SUMMARY
Wnt/β-catenin signaling is a central regulator of adult stem cells. Variable sensitivity of Wnt reporter transgenes, β-catenin’s dual roles in adhesion and signaling, and hair follicle degradation and inflammation resulting from broad deletion of epithelial β-catenin, have precluded clear understanding of Wnt/β-catenin’s functions in adult skin stem cells. By inducibly deleting β-catenin globally in skin epithelia, only in hair follicle stem cells, or only in interfollicular epidermis, and comparing the phenotypes with those caused by ectopic expression of the Wnt/β-catenin inhibitor Dkk1, we show that this pathway is necessary for hair follicle stem cell proliferation. However, β-catenin is not required within hair follicle stem cells for their maintenance, and follicles resume proliferating after removal of ectopic Dkk1, indicating persistence of functional progenitors. We further unexpectedly discovered a broader role for Wnt/β-catenin signaling in contributing to progenitor cell proliferation in non-hairy epithelia and interfollicular epidermis under homeostatic, but not inflammatory, conditions.
INTRODUCTION
The Wnt/β-catenin signaling pathway is broadly utilized in development and controls the activity of embryonic and adult stem cell populations. Signaling is initiated by Wnt ligands that bind Frizzled (FZD) receptors and LRP5/6 co-receptors, resulting in inactivation of a complex of proteins that targets cytoplasmic β-catenin for degradation. As a consequence, β-catenin accumulates in the cytoplasm, and translocates to the nucleus where it partners with members of the LEF/TCF family of DNA binding factors to activate target gene expression (McNeill and Woodgett, 2010). Members of the Dickkopf (DKK) family of secreted Wnt inhibitors specifically inhibit Wnt/LRP signaling by forming a complex with LRP and Kremen (KRM) receptors that is internalized, removing LRP from the membrane (Mao et al., 2002).
Certain Wnt ligands, and Wnt-FZD pairings, initiate signaling through non-canonical pathways that are independent of both LRP and β-catenin. Furthermore, the β-catenin pathway can be activated by non-Wnt ligands (McNeill and Woodgett, 2010). Adding to the complexity of its biological functions, β-catenin is a component of adherens junctions, and plays dual roles in adhesion and signaling (Incassati et al., 2010). Thus manipulation of β-catenin may lead to phenotypes that are independent of Wnt ligands and LRP.
Hair follicles (HFs) regenerate periodically throughout life, providing an accessible system for delineating molecular mechanisms controlling adult stem cell proliferation and maintenance. In mice, HF morphogenesis is completed in the two weeks following birth, when rapidly proliferating epithelial matrix cells almost completely surround the mesenchymal component of the HF, known as the dermal papilla (DP), and differentiate to produce the hair shaft and an inner root sheath (IRS) that molds the hair shaft as it emerges from the follicle. The follicle is bounded by an outer root sheath (ORS) that is contiguous with the epidermis, and contains a population of rarely cycling epithelial stem cells in a specialized niche known as the bulge (Myung and Ito, 2012) (Fig. 1A).
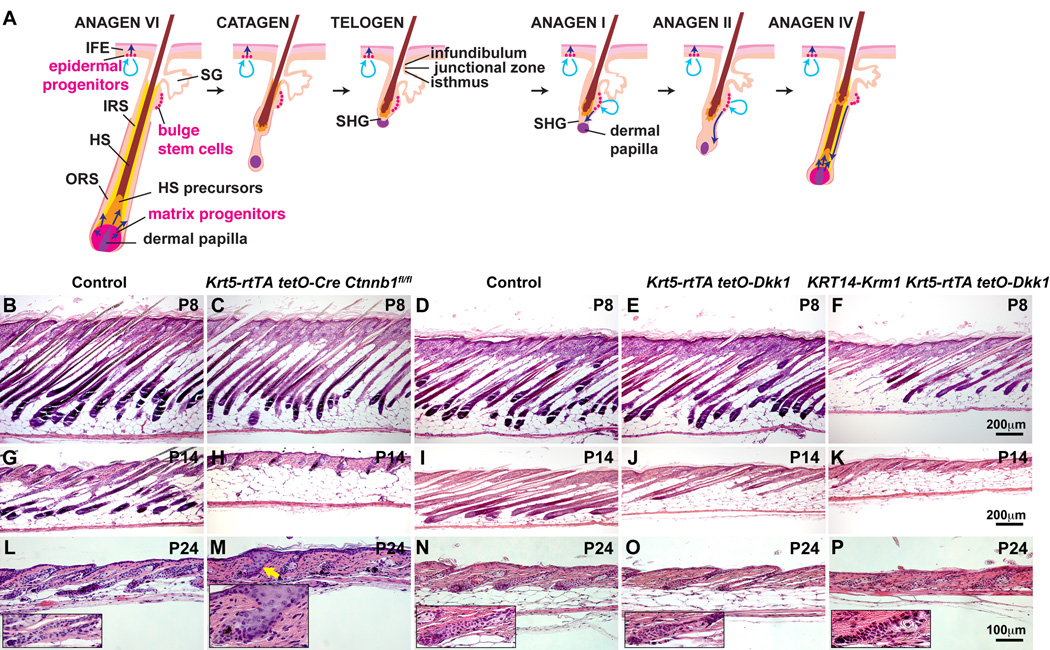
Fig. 1. Inducible postnatal ectopic expression of Dkk1, or deletion of epithelial β-catenin causes rapid regression of anagen HFs.
(A) Schematic of HF growth cycle and structure. Light blue arrows indicate stem cell self-renewal. Dark blue arrows indicate cell movements. (B–P) Krt5-rtTA tetO-Cre Ctnnb1fl/fl mutants, Dkk1 transgenics and Dkk1/Krm1 transgenics, and their respective littermate controls were induced from P4 (B–K) or P7 (L–P) and H&E stained skin sections photographed at the stages indicated. Arrow (M) indicates structural abnormalities in β-catenin mutant HF. Insets in (L–P) represent higher magnification photographs. Scale bars: (B–K) 200 µm and (L–P) 100 µm. See also Fig. S1.
At the end of the growth phase (anagen), the lower two thirds of the follicle, including the matrix and lower part of the IRS, undergo programmed regression (catagen), before entering a telogen resting phase (Fig. 1A). The telogen HF retains bulge stem cells, and a distinct population of secondary hair germ (SHG) cells that abut the DP. SHG cells possess lower proliferative potential than bulge cells in vitro, but in vivo they can replenish the bulge following its destruction, indicating that they hold stem cell potential (Myung and Ito, 2012). Onset of a new anagen growth phase is preceded by proliferation of SHG cells, which begin to populate a new matrix, while transient proliferation of bulge cells occurs in very early anagen (Myung and Ito, 2012). Additional stem cell populations in the HF include Lrig1-expressing cells in the junctional zone between the bulge and the infundibulum that can contribute to adjacent interfollicular epidermis (IFE) but do not give rise to the bulge or lower follicle, and Lgr6-positive cells in the isthmus that can contribute to sebaceous gland and IFE (Myung and Ito, 2012). Despite intense investigation, the molecular signals regulating HF proliferation and maintenance of the bulge stem cell population are not fully understood.
Wnt/LRP/β-catenin signaling is required for embryonic HF morphogenesis but is dispensable for development of IFE (Andl et al., 2002; Huelsken et al., 2001). Forced activation of β-catenin signaling converts embryonic ectoderm to a HF-like fate (Narhi et al., 2008; Zhang et al., 2008), and in adult skin promotes de novo HF formation from epidermal cells (Gat et al., 1998), indicating that in favorable developmental contexts, high levels of β-catenin signaling direct acquisition of appendage identity.
Nuclear-localized β-catenin and/or Wnt reporter transgene activity have been described in HF SHG at anagen onset, and in the matrix, DP and hair shaft precursor cells during anagen, but are low or undetectable in telogen HFs (DasGupta and Fuchs, 1999; Maretto et al., 2003). Loss of β-catenin in postnatal DP or epithelial deletion of Wntless (WLS), a protein required for efficient secretion of both canonical and non-canonical Wnt ligands, cause failure of matrix cell proliferation and premature catagen (Enshell-Seijffers et al., 2010; Myung et al., 2012). It is not clear whether the effects of Wls deletion are mediated primarily through the DP or HF epithelia, or reflect contributions of non-canonical Wnt signaling. However, proliferation of progenitor cells in response to forced expression of stabilized β-catenin, and the effects of injection of recombinant DKK1 on hair follicle growth, suggest functions for Wnt/β-catenin signaling in HF epithelial cells during anagen (Kwack et al., 2012; Lowry et al., 2005; Van Mater et al., 2003). Global deletion of epithelial β-catenin in telogen causes stem cell depletion (Lowry et al., 2005), but whether this is due to a direct requirement for β-catenin in stem cells is unknown. Furthermore, the effects of epithelial β-catenin deletion at other stages of the growth cycle, and the consequences of specifically inhibiting canonical Wnt signaling upstream of β-catenin, have not been systematically investigated.
Unlike the HF, which proliferates periodically, basal IFE is active throughout life, both renewing itself and generating cells that differentiate to form a cornified layer that is continuously shed. While expression of the TOPGAL Wnt reporter transgene is undetectable in the IFE (DasGupta and Fuchs, 1999), expression of other, more sensitive reporters, and possible functions of β-catenin signaling in adult IFE in vivo, have not been examined.
Here we show, using two, independent, sensitive in vivo reporters, that Wnt/β-catenin signaling is active in IFE and specialized non-hairy epithelia as well as in anagen HFs. Using multiple genetic approaches to manipulate signaling in specific cell types, we demonstrate that epithelial β-catenin signaling is required for maintenance of proliferation in anagen HFs and contributes to proliferation of footpad and tongue, but is not required within the HF bulge and SHG for stem cell survival. Consistent with this, hair re-growth occurs spontaneously after removal of Wnt/β-catenin signaling inhibition. To analyze the role of β-catenin in the IFE of hairy skin, we developed a novel system that permits gene deletion specifically in IFE while sparing the hair follicle bulge, SHG and DP, allowing for analysis of IFE phenotypes in the absence of inflammatory reactions associated with HF degradation. These experiments revealed a previously unknown role for β-catenin signaling in contributing to proliferation of IFE in vivo.
