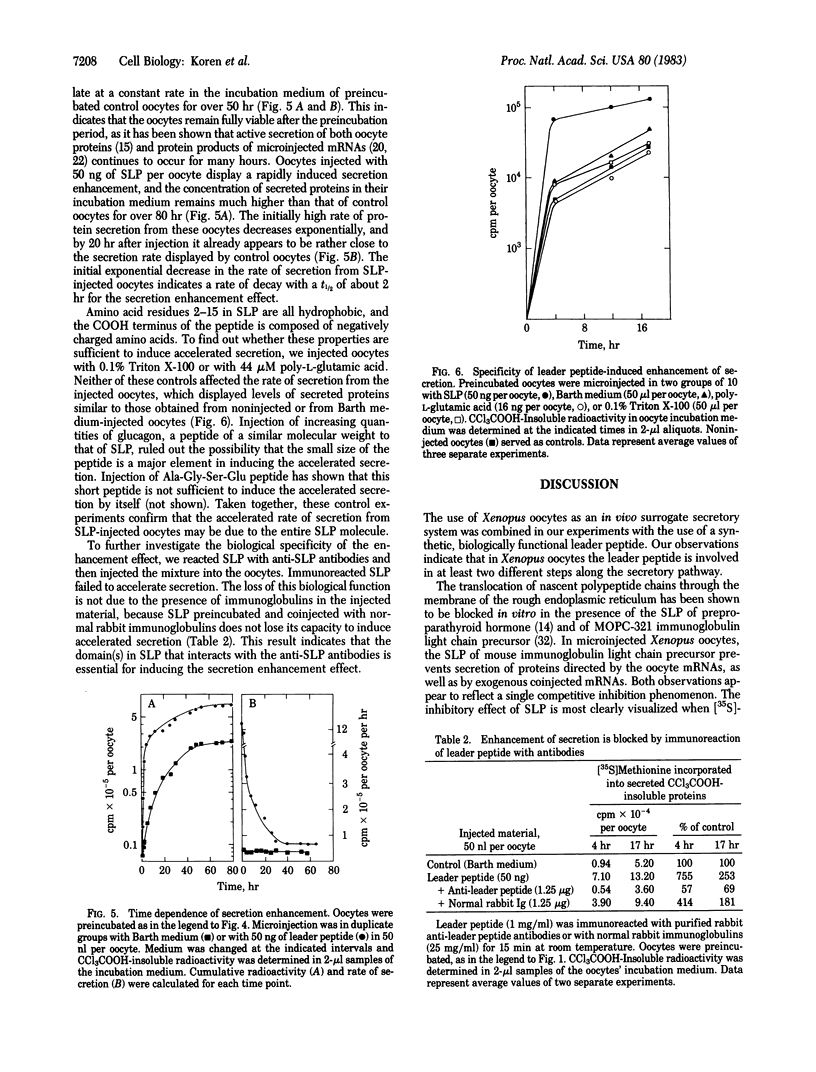
To investigate the role of the leader peptide in modulating secretion from living cells, we injected a synthetic peptide into Xenopus oocytes. The peptide consisted of the NH2-terminal leader sequence of mouse immunoglobulin light chain precursor. We found that the leader peptide has two different roles in regulating secretion from the oocytes. First, it competitively inhibits the synthesis of secretory and membrane proteins but not of cytoplasmic proteins. The inhibition occurs both with oocyte proteins and with proteins directed by coinjected myeloma mRNA. The inhibition reaches a maximum 2 hr after injection and decays within 3 hr. It appears to be mediated through the cell membrane, because 125I-labeled leader peptide segregates into the membrane fraction of microinjected oocytes simultaneously with the interference with methionine incorporation. A second role of the microinjected leader peptide is to induce a rapid acceleration in the rate of export of secretory proteins from the oocyte. The maximal enhancement effect is obtained upon injection of 50 ng of leader peptide per oocyte. It is not merely due to the small size, negative charge, or hydrophobicity of the peptide, because enhanced secretion does not occur when glucagon, poly-L-glutamic acid, or Triton X-100 is injected. Furthermore, immunoreaction of the peptide with specific antibodies prior to microinjection prevents the accelerated export. Our observations indicate that in Xenopus oocytes, the leader peptide is involved in both translocation and later step(s) in the secretory pathway.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedouelle H., Bassford P. J., Jr, Fowler A. V., Zabin I., Beckwith J., Hofnung M. Mutations which alter the function of the signal sequence of the maltose binding protein of Escherichia coli. Nature. 1980 May 8;285(5760):78–81. doi: 10.1038/285078a0. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman A., Lane C. D., Craig R., Boulton A., Mohun T., Morser J. The influence of topology and glycosylation on the fate of heterologous secretory proteins made in Xenopus oocytes. Eur J Biochem. 1981 Jan;113(2):339–348. doi: 10.1111/j.1432-1033.1981.tb05072.x. [DOI] [PubMed] [Google Scholar]
- Colman A., Morser J. Export of proteins from oocytes of Xenopus laevis. Cell. 1979 Jul;17(3):517–526. doi: 10.1016/0092-8674(79)90260-5. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Farquhar M. G., Palade G. E. The Golgi apparatus (complex)-(1954-1981)-from artifact to center stage. J Cell Biol. 1981 Dec;91(3 Pt 2):77s–103s. doi: 10.1083/jcb.91.3.77s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habener J. F., Rosenblatt M., Dee P. C., Potts J. T., Jr Cellular processing of pre-proparathyroid hormone involves rapid hydrolysis of the leader sequence. J Biol Chem. 1979 Nov 10;254(21):10596–10599. [PubMed] [Google Scholar]
- Katz F. N., Rothman J. E., Lingappa V. R., Blobel G., Lodish H. F. Membrane assembly in vitro: synthesis, glycosylation, and asymmetric insertion of a transmembrane protein. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3278–3282. doi: 10.1073/pnas.74.8.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreil G. Transfer of proteins across membranes. Annu Rev Biochem. 1981;50:317–348. doi: 10.1146/annurev.bi.50.070181.001533. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane C. D., Colman A., Mohun T., Morser J., Champion J., Kourides I., Craig R., Higgins S., James T. C., Applebaum S. W. The Xenopus oocyte as a surrogate secretory system. The specificity of protein export. Eur J Biochem. 1980 Oct;111(1):225–235. doi: 10.1111/j.1432-1033.1980.tb06097.x. [DOI] [PubMed] [Google Scholar]
- Lane C. D., Marbaix G., Gurdon J. B. Rabbit haemoglobin synthesis in frog cells: the translation of reticulocyte 9 s RNA in frog oocytes. J Mol Biol. 1971 Oct 14;61(1):73–91. doi: 10.1016/0022-2836(71)90207-5. [DOI] [PubMed] [Google Scholar]
- Lane C., Shannon S., Craig R. Sequestration and turnover of guinea-pig milk proteins and chicken ovalbumin in Xenopus oocytes. Eur J Biochem. 1979 Nov;101(2):485–495. doi: 10.1111/j.1432-1033.1979.tb19743.x. [DOI] [PubMed] [Google Scholar]
- Li C. H., Yamashiro D., Lemaire S. Total synthesis of camel beta-melanotropin by the solid-phase method. Biochemistry. 1975 Mar 11;14(5):953–956. doi: 10.1021/bi00676a012. [DOI] [PubMed] [Google Scholar]
- Majzoub J. A., Rosenblatt M., Fennick B., Maunus R., Kronenberg H. M., Potts J. T., Jr, Habener J. F. Synthetic pre-proparathyroid hormone leader sequence inhibits cell-free processing of placental, parathyroid, and pituitary prehormones. J Biol Chem. 1980 Dec 10;255(23):11478–11483. [PubMed] [Google Scholar]
- Meyer D. I., Krause E., Dobberstein B. Secretory protein translocation across membranes-the role of the "docking protein'. Nature. 1982 Jun 24;297(5868):647–650. doi: 10.1038/297647a0. [DOI] [PubMed] [Google Scholar]
- Meyer D. I., Louvard D., Dobberstein B. Characterization of molecules involved in protein translocation using a specific antibody. J Cell Biol. 1982 Feb;92(2):579–583. doi: 10.1083/jcb.92.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Mohun T. J., Lane C. D., Colman A., Wylie C. C. The secretion of proteins in vitro from Xenopus oocytes and their accessory cells: a biochemical and morphological study. J Embryol Exp Morphol. 1981 Feb;61:367–383. [PubMed] [Google Scholar]
- Rapoport T. A. Intracellular compartmentation and secretion of carp proinsulin synthesized in Xenopus oocytes. Eur J Biochem. 1981 Apr;115(3):665–669. doi: 10.1111/j.1432-1033.1981.tb06254.x. [DOI] [PubMed] [Google Scholar]
- Richter J. D., Smith L. D. Differential capacity for translation and lack of competition between mRNAs that segregate to free and membrane-bound polysomes. Cell. 1981 Nov;27(1 Pt 2):183–191. doi: 10.1016/0092-8674(81)90372-x. [DOI] [PubMed] [Google Scholar]
- Richter J. D., Wasserman W. J., Smith L. D. The mechanism for increased protein synthesis during Xenopus oocyte maturation. Dev Biol. 1982 Jan;89(1):159–167. doi: 10.1016/0012-1606(82)90304-9. [DOI] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreq H., Parvari R., Silman I. Biosynthesis and secretion of catalytically active acetylcholinesterase in Xenopus oocytes microinjected with mRNA from rat brain and from Torpedo electric organ. Proc Natl Acad Sci U S A. 1982 Feb;79(3):830–834. doi: 10.1073/pnas.79.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczesna E., Boime I. mRNA-dependent synthesis of authentic precursor to human placental lactogen: conversion to its mature hormone form in ascites cell-free extracts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1179–1183. doi: 10.1073/pnas.73.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge K., Stahl S., Gilbert W. Eukaryotic signal sequence transports insulin antigen in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3369–3373. doi: 10.1073/pnas.77.6.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum. II. Signal recognition protein (SRP) mediates the selective binding to microsomal membranes of in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):551–556. doi: 10.1083/jcb.91.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Ibrahimi I., Blobel G. Translocation of proteins across the endoplasmic reticulum. I. Signal recognition protein (SRP) binds to in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):545–550. doi: 10.1083/jcb.91.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehavi-Willner T., Lane C. Subcellular compartmentation of albumin and globin made in oocytes under the direction of injected messenger RNA. Cell. 1977 Jul;11(3):683–693. doi: 10.1016/0092-8674(77)90085-x. [DOI] [PubMed] [Google Scholar]