RESULTS
Wnt/β-catenin signaling is active in basal IFE and non-hairy epithelia as well as in anagen HFs
To assay for Wnt/β-catenin signaling in postnatal skin, we utilized Axin2lacZ, in which lacZ is inserted into the endogenous Axin2 locus, a ubiquitous Wnt target (Lustig et al., 2002; Yu et al., 2005), and TCF/Lef:H2B-GFP (TL-GFP) in which six copies of a TCF/LEF responsive element and an hsp68 minimal promoter drive expression of an H2B-GFP fusion protein (Ferrer-Vaquer et al., 2010). Sensitivity of the TL-GFP reporter is supported by its activity at sites of Wnt/β-catenin signaling not documented with other reporters but confirmed through genetic analysis (Ferrer-Vaquer et al., 2010). Expression of Axin2lacZ and TL-GFP was low or undetectable in the bulge, SHG and DP of telogen HFs (Fig. S1D,H). Both reporters were expressed in the DP, SHG and matrix in early and mid-anagen, and very strongly in hair shaft precursor cells at midanagen (Fig. S1A,B,C,E,G). Unexpectedly, both Axin2lacZ and TL-GFP were expressed at low levels in IFE (Fig. S1B,D,E,F,H). TL-GFP-expressing IFE cells were observed in basal and suprabasal layers. Axin2lacZ-positive cells were confined to basal cells, suggesting that suprabasal expression of TL-GFP may be due to perdurance of H2BGFP. The number of positive cells and intensity of reporter expression in IFE increased with age (Fig. S1F,H). Axin2lacZ and TL-GFP were also expressed in stratified tongue epithelia (Fig. S1I,K). Axin2lacZ, but not TL-GFP, was expressed in adult footpad epidermis (Fig. S1J,L), perhaps reflecting greater sensitivity of the Axin2lacZ reporter in the footpad, or increased signaling in mice heterozygous for loss of Axin2 function. Consistent with Wnt/β-catenin signaling in IFE, several Wnt ligands and FZD receptors are expressed in embryonic and adult IFE as well as in HFs (Reddy et al., 2001; Reddy et al., 2004) (Fig. S1M,N).
Epithelial β-catenin deletion or ectopic Dkk1 expression induced during embryonic anagen causes rapid hair follicle regression
To delineate the requirements for Wnt/β-catenin signaling at successive stages of the embryonic hair growth cycle, we compared the effects of doxycycline inducible deletion of β-catenin and inducible ectopic expression of DKK1, which inhibits signaling at the level of the LRP co-receptor. Mice carrying a Krt5-rtTA transgene (Diamond et al., 2000) in which a reverse tet transactivator is expressed in basal epidermis and HF ORS including bulge stem cells were mated to mice carrying tetO-Cre (Mucenski et al., 2003) and a conditional null allele of Ctnnb1 (Brault et al., 2001), or to tetO-Dkk1 mice (Chu et al., 2004) (Fig. S1O–S). Inducible Dkk1 was expressed at higher levels in HFs than in IFE (Fig. S1S). In contrast with published data (Kwack et al., 2012), we were not able to detect significant levels of expression of endogenous Dkk1 by in situ hybridization at any stage of the adult HF growth cycle. The Wnt/LRP inhibitory actions of DKK1 require interaction with KRM (Mao et al., 2002), which is expressed at low levels in postnatal skin (Fig. S1T,U). As limiting levels of KRM may restrict the effectiveness of DKK1-mediated inhibition, we generated KRT14-Krm1 mice that constitutively expressed high levels of Krm1 in epithelial cells (Fig. S1V) and assayed the effects of co-expressed Krm1 and Dkk1 on hair growth. KRT14-Krm1 mice did not display detectable abnormalities in skin histology or in timing of the hair growth cycle in the absence of co-expressed Dkk1 (Fig. S1W,X).
Experimental Krt5-rtTA tetO-Cre Ctnnb1fl/fl (β-catenin mutant), Krt5-rtTA tetO-Dkk1 (Dkk1 transgenic), or Krt14-Krm1 Krt5-rtTA tetO-Dkk1 (Dkk1/Krm1 transgenic) mice and their respective control littermates were doxycycline treated from postnatal day (P) 4 (Fig. 1B–K) or 7 (Fig. 1L–P), and dorsal skin was harvested at P8, P14, and P24. Loss of epithelial β-catenin or forced expression of Dkk1 caused rapid cessation of anagen and entry into a premature regression phase compared with controls (Fig. 1B–E;G–J;L–O). The effects of Dkk1 were enhanced by co-expression with Krm1 (Fig. 1F,K,P). As HF regression is caused by either epithelial β-catenin deletion or LRP signaling inhibition, these data indicate that canonical Wnt/LRP/β-catenin pathway activity is required within epithelial cells to maintain anagen. At P24, HFs in epithelial β-catenin-deleted, but not Dkk1 or Dkk1/Krm1-expressing skin displayed structural defects including a widened infundibulum and loss of a clearly distinguishable SHG (Fig. 1M, arrow; see inset), suggesting additional, LRP-independent roles for β-catenin in telogen (Fig. 1L–P, see insets).
Rapid regression of mutant hair follicles is associated with inhibition of cell proliferation but bulge stem cell markers are maintained
To determine the underlying cause of rapid regression of β-catenin deficient and Dkk1 expressing HFs, we assayed for changes in cell proliferation and apoptosis. Ki67 immunofluorescence at P8 or P14 revealed greatly reduced proliferation in both β-catenin mutant (Fig. 2B,G) and Dkk1 or Dkk1/Krm1 transgenic (Fig. 2D,E,I,J) follicles compared with controls (Fig. 2A,C,F,H). Similarly, Dkk1-expressing hair bulb sections contained a mean of 16±3% BrdU-positive nuclei after 4 days of induction at P8, compared with 36±5% in controls (n=17 HFs from 2 control mice and 13 HFs from 2 transgenic mice) (Fig. S2A–C). β-catenin-deleted and Dkk1-expressing HFs showed greatly diminished expression of cyclin D1, a direct Wnt/β-catenin target gene that helps initiate transition from late G1 to S phase of the cell cycle (Kobielak et al., 2003; Tetsu and McCormick, 1999), likely contributing to decreased HF matrix proliferation (Fig. S2D–G).
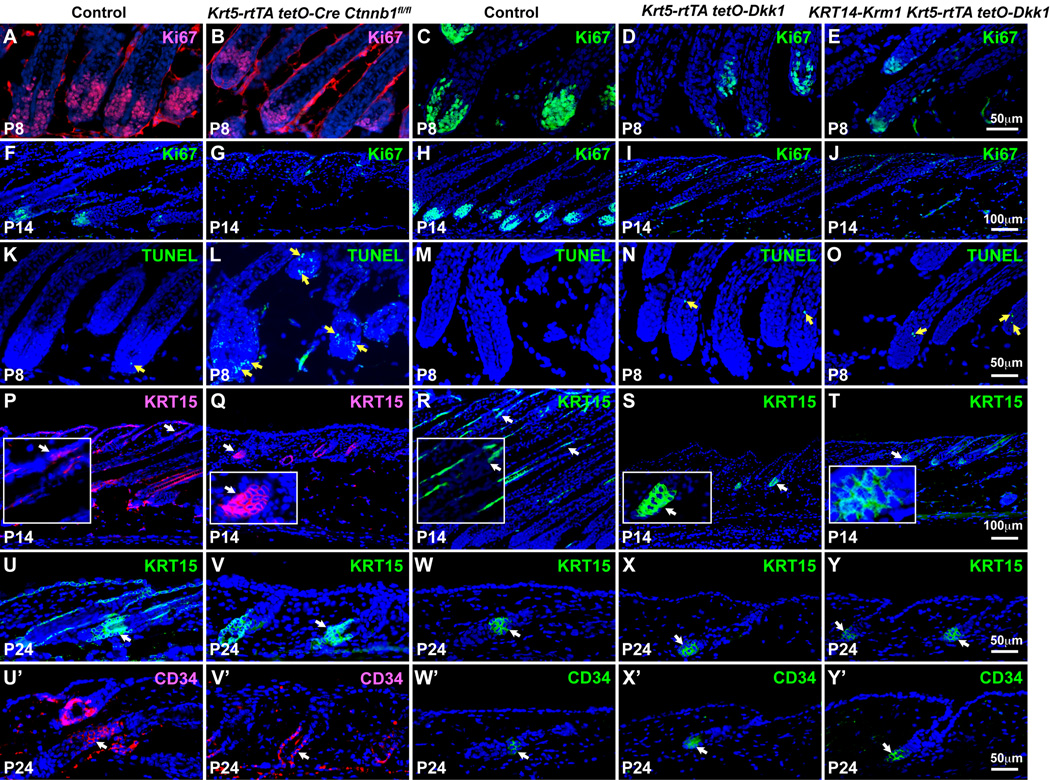
Fig. 2. Inducible β-catenin deletion or Dkk1 expression in anagen inhibits hair follicle matrix proliferation without loss of stem cells.
Krt5-rtTA tetO-Cre Ctnnb1fl/flmutants, Dkk1 transgenics and Dkk1/Krm1 transgenics, and their respective littermate controls mice were induced from P4 (A–T) or P7 (U–Y’) and skin samples harvested at the stages indicated. (A–J) Ki67 immunofluorescence (A,B, red; C–J, green). (K–O) TUNEL staining (green) (yellow arrows). (P–Y) KRT15 immunofluorescence (P,Q, red; RY, green) (white arrows). Insets in (P–T) represent higher magnification photographs of regions indicated by arrows. (U’–Y’) CD34 immunofluorescence (U’,V’, red; W’–Y’, green) (white arrows). Scale bars: 50 µm (A–E, K–O, U–Y’); 100 µm (F–J, P–T). See also Fig. S2.
Consistent with accelerated entry into catagen, P8 Dkk1- and Dkk1/Krm1-expressing follicles contained slightly increased numbers of apoptotic cells compared with controls (Fig. 2M–O, yellow arrows). Although the timing of regression was similar in β-catenin-deleted and Dkk1/Krm1-expressing follicles, many more TUNEL-positive cells were observed in β-catenin mutant HF matrix (Fig. 2L,O).
Interestingly, expression of both KRT15, a marker for epithelial stem cells in the HF bulge and SHG (Morris et al., 2004), and CD34, which specifically marks bulge stem cells (Trempus et al., 2003), was readily detected at P14 and P24 in β-catenin mutants and Dkk1 transgenics induced from P4 and P7, respectively (Fig. 2P–Y’), indicating that cessation of anagen was not due to immediate loss of the stem cell compartment.
β-catenin deletion or ectopic Dkk1 block both plucking-induced and spontaneous anagen
To determine whether adult anagen onset requires β-catenin or signaling through LRP we doxycycline treated Krt5-rtTA tetO-Cre Ctnnb1fl/fl and Dkk1 or Dkk1/Krm1 transgenic mice and their respective littermate controls from P50 (telogen) and plucked hair at P54 to induce a new growth phase. At three days post-plucking (3DPP), SHG cells in control follicles gave rise to a primitive matrix that began to surround the DP (Fig. 3A,C). High levels of proliferation were observed in the developing matrix and in the upper ORS (Fig. 3F,H), and nuclear localized β-catenin was prominently present in the matrix and at lower levels in the DP (Fig. 3K,M). Deletion of epithelial β-catenin caused failure of matrix cells to migrate around the DP (Fig. 3B). Upper regions of the mutant HFs proliferated in response to plucking; however, proliferation of the SHG was markedly decreased or absent (Fig. 3G).
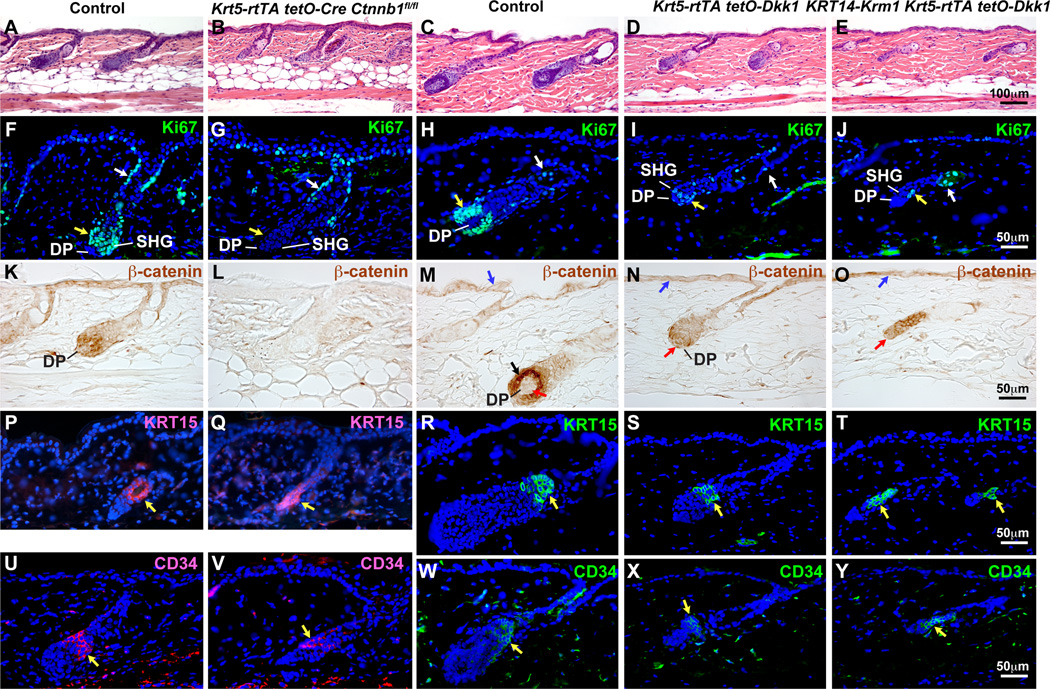
Fig. 3. Inducible β-catenin deletion or ectopic Dkk1 expression blocks plucking-induced anagen.
Krt5-rtTA tetO-Cre Ctnnb1fl/flmutants, Dkk1 transgenics and Dkk1/Krm1 transgenics, and their respective littermate controls were induced from P50, hair plucked at P54, and dorsal skin analyzed at P57. Paraffin sections were stained with H&E (A–E), or immunostained for Ki67 (green) (F–J), β-catenin (brown) (K–O), KRT15 (P,Q, red; R–T, green), or CD34 (U,V, red; W–Y, green). In (F–G), yellow arrows indicate SHG; white arrows indicate upper ORS. In (M–O), black arrow indicates SHG; blue arrows indicate IFE; red arrows indicate DP. Yellow arrows in (P–Y) indicate positive signals. Scale Bars: 100µm (A–E); 50µm (F–Y). See also Figs. S2 and S3.
Dkk1 double transgenic and Dkk1 Krm1 triple transgenic follicles displayed signs of very early anagen at 3DPP with limited migration of epithelial cells around the DP (Fig. 3C–E). Proliferation of the SHG was severely reduced compared with controls, but not completely absent (Fig. 3H–J). Presence of β-catenin at the cell membrane was unaffected by ectopic Dkk1 (Fig. 3M–O, blue arrows); however nuclear β-catenin was dramatically reduced in Dkk1 expressing matrix cells (Fig. 3M–O, black arrow; Fig. S3A,B), and expression of the Wnt target gene cyclin D1 was almost completely absent (Fig. S3C,D). Dkk1-expressing HF failed to progess through anagen, indicated by decreased BMP signaling, which is required for differentiation of hair shaft and IRS (Andl et al., 2004; Kobielak et al., 2003) (Fig. S3I,J), and absence of markers for IRS and hair shaft differentiation (Fig. S3K–N). Nuclear β-catenin and Axin2lacZ reporter expression were reduced in the epithelium but still present in the DP of most Dkk1-expressing follicles (Fig. 3M–O; Fig. S3A,B,E–H, red arrows) suggesting that Dkk1-mediated inhibition did not extend effectively to DP cells.
As plucking-induced anagen may induce a wound-like response in the skin, we also tested whether epithelial β-catenin deletion or ectopic Dkk1 or Dkk1/Krm1 blocked entry of HFs into spontaneous anagen. In mice induced from P17 (catagen), control HFs entered early anagen by P25 and were in full anagen at P30. By contrast, β-catenin mutant follicles failed to enter anagen (Fig. S2H–K). Dkk1-expressing follicles were arrested in early anagen at P30; this effect was more pronounced in follicles coexpressing Dkk1 and Krm1 (Fig. S3O–T).
β-catenin is required within bulge and SHG cells for anagen initiation
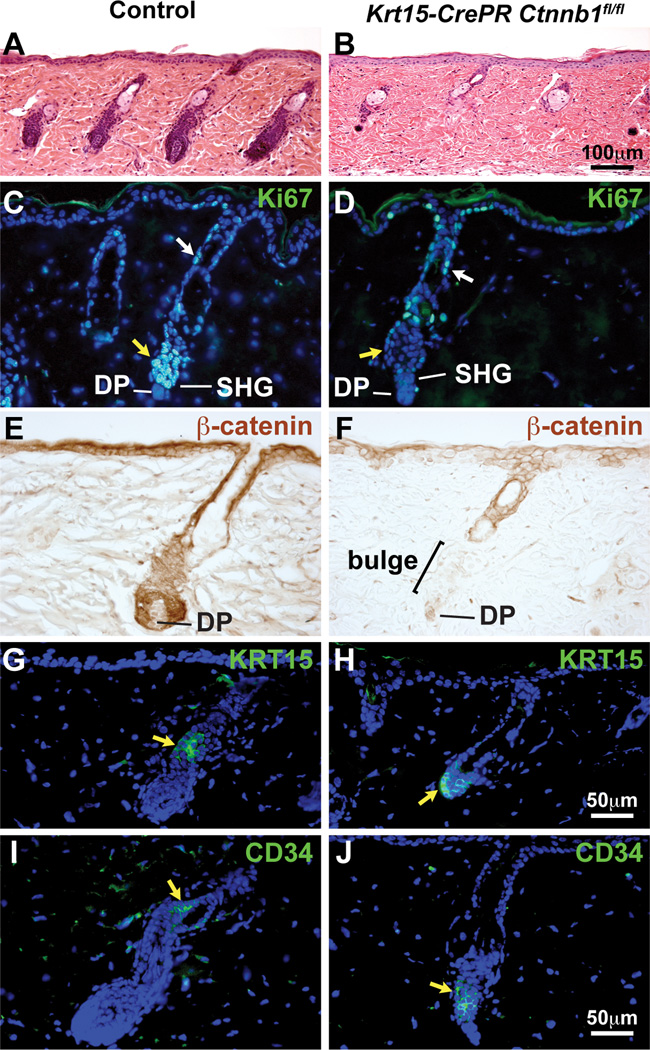
To determine whether β-catenin is required within HF epithelial stem cells for anagen initiation, we generated Krt15-CrePR1 Ctnnb1fl/fl mice in which deletion of β-catenin can be induced specifically in Krt15 promoter-active bulge and SHG cells by topical treatment with mifepristone (RU486). Krt15-CrePR1 Ctnnb1fl/fl mice were induced for 5 days prior to hair plucking at P54. β-catenin was efficiently deleted in CD34-positive HF bulge and SHG cells in mutant skin at 5DPP but was retained in the upper HF above the bulge (Fig. S2L,M; Fig. 4E,F). Unlike littermate controls, which displayed robust hair regrowth by 14DPP, Krt15-CrePR1 Ctnnb1fl/fl mutant HF did not progress through anagen (Fig. 4A,B), SHG proliferation was severely decreased or absent (Fig. 4C,D), and external hair growth failed (Fig. S2N). Expression of KRT15 and CD34 was maintained at early stages after hair plucking in adult Krt5-rtTA tetO-Cre Ctnnb1fl/fl, Krt15-CrePR1 Ctnnb1fl/fl, Dkk1-expressing and Dkk1/Krm1-expressing mice (Fig. 3P–Y; Fig. 4G–J). Thus, short-term deletion of epithelial β-catenin or inhibition of LRP signaling produced dramatic defects in SHG and matrix proliferation without loss of the stem cell compartment.
Fig. 4. β-catenin is required in HF stem cells for anagen onset.
Krt15-CrePR1 Ctnnb1fl/fl mice were induced for 5 days prior to plucking at P54 and skin was harvested at P59. Krt15-CrePR1 Ctnnb1fl/fl mutant HF fail to enter anagen (A,B). The upper ORS proliferates in mutants (white arrows), but proliferation of the SHG (yellow arrows) is severely reduced or absent compared with controls (C,D). β-catenin protein (brown) is absent in mutant HF SHG and bulge, but not in IFE or HF epithelia above the bulge (E,F). KRT15 (G,H) or CD34 (I,J) immunofluorescence (green) (yellow arrows) reveals stem cell persistence in control (G,I) and Krt15-CrePR1 Ctnnb1fl/fl mutant (H,J) HFs at 5DPP. Scale Bars: 100µm (A,B); 50µm (C–J). See also Fig. S2.
Hair follicle stem cells are maintained during long-term induction of Dkk1, but are eventually lost following broad deletion of β-catenin
External hair was almost completely absent in epithelial β-catenin mutants following long periods of deletion (Fig. S4A,B). To ask whether maintenance of HF structures and their associated stem cells was affected by long-term loss of β-catenin, we induced deletion from P4 and examined skin histology and expression of KRT15 and CD34 at P60. HF density was greatly reduced at this stage compared with controls, and remaining structures appeared degraded or formed cysts (Fig. 5A,B). Expression of KRT15 and CD34 was robust in control littermate HFs, but completely absent from β-catenin-deficient skin (Fig. 5K,K’,L,L’). Similarly, in β-catenin mutants induced from P30 and examined at P100 or P180, HFs were degraded and/or had formed utricles, and expression of KRT15 and CD34 was absent (Fig. S4A’,B’,F–G’,K,L,P–Q’,U,V). Thus, epithelial β-catenin is required for long-term maintenance of HF structures and their associated stem cells. DP cells were frequently noted stranded in the dermis or at the tail end of “streamers” of degenerating HFs (Fig. S4W,X), suggesting that follicular degradation may have been caused in part by loss of contact of HF epithelia with the DP.
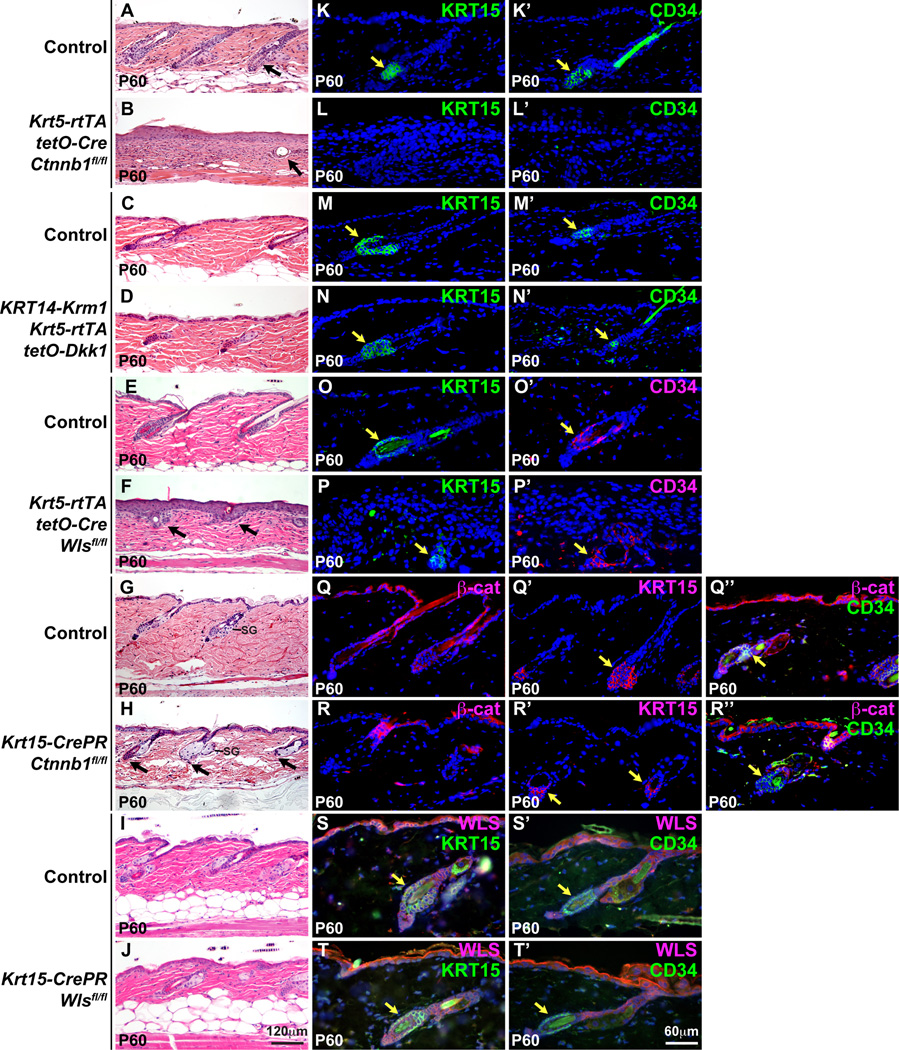
Fig. 5. HF stem cells disappear after long-term broad deletion of epithelial β-catenin, but persist when β-catenin deletion is restricted to the bulge and SHG.
Krt5-rtTA tetO-Cre Ctnnb1fl/fl mutants, Dkk1 Krm1 triple transgenics, and control littermates were doxycycline treated from P4-P60 and Krt5-rtTA tetO-Cre Wlsfl/fl and control mice from P4-P18. Krt15-CrePR1 Wlsfl/fl and control littermates were treated topically with 1% mifepristone from P19-P25. Krt15-CrePR1 Ctnnb1fl/fl mice and control littermates were treated topically with 1% mifepristone from P20 to P27. All skin samples were analyzed at P60 (telogen) by H&E staining (A–J), or immunofluorescence for KRT15 (K–P, S,T, green; Q’,R’, red), CD34 (K’-N’,Q’’,R’’,S’,T’, green; O’,P’, red), β-catenin (Q,R,Q’’,R’’, red) or WLS (S,T,S’,T’, red). Yellow arrows indicate positive staining for KRT15 or CD34. Scale bars: 120µm (A–J) and 60µm (K–T’, Q’’,R’’). See also Figs. S2, S4, S6 and S7.
Similar to epithelial β-catenin mutants, mice co-expressing ectopic Dkk1 and Krm1 had completely lost external hair by P103, following doxycycline treatment from P4 (Fig. S4E). However, analysis of skin histology at P60 revealed maintenance of HF structures that were apparently arrested in early anagen (Fig. 5C,D), and KRT15 and CD34 stem cell markers were expressed at similar levels to those observed in control littermate HFs (Fig. 5M,M’,N,N’). Remarkably, HF structures and associated KRT15 and CD34-positive stem cells were still maintained at P102 and P185 in mice induced to express Dkk1 or both Dkk1 and Krm1 from P1 (Fig. S4C’–E’, H–J’, M–O, R–T’). Therefore, over long periods of time, broad loss of epithelial β-catenin, but not ectopic Dkk1-mediated inhibition of Wnt signaling, causes loss of HF structures and stem cells.
To determine whether long-term stem cell maintenance requires epithelial Wnt ligands, we directly compared the effects of deletion of epithelial WLS on maintenance of HF stem cells at stages when these are lost in induced β-catenin mutants. Consistent with published data (Myung et al., 2012), broad deletion of epithelial Wls in K5-rtTA tetO-Cre Wlsfl/fl mutants induced from P4 resulted in premature regression of HFs by P14 without loss of bulge stem cell markers (Fig. S2S–X). At P60, WLS protein was absent from mutant skin epithelial cells (Fig. S2O,P), external hair was lost, and HF structures were retained but showed marked abnormalities including the formation of cysts (Fig. 5E,F). Despite these defects, strong expression of KRT15 and CD34 was associated with follicle structures in Wls mutant skin (Fig. 5O–P’). KRT15 and CD34 were also detected at P60 following specific deletion of WLS in the bulge and SHG of Krt15-CrePR1 Wlsfl/fl mutant HFs (Fig. S2Q,R; Fig. 5S,S’,T,T’). Thus, long-term broad deletion of β-catenin, but not inhibition of LRP function or deletion of epithelial Wls, prevents stem cell maintenance.
Stem cells are maintained following specific deletion of β-catenin in the bulge and SHG
To determine whether eventual loss of HF stem cell markers in epithelial β-catenin mutants was due to a requirement for β-catenin within bulge stem cells and SHG, we examined their expression in P60 Krt15-CrePR1 Ctnnb1fl/fl mice, following topical 1% mifepristone treatment from P20–27. Double immunofluorescence for β-catenin and CD34 revealed maintenance of CD34 immunoreactivity in β-catenin-deleted bulge cells at this stage (Fig. 5Q’’,R’’). Staining of serial sections for β-catenin and KRT15 indicated that expression of KRT15 persisted in β-catenin-deleted HFs (Fig. 5Q,Q’,R,R’). Similar results were obtained with another bulge stem cell marker, S100A4 (data not shown). Despite persistence of bulge stem cell marker expression, the SHG of Krt15-CrePR1 Ctnnb1fl/fl HF appeared abnormal or even absent in some HF; however the upper follicles and sebaceous glands (SG) remained intact (Fig. 5G,H). These data, and the slow time course of bulge stem cell disappearance in K5-rtTA tetO-Cre Ctnnb1fl/fl mice, suggested that bulge cell loss in K5-rtTA tetO-Cre Ctnnb1fl/fl mutants occured secondary to follicular degradation resulting from combined absence of β-catenin in the HF bulge/SHG and KRT15-negative cell populations, such as those in the junctional zone and isthmus (Myung and Ito, 2012).
Long-term Dkk1-mediated inhibition of hair follicle growth is reversible
Our observation that KRT15 and CD34 expression persisted even following very long periods of Dkk1 induction raised the possibility that functional stem cells were maintained in Dkk1-expressing follicles. To test this, we asked whether hair growth was reversible following removal of the inhibitor. Control and Dkk1 transgenic mice were maintained on doxycycline from postnatal day 21 until 15.5 months of age. At this stage, the experimental mice completely lacked external hair (Fig. 6A,B), but maintained HF structures (Fig. 6C,D) with associated stem cells (Fig. 6E–H). Ectopic expression of Dkk1 was readily detected in induced double transgenic skin by in situ hybridization (Fig. 6I).
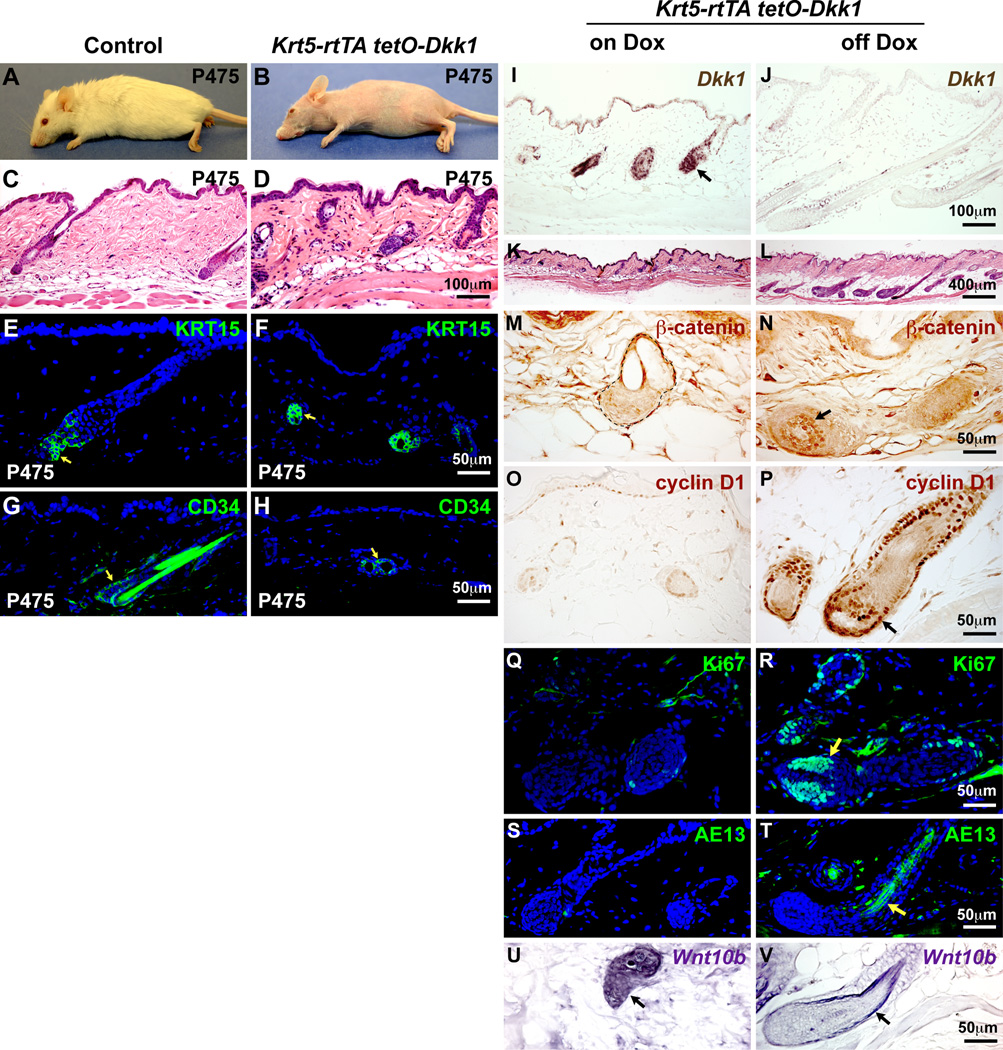
Fig. 6. Dkk1-mediated hair growth inhibition is reversible.
Control and Dkk1 double transgenic mice were induced from P21-P475, and skin biopsies were performed at P475 (on Dox) (A–H,I,K,M,O,Q,S,U) and at P489, 14 days after doxycycline withdrawal (off Dox) (J,L,N,P,R,T,V). Dkk1 transgenic mice completely lacked external hair at P475 (A,B) but HF structures (C,D) and expression of KRT15 (E,F) and CD34 (G,H) (green, arrows) were maintained. (I,J) In situ hybridization reveals Dkk1 expression (brown) in IFE and HFs (arrow) of Dkk1 transgenic skin on Dox (I) and absence of Dkk1 expression following Dox withdrawal (J). (K,L) HFs were arrested in early anagen prior to Dox withdrawal (K) and progressed into full anagen following Dox removal (L). (M–R) Immunostaining reveals absence of nuclear-localized β-catenin (M, brown), cyclin D1 (O, brown), Ki67 (Q, green), and AE13 (S, green) expression in HFs prior to Dox withdrawal, and presence of these markers in HFs following Dox withdrawal (N,P,R,T). (U,V) In situ hybridization for Wnt10b shows its persistent expression (purple) (black arrows) in HFs of Dkk1 transgenic skin on Dox (U) and after Dox withdrawal (V). Arrows indicate positive signals. Scale bars: 400µm (K,L); 100µm (C,D,I,J); 50µm (E–H and M–V). See also Fig. S5.
Mice were sacrificed for analysis 14 days after doxycycline withdrawal. At this timepoint, expression of Dkk1 was no longer detected (Fig. 6J) and, remarkably, growth of numerous follicles had occurred spontaneously (Fig. 6K,L). HF growth following doxycycline withdrawal was accompanied by resumption of Wnt/β-catenin signaling, indicated by accumulation of nuclear β-catenin in the HF matrix and precortical regions (Fig. 6M,N), expression of cyclin D1 (Fig. 6O,P), and high levels of matrix cell proliferation (Fig. 6Q,R). To avoid multiple survival biopsies, we did not test whether external hair growth occurred following removal of the inhibitor. However, the reactivated matrix cells were capable of differentiating, as indicated by positive staining for hair shaft precursor cells with the hair keratin marker AE13 (Fig. 6S,T). These data suggested persistence in Dkk1-inhibited follicles of Wnt ligands capable of re-activating HF growth following removal of the inhibitor. Consistent with this, Wnt10b, a ligand specifically expressed in anagen HFs within the skin (Reddy et al., 2001), was expressed in double transgenic HFs both before and after doxycycline withdrawal (Fig. 6U,V).
To test whether HFs co-expressing Dkk1 and Krm1 were also capable of re-initiating follicular growth following removal of the inhibitor, control littermate, Dkk1 transgenic and Dkk1/Krm1 transgenic mice were induced from birth until P100. Analysis of histology, and immunostaining for KRT15, CD34, β-catenin, cyclin D1, Ki67 and AE13 confirmed maintenance of HF structures and stem cell marker expression in Dkk1/Krm1 as well as Dkk1 transgenic and control mice at this stage, with lack of nuclear β-catenin or cyclin D1 expression, absent or low levels of proliferation, and lack of hair shaft differentiation (Fig. S5A–Q). Following 15 days of doxycycline withdrawal, both Dkk1 and Dkk1/Krm1 skin contained numerous HFs that had spontaneously entered anagen, compared with control littermate follicles that remained in telogen (Fig. S5A’–C’). Hair follicle re-growth was less synchronous in Dkk1/Krm1 skin than in skin that had expressed Dkk1 alone, consistent with the stronger inhibitory effects of Dkk1/Krm1. Dkk1 and Dkk1/Krm1 transgenic follicles displayed an extended pattern of KRT15 and CD34 staining that is characteristic of anagen, rather than the compact staining pattern seen in control telogen stage follicles (Fig. S5D’–I’). This was distinct from the staining observed in Dkk1 and Dkk1/Krm1 transgenics prior to doxycycline removal, which was intermediate between telogen and full anagen patterns, consistent with arrest in early anagen (Fig. S5E,F,H,I). Both Dkk1 and Dkk1/Krm1 transgenic samples displayed accumulation of nuclear β-catenin and expression of cyclin D1 in HF matrix regions following doxycycline withdrawal (Fig. S5J’–M’), as well as robust matrix proliferation (Fig. S5N’,O’) and expression of hair shaft keratins (Fig. S5P’,Q’).
These data indicated functional persistence of HF bulge and/or SHG cells and reversibility of the effects of LRP inhibition even following very long-term Dkk1 induction, with or without co-expressed Krm1. Histological analyses, the intermediate expression pattern of KRT15, persistent expression of Wnt10b, and the ability of HFs to spontaneously resume growth immediately following removal of the inhibitor, supported the interpretation that Dkk1-inhibited HFs were arrested in early anagen and were primed for hair re-growth rather than being maintained in a normal telogen resting phase.
β-catenin contributes to proliferation of the IFE during homeostasis
The epidermis of mice with long term broad deletion of epithelial β-catenin or Wls displayed marked thickening and hyperproliferation, expansion of p63-positive basal and KRT10-positive suprabasal layers, and ectopic expression of hyperproliferation markers including KRT6 and KRT17 (Fig. 5A,B,E,F; Fig. S4A’,B’; Fig. S6A,C). Induced Krt14-CreERT2 Ctnnb1fl/fl and K5-rtTA tetO-Cre Ctnnb1fl/fl mutants did not display overt blistering, and α-catenin and E-cadherin were present at cell membranes in β-catenin mutant as well as control epidermis (Fig. S7A–D). Transmission electron micrography revealed the presence of enlarged intercellular spaces in β-catenin mutant epidermis following long periods of broad depletion of epithelial β-catenin. In some cases, mutant keratinocytes displayed long membranous protrusions (Fig. S7E,F) that were not seen in control skin, or in induced Krt14-Krm1/Dkk1 triple transgenics (Fig. S7G,H), or following bulge cell-restricted deletion of β-catenin (not shown). Interestingly, however, IFE from induced Krt5-rtTA tetO-Cre Wlsfl/fl mutants displayed similar abnormalities at the ultrastructural level (Fig. S7I,J), but retained β-catenin protein (Fig. S6C), suggesting that these apparent defects in cell adhesion were not directly caused by absence of β-catenin.
As degrading HF elicit inflammation (Kirkham, 2009), epidermal hyperproliferation could be a consequence of an inflammatory response to HF disintegration (Augustin et al., 2013), or might reflect a direct requirement for β-catenin signaling in the IFE. Epidermal hyperproliferation was not observed in induced Krt15-CrePR1 Ctnnb1fl/fl or Krt15-CrePR1 Wlsfl/fl mutants that retained β-catenin or WLS in the IFE, or in Krt14-Krm1 Krt5-rtTA tetO-Dkk1 mice, which express lower levels of Dkk1 in IFE than in HFs (Fig. S6B,D,E; Fig. S1S). However these mutants also lacked overt HF structural defects and displayed lower levels of inflammatory cells than those seen in Krt5-rtTA tetO-Cre-driven mutants (Fig. S7K–T).
To test directly whether β-catenin is required for IFE homeostasis we generated a novel Axin2CreERT2/tdT knockin line by insertion of a CreERT2/tdTomato fusion cDNA downstream of the first codon of the endogenous mouse Axin2 gene (Fig. S7U). This line expresses cytoplasmic tandem dimer Tomato (tdT) and tamoxifen-inducible Cre-recombinase (CreERT2) in Axin2 promoter-active cells (not shown). To assess the efficiency of inducible deletion, we bred Axin2CreERT2/tdT mice with the R26RmTmG Cre reporter line that expresses membrane-targeted tandem dimer Tomato (mT) prior to Cremediated excision and membrane-targeted GFP (mG) after excision (Muzumdar et al., 2007). Following induction of Cre activity by treatment with tamoxifen during telogen, GFP-positive cells were present in the IFE and HF infundibulum, but no excision was observed in the HF bulge, SHG or DP (Fig. 7A). Clones of GFP-positive deleted cells were also observed in tongue and footpad epidermis (Fig. 7B,C), consistent with Axin2lacZ expression in basal progenitor cells of these tissues. To determine whether Axin2 promoter-active cells give rise to clones in the IFE, we crossed Axin2CreERT2/tdT mice with R26R-Confetti mice that function as a stochastic multicolor Cre reporter, permitting clonal analysis of cells expressing GFP, YFP, RFP or CFP under the control of a CAG promoter (Snippert et al., 2010). RFP was expressed much more strongly than Axin2 promoter-driven tdT in epithelial cells, allowing us to easily identify RFP-positive, as well as other fluorescently marked clones. Following tamoxifen induction in telogen, we observed clones of fluorescently marked cells originating in the basal progenitor layer of the IFE (Fig. 7D,D’).
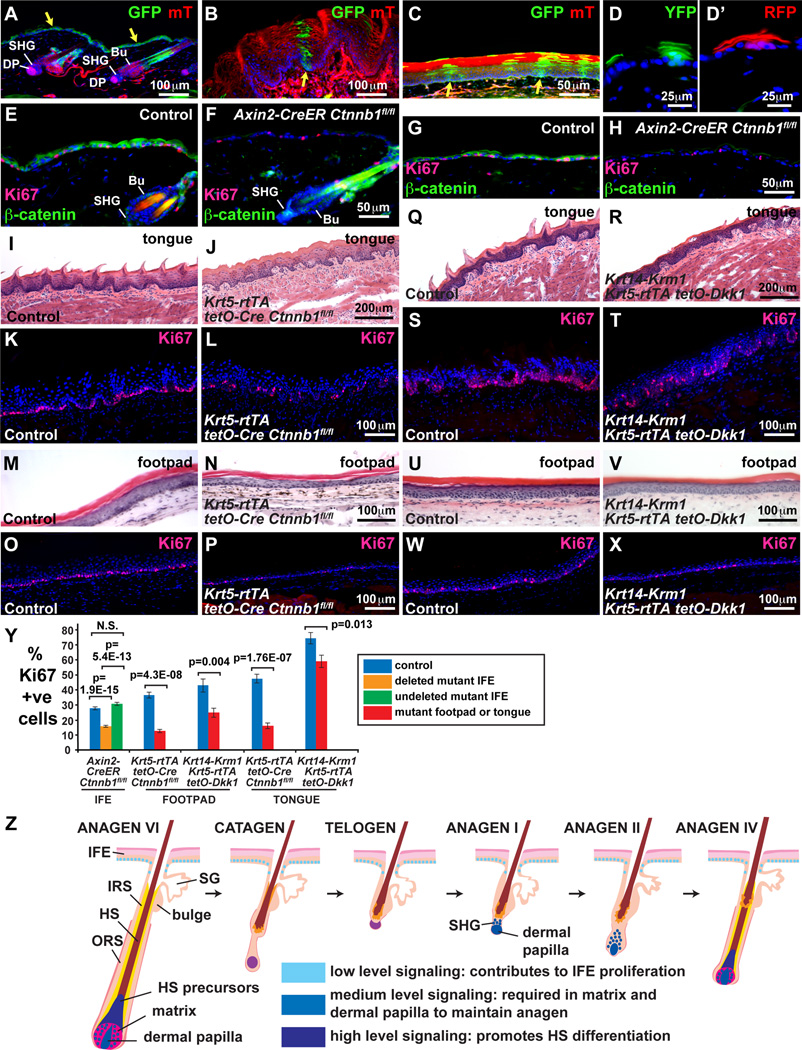
Fig. 7. β-catenin contributes to proliferation of IFE and non-hairy epithelia.
(A–C) Axin2CreERT2/tdT R26RmTmG mice were tamoxifen treated from P43–45 and frozen sections analyzed for expression of membrane-localized GFP (deleted cells) and mT (undeleted cells) at P71. (A) Dorsal skin: GFP was expressed in IFE (arrow) and upper HF; no excision was observed in the HF bulge, SHG or DP. (B,C) Dorsal tongue (B) and footpad (C) epithelia contained clones of GFP-positive deleted cells originating in the basal layer (arrows). (D,D’) cytoplasmic YFP- and RFP-marked clones originating in whisker pad basal IFE of Axin2CreERT2/tdT R26R-Confetti mice tamoxifen treated for 3 days at 16 weeks and analyzed 5 weeks later. (E–H) Axin2CreERT2/tdT Ctnnb1fl/fl (F,H) and control littermate Axin2CreERT2/tdT Ctnnb1fl/+ (E,G) mice were tamoxifen treated at P43–45 and skin sections analyzed at P71 by immunofluorescence for β-catenin (green) and Ki67 (red) after microwave pre-treatment to remove tdT fluorescence. β-catenin was mosaically deleted in mutant IFE (E–H) and upper HF (E,F) but was retained in HF bulge and SHG. β-catenin-deleted IFE displayed fewer Ki67-positive cells than controls. (I–P) Absent tongue filiform papillae (I,J), decreased footpad epidermal thickness (M,N) and decreased proliferation of tongue (K,L) and footpad (O,P) epithelia in P100 Krt5-rtTA tetO-Cre Ctnnbfl/fl mice doxycycline treated from P20 compared with control littermates. (Q–X) Defective tongue filiform papillae (Q,R), mildly decreased footpad epidermal thickness (U,V) and decreased proliferation of dorsal tongue (S,T) and footpad (W,X) epithelia in P360 KRT14-Krm1 Krt5-rtTA tetO-Dkk1 mice doxycycline treated from P20 compared with control littermates. (Y) Quantification reveals statistically significant reductions in proliferation of β-catenin-deleted and Dkk1/Krm1-expressing IFE, footpad and dorsal tongue epithelia. Blue bars represent littermate controls; orange and green bars represent regions of β-catenin-deleted and undeleted epidermis, respectively, in Axin2CreERT2/tdT Ctnnb1fl/fl mutants; red bars represent Krt5-rtTA tetO-Cre Ctnnbfl/fl or KRT14-Krm1 Krt5-rtTA tetO-Dkk1 samples as indicated. Results are shown as mean ± S.E.M. P-values were calculated using a two-tailed Student's t-test. N.S., not significant. (Z) Model depicting functions of low, medium and high level Wnt/β-catenin signaling in skin epithelia. Scale bars: 200µm (I,J,Q,R); 100µm (A,B,K–P,S–X); 50µm (C,E–H); 25µm (D,D’). See also Fig. S6 and Fig. S7.
As efficient Axin2CreERT2/tdT-mediated deletion of β-catenin may cause systemic defects, we used conditions under which mice exhibited mosaic deletion. Axin2CreERT2/tdT Ctnnb1fl/fl mice and Axin2CreERT2/tdT Ctnnb1fl/+ control littermates were injected with 200mg/kg tamoxifen daily at P43, P44 and P45 and tissue was harvested 4 weeks later. Immunofluorescence for β-catenin revealed mosaic absence of β-catenin in IFE but not in HF bulge or SHG (Fig. 7E–H). Histological analysis did not reveal gross HF structural defects in these animals (not shown). We quantified proliferation by assaying for Ki67 immunofluorescence in non-serial sections of IFE, choosing regions where β-catenin was either efficiently deleted in continuous strips of at least 20 cells (n=75 non-adjacent strips of 22–67 cells, total of 2,965 cells analyzed), or was retained in continuous strips of at least 20 cells (n=23 non-adjacent strips of 20–65 cells, total of 903 cells analyzed), and compared proliferation rates with those seen in littermate control epidermis (n=52 non-adjacent strips of 38–85 cells, total of 2,780 cells analyzed). Surprisingly, rather than exhibiting hyperproliferation, regions of β-catenin-deleted epidermis showed a statistically significant decrease in proliferation rate of more than 40% when compared with either non-deleted mutant epidermis (p=5.4×10−13) or control epidermis (p=1.9×10−15) (Fig. 7E–H,Y). The proliferation rate of non-deleted mutant epidermis was similar to that of control epidermis, indicating that decreased proliferation of deleted cells was not due to systemic defects (Fig. 7Y).
Non-hairy epithelia of the footpad and tongue lacked obvious inflammation; however, filiform papillae were reduced or absent in induced K5-rtTA tetO-Cre Ctnnbfl/fl and K5-rtTA tetO-Dkk1 Krt14-Krm1 mice compared with controls (Fig. 7I,J,Q,R). We assayed for epithelial proliferation in n=10 non-adjacent samples of footpad and dorsal tongue from each littermate control and mutant or transgenic, with each sample containing 60 DAPI-stained basal nuclei. Proliferation was significantly reduced in footpad and tongue of K5-rtTA tetO-Cre Ctnnbfl/fl mutants (Fig. 7K–P,Y); similar though less pronounced, defects were observed in the footpad and tongue of induced K5-rtTA tetO-Dkk1 Krt14-Krm1 mice (Fig. 7S–Y), indicating that they were caused by decreased Wnt/LRP/β-catenin signaling, rather than being directly due to loss of β-catenin’s functions in adhesion. Thus, in addition to controlling HF matrix proliferation, Wnt/β-catenin signaling contributes to proliferation of IFE and specialized non-hairy epithelia.
In summary, our data suggest a model in which high levels of Wnt/β-catenin signaling in the HF matrix drive proliferation. As cells exit the matrix compartment, a further elevation of Wnt/β-catenin signaling levels causes them to terminally differentiate (Zhang et al., 2008; Zhou et al., 1995). Ectopic Dkk1, or deletion of Wls or β-catenin, inhibits these high levels of signaling, preventing matrix proliferation and differentiation. Unlike broad loss of epithelial β-catenin, bulge/SHG-restricted β-catenin deletion does not cause loss of stem cells, indicating that β-catenin is not required within bulge/SHG cells for their maintenance. Unexpectedly, our data also reveal low level Wnt/β-catenin signaling in IFE that is required to maintain normal levels of proliferation under homeostatic conditions (Fig. 7Z), and demonstrate that Wnt/β-catenin signaling contributes to proliferation in specialized non-hairy epithelia. Despite these functions, complete loss of epithelial β-catenin does not prevent long-term maintenance of IFE, or footpad and tongue epithelia, and β-catenin-deleted IFE can still mount a hyperproliferative response to inflammation associated with HF degradation.
DISCUSSION
Here we describe the sites and levels of Wnt/β-catenin signaling in postnatal skin using two independent sensitive in vivo reporter assays. High levels of signaling were observed in the HF DP, SHG and matrix at early stages of anagen. As matrix cells differentiated, they showed a further increase in signaling levels. Reporter gene expression was not observed in telogen HF bulge, SHG or DP, but was detected at low levels in the infundibulum and IFE. Reporter expression was also detected in non-hairy basal epithelia of footpad and tongue.
To delineate the functions of high and low level signaling in different epithelial cell types, we directly compared the effects of three independent genetic manipulations: deletion of epithelial β-catenin, which abolishes all signaling through the Wnt/β-catenin pathway; deletion of epithelial Wls, which dramatically decreases the availability of ligands for paracrine signaling (Ching and Nusse, 2006); and ectopic expression of the secreted Wnt/LRP inhibitor DKK1 which inhibits signaling depending on the levels of DKK1 expression and availability of the DKK1, receptor KRM.
During an established anagen phase, either deletion of epithelial β-catenin or expression of Dkk1 caused HFs to rapidly cease proliferating and enter premature catagen. The similar phenotypes resulting from these two independent manipulations indicate that maintenance of proliferation during anagen requires signaling within epithelial cells through the canonical Wnt/LRP/β-catenin pathway. The KRT15-positive stem cell compartment was maintained during short-term deletion of β-catenin or expression of Dkk1. This observation, together with the strong localization of nuclear β-catenin and Wnt reporter gene expression in normal matrix cells, suggests that the immediate effects of Wnt pathway inhibition are manifest directly in the matrix, rather than via acute loss of bulge stem cells.
As β-catenin is cell autonomous, the block to proliferation caused by deletion of epithelial β-catenin in anagen was caused by its loss within epithelial cells. Interestingly, however, the observed effects on proliferation and catagen induction are similar to those noted when β-catenin is specifically deleted in the anagen DP (Enshell-Seijffers et al., 2010). Thus β-catenin is required in both these HF compartments for continued hair growth. Our results further suggest that antagonism of Wnt signaling in the epithelium and DP could constitute part of the normal mechanism of catagen induction, and prompt study of endogenous Wnt inhibitors that might promote the anagen-catagen transition.
Epithelial β-catenin mutants and mice expressing Dkk1 displayed subtle differences in response to hair plucking: in β-catenin mutants cells proliferated in the ORS of plucked HFs, but not in the SHG. By contrast, expression of ectopic Dkk1, even when concomitant with forced expression of the DKK1 receptor Kremen1, permitted some degree of proliferation of SHG as well as ORS cells. Similarly, following deletion of epithelial Wls many follicles enter anagen but are arrested in anagen I (Myung et al., 2012). These observations provide circumstantial evidence that the initial burst of β-catenin-dependent proliferation at anagen onset is controlled by mechanisms other than Wnt/LRP signaling, consistent with prior data indicating that HF stem cells are initially activated by downregulation of dermal BMP signaling (Plikus et al., 2008), and that impaired BMP signaling stabilizes β-catenin through a Wnt ligand-independent pathway (Kobielak et al., 2007).
In mice expressing epithelial Dkk1, with or without forced expression of Krm1, HFs and their associated stem cells were maintained for more than a year of Dkk1 expression. Stem cells were also maintained following long-term deletion of epithelial Wls, either throughout skin epithelia, or specifically in the bulge/SHG stem cell compartment, and following specific deletion of β-catenin in bulge/SHG stem cells. These observations suggest that the degradation of HF structures and complete loss of bulge stem cells seen following broad deletion of β-catenin results from combined absence of β-catenin in the HF bulge/SHG, junctional zone, isthmus and/or infundibulum. Furthermore, long-term maintenance of follicular structures and their associated stem cells occurs via a mechanism distinct from that controlling proliferation, and requires either very low levels of Wnt/LRP/β-catenin signaling that persist in the presence of ectopic Dkk1 or absence of epithelial Wls, or is independent of LRP and epithelial Wnt ligands.
Remarkably, removal of the inducing agent in aged Dkk1 transgenic mice following long periods of Dkk1 expression lead to spontaneous resumption of hair germ proliferation, formation of a new matrix, and differentiation of hair shaft and IRS cells, providing evidence that functional bulge/SHG stem cells were maintained in the absence of Wnt/β-catenin signaling. These data suggest graded inhibition of Wnt/LRP signaling as a promising method for reversibly inhibiting the growth of unwanted hair without causing permanent follicle destruction and skin damage. Small molecule Wnt inhibitors have been identified (Voronkov and Krauss, 2013) and could potentially be tested as topical agents for prevention of growth of unwanted hair in humans. Use of such approaches would require tight control of the levels of applied Wnt inhibitor to avoid causing hair follicle abnormalities.
Unexpectedly, we detected activity of Wnt reporter genes in adult IFE and in specialized non-hairy epithelia. Using a novel approach, we discovered that specific loss of β-catenin in the IFE of hairy skin, sparing the HF bulge and SHG, caused significantly reduced IFE proliferation. Similarly, β-catenin deletion or forced expression of Dkk1/Krm1 decreased the thickness and proliferation of specialized non-hairy epithelia of the footpad and dorsal tongue. In line with these observations, human patients with mutations in the canonical Wnt gene WNT10A display epidermal defects, smooth tongues and palmoplantar keratoderma in addition to hair growth defects (Adaimy et al., 2007; Petrof et al., 2011). Furthermore, inducible deletion of epithelial β-catenin causes regression of squamous cell carcinoma (SCC) in mice (Malanchi et al., 2008). By contrast, broad epithelial deletion of β-catenin in IFE and HFs was associated with HF disintegration, an inflammatory response, and IFE hyperproliferation. Taken together, these data indicate that Wnt/β-catenin signaling contributes to adult IFE proliferation under homeostatic conditions and in SCC, but is not required for long-term maintenance of the IFE and is bypassed during hyperproliferative responses to inflammation. The approaches described here will be generally useful for determining the effects of gene deletion in adult IFE in cases where HF degradation and inflammation resulting from broad epithelial deletion complicate analysis of IFE phenotypes.
EXPERIMENTAL PROCEDURES
Mouse strains, transgene induction, depilation, skin biopsies, and genotyping
Krt5-rtTA tetO-Dkk1 mice were generated as described previously (Chu et al., 2004). A KRT14-Krm1 transgene was constructed by cloning full-length mouse Krm1 cDNA (NM_032396) into KRT14 promoter vector (Saitou et al., 1995). Ctnnb1fl/fl, Wlsfl/fl, Krt15-CrePR1, Krt14-CreER, R26R-Confetti, and R26RmTmG mice were obtained from Jackson Labs (Bar Harbor, ME, USA). Axin2-CreERT2/tdT mice were generated by insertion of a CreERT2/tdTomato fusion cDNA downstream of the first ATG of the mouse Axin2 gene using homologous recombination in mouse ES cells. Transgene induction was performed as described previously (Chu et al., 2004; Ito et al., 2005). Skin biopsies were taken from euthanized mice or under anesthesia. Detailed methods are provided in Supplemental Data. The IACUC committees of the University of Pennsylvania and the University of Cincinnati approved all experimental procedures involving mice.
Histology, staining procedures and transmission electron micrography (TEM)
Preparation of paraffin sectioned skin, histological analysis, BrdU incorporation assays, TUNEL assays, immunostaining, in situ hybridization, X-gal staining, alkaline phosphatase staining, and TEM were performed according to published protocols (Andl et al., 2006; Zhang et al., 2009). Details are provided in Supplemental Data.
Supplementary Material
Highlights.
-
·
Epithelial Wnt/β-catenin signaling is required for hair matrix cell proliferation
-
·
LRP inhibition permits stem cell survival and blocks hair growth reversibly
-
·
β-catenin is required in bulge stem cells for their proliferation but not survival
-
·
β-catenin signaling contributes to proliferation of interfollicular epidermis
ACKNOWLEDGEMENTS
We thank Adam Glick for Krt5-rtTA mice; Boris Jerchow and Walter Birchmeier for Axin2lacZ mice; Pierre Coulombe for anti-KRT17; and Jean Richa for production of transgenic mice. This work was supported by NIH R37AR47709 and the Penn SDRC P30AR057217 (S.E.M); University of Cincinnati NIEHS P30-ES006096 (Y.Z.); and UL1RR026314 (E.E.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
REFERENCES
- Adaimy L, Chouery E, Megarbane H, Mroueh S, Delague V, Nicolas E, Belguith H, de Mazancourt P, Megarbane A. Mutation in WNT10A is associated with an autosomal recessive ectodermal dysplasia: the odonto-onycho-dermal dysplasia. Am J Hum Genet. 2007;81:821–828. doi: 10.1086/520064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andl T, Ahn K, Kairo A, Chu EY, Wine-Lee L, Reddy ST, Croft NJ, Cebra-Thomas JA, Metzger D, Chambon P, et al. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131:2257–2268. doi: 10.1242/dev.01125. [DOI] [PubMed] [Google Scholar]
- Andl T, Murchison EP, Liu F, Zhang Y, Yunta-Gonzalez M, Tobias JW, Andl CD, Seykora JT, Hannon GJ, Millar SE. The miRNA-processing enzyme dicer is essential for the morphogenesis and maintenance of hair follicles. Curr Biol. 2006;16:1041–1049. doi: 10.1016/j.cub.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andl T, Reddy ST, Gaddapara T, Millar SE. WNT Signals Are Required for the Initiation of Hair Follicle Development. Dev Cell. 2002;2:643–653. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Augustin I, Gross J, Baumann D, Korn C, Kerr G, Grigoryan T, Mauch C, Birchmeier W, Boutros M. Loss of epidermal Evi/Wls results in a phenotype resembling psoriasiform dermatitis. J Exp Med. 2013 doi: 10.1084/jem.20121871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Ching W, Nusse R. A dedicated Wnt secretion factor. Cell. 2006;125:432–433. doi: 10.1016/j.cell.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Chu EY, Hens J, Andl T, Kairo A, Yamaguchi TP, Brisken C, Glick A, Wysolmerski JJ, Millar SE. Canonical WNT signaling promotes mammary placode development and is essential for initiation of mammary gland morphogenesis. Development. 2004;131:4819–4829. doi: 10.1242/dev.01347. [DOI] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Diamond I, Owolabi T, Marco M, Lam C, Glick A. Conditional gene expression in the epidermis of transgenic mice using the tetracycline-regulated transactivators tTA and rTA linked to the keratin 5 promoter. J Invest Dermatol. 2000;115:788–794. doi: 10.1046/j.1523-1747.2000.00144.x. [DOI] [PubMed] [Google Scholar]
- Enshell-Seijffers D, Lindon C, Kashiwagi M, Morgan BA. beta-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev Cell. 2010;18:633–642. doi: 10.1016/j.devcel.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer-Vaquer A, Piliszek A, Tian G, Aho RJ, Dufort D, Hadjantonakis AK. A sensitive and bright single-cell resolution live imaging reporter of Wnt/ss-catenin signaling in the mouse. BMC Dev Biol. 2010;10:121. doi: 10.1186/1471-213X-10-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. Beta-catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Incassati A, Chandramouli A, Eelkema R, Cowin P. Key signaling nodes in mammary gland development and cancer: beta-catenin. Breast Cancer Res. 2010;12:213. doi: 10.1186/bcr2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- Kirkham N. Tumors and Cysts of the Epidermis. In: Elder DE, Elenitsas R, Johnson BL, Murphy GF, Xu X, editors. Lever's Histopathology of the Skin. Philadelphia: Lippincott Williams & Wilkins; 2009. pp. 791–849. [Google Scholar]
- Kobielak K, Pasolli HA, Alonso L, Polak L, Fuchs E. Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA. J Cell Biol. 2003;163:609–623. doi: 10.1083/jcb.200309042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobielak K, Stokes N, de la Cruz J, Polak L, Fuchs E. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. Proc Natl Acad Sci U S A. 2007;104:10063–10068. doi: 10.1073/pnas.0703004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwack MH, Kim MK, Kim JC, Sung YK. Dickkopf 1 promotes regression of hair follicles. J Invest Dermatol. 2012;132:1554–1560. doi: 10.1038/jid.2012.24. [DOI] [PubMed] [Google Scholar]
- Lowry WE, Blanpain C, Nowak JA, Guasch G, Lewis L, Fuchs E. Defining the impact of beta-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 2005;19:1596–1611. doi: 10.1101/gad.1324905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malanchi I, Peinado H, Kassen D, Hussenet T, Metzger D, Chambon P, Huber M, Hohl D, Cano A, Birchmeier W, et al. Cutaneous cancer stem cell maintenance is dependent on beta-catenin signalling. Nature. 2008;452:650–653. doi: 10.1038/nature06835. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, Delius H, Hoppe D, Stannek P, Walter C, et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill H, Woodgett JR. When pathways collide: collaboration and connivance among signalling proteins in development. Nat Rev Mol Cell Biol. 2010;11:404–413. doi: 10.1038/nrm2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- Mucenski ML, Wert SE, Nation JM, Loudy DE, Huelsken J, Birchmeier W, Morrisey EE, Whitsett JA. beta -catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J Biol Chem. 2003 doi: 10.1074/jbc.M305892200. [DOI] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Myung P, Ito M. Dissecting the bulge in hair regeneration. J Clin Invest. 2012;122:448–454. doi: 10.1172/JCI57414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myung PS, Takeo M, Ito M, Atit RP. Epithelial Wnt Ligand Secretion Is Required for Adult Hair Follicle Growth and Regeneration. J Invest Dermatol. 2012 doi: 10.1038/jid.2012.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narhi K, Jarvinen E, Birchmeier W, Taketo MM, Mikkola ML, Thesleff I. Sustained epithelial beta-catenin activity induces precocious hair development but disrupts hair follicle down-growth and hair shaft formation. Development. 2008;135:1019–1028. doi: 10.1242/dev.016550. [DOI] [PubMed] [Google Scholar]
- Petrof G, Fong K, Lai-Cheong JE, Cockayne SE, McGrath JA. Schopf-Schulz-Passarge syndrome resulting from a homozygous nonsense mutation, p.Cys107X, in WNT10A. Australas J Dermatol. 2011;52:224–226. doi: 10.1111/j.1440-0960.2011.00788.x. [DOI] [PubMed] [Google Scholar]
- Plikus MV, Mayer JA, de la Cruz D, Baker RE, Maini PK, Maxson R, Chuong CM. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy S, Andl T, Bagasra A, Lu MM, Epstein DJ, Morrisey EE, Millar SE. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech Dev. 2001;107:69–82. doi: 10.1016/s0925-4773(01)00452-x. [DOI] [PubMed] [Google Scholar]
- Reddy ST, Andl T, Lu MM, Morrisey EE, Millar SE. Expression of frizzled genes in developing and postnatal hair follicles. J Invest Dermatol. 2004;123:275–282. doi: 10.1111/j.0022-202X.2004.23215.x. [DOI] [PubMed] [Google Scholar]
- Saitou M, Sugai S, Tanaka T, Shimouchi K, Fuchs E, Narumiya S, Kakizuka A. Inhibition of skin development by targeted expression of a dominant-negative retinoic acid receptor. Nature. 1995;374:159–162. doi: 10.1038/374159a0. [DOI] [PubMed] [Google Scholar]
- Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Trempus CS, Morris RJ, Bortner CD, Cotsarelis G, Faircloth RS, Reece JM, Tennant RW. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J Invest Dermatol. 2003;120:501–511. doi: 10.1046/j.1523-1747.2003.12088.x. [DOI] [PubMed] [Google Scholar]
- Van Mater D, Kolligs FT, Dlugosz AA, Fearon ER. Transient activation of beta -catenin signaling in cutaneous keratinocytes is sufficient to trigger the active growth phase of the hair cycle in mice. Genes Dev. 2003;17:1219–1224. doi: 10.1101/gad.1076103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronkov A, Krauss S. Wnt/beta-catenin signaling and small molecule inhibitors. Curr Pharm Des. 2013;19:634–664. doi: 10.2174/138161213804581837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HM, Jerchow B, Sheu TJ, Liu B, Costantini F, Puzas JE, Birchmeier W, Hsu W. The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development. 2005;132:1995–2005. doi: 10.1242/dev.01786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Andl T, Yang SH, Teta M, Liu F, Seykora JT, Tobias JW, Piccolo S, Schmidt-Ullrich R, Nagy A, et al. Activation of beta-catenin signaling programs embryonic epidermis to hair follicle fate. Development (Cambridge, England) 2008;135:2161–2172. doi: 10.1242/dev.017459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Tomann P, Andl T, Gallant NM, Huelsken J, Jerchow B, Birchmeier W, Paus R, Piccolo S, Mikkola ML, et al. Reciprocal requirements for EDA/EDAR/NF-kappaB and Wnt/beta-catenin signaling pathways in hair follicle induction. Dev Cell. 2009;17:49–61. doi: 10.1016/j.devcel.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Byrne C, Jacobs J, Fuchs E. Lymphoid enhancer factor 1 directs hair follicle patterning and epithelial cell fate. Genes Dev. 1995;9:700–713. doi: 10.1101/gad.9.6.700. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.