The heme-thiolate peroxygenase from Agrocybe aegerita (AaeAPO, EC 1.11.2.1) is a versatile biocatalyst and cytochrome P450 analog that catalyzes a variety of oxygenation reactions with high efficiency and selectivity.[1] Our recent kinetic characterization of AaeAPO-catalyzed reactions has shown that AaeAPO compound I is an oxo-FeIV porphyrin radical cation.[2] The reactivity of AaeAPO-I toward a panel of substrates showed very fast C–H hydroxylation rates, similar to those of cytochrome P450 (CYP119-I),[3] and much faster than chloroperoxidase compound I (CPO-I).[4] Mechanistic probes have revealed a large hydrogen isotope effect for aliphatic C–H hydroxylation and rearranged products from the hydroxylation of norcarane.[1b] There is, however, very little information available regarding the thermodynamic properties of such highly reactive oxoiron species for any heme-thiolate proteins.
For hydrogen abstraction reactions, the redox potential of the oxidant is correlated with the rates of C–H activation.[5] Yet these values are often not accessible, especially for highly reactive oxidants. We have developed a method to measure redox potentials for oxometalloporphyrin model compounds that takes advantage of the rapid, reversible oxygen atom transfer between oxo-metal complexes and halide ions.[6] By using rapid-mixing stopped-flow spectroscopy, rate constants of both forward and reverse reactions are measured. Thus, the driving force of the unknown oxo-transfer redox couple (Mn+2=O/Mn) is obtained from the equilibrium constants for the reaction and the known potentials of the HOX/X− couples. Using this method, the oxo-transfer driving force for several heme-enzyme model complexes have been measured, such as oxo-MnVTDMImP[6-7] and [oxo-FeIV-4-TMPyP]+.[8]
Here, we describe measurements of the driving force for oxygen atom transfer by the heme-thiolate proteins AaeAPO and CPO. We have found that oxo-transfer between AaeAPO-I and chloride or bromide ions is fast and reversible (Scheme 1). The redox potential of the couple AaeAPO-I/ferric-AaeAPO has been obtained over a wide pH range from the rate constants of the forward and reverse reactions. Thus, the highly reactive AaeAPO-I can be placed on an absolute energy scale and compared with those of CPO and HRP for the first time.
Scheme 1.
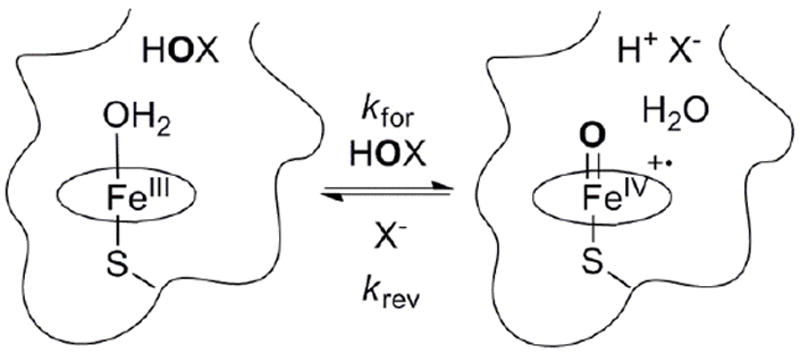
Reversible oxygen atom transfer between ferric AaeAPO and HOBr or HOCl (kfor), and AaeAPO-I with halide ions (krev).
AaeAPO-I was generated by the stoichiometric reaction of FeIII-AaeAPO with HOCl or HOBr and characterized by rapid mixing, stopped-flow spectroscopy. The UV/Vis spectral features of AaeAPO-I generated with these hypohalous acids (Figure 1) are the same as those we recently reported for peroxyacid oxidations.[2] The Soret band of the ferric enzyme at 417 nm diminished over the first 50 ms after mixing while new absorbances characteristic of the formation of an oxo-FeIV porphyrin radical cation appeared at 361 and 694 nm. AaeAPO-I subsequently decayed in a second, slower phase. SVD analysis of these transient spectra indicated that only two species were present in significant amounts during this transformation. The AaeAPO-I formation rate was directly measured by monitoring the conversion of the ferric enzyme to oxo-FeIV radical cation. Binding of HOX to the heme iron is a rapid step and heterolytic FeO–X bond cleavage is rate-limiting.[2] Plotting the initial absorbance change at 417 nm against the HOX concentration afforded a linear relationship with no evidence of saturation. Second-order rate constants were obtained from the slopes (Figure S1).
Figure 1.
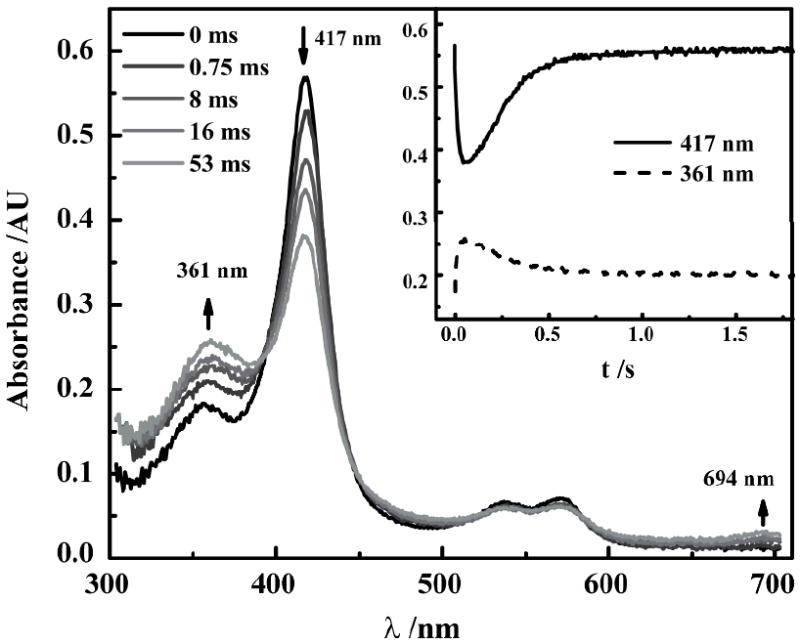
UV/vis spectra observed upon 1:1 mixing of 5 μM AaeAPO with 15 μM NaOBr at pH 5.0, 4°C. Inset: Time courses of data obtained at 417 nm (ferric AaeAPO) and 361 nm (AaeAPO-I).
The oxidation of AaeAPO with HOCl or HOBr was examined over a range of pH as shown in Table 1. At pH 3.0, HOCl was used because HOBr is not stable at this pH. The slightly milder oxidant, HOBr, was used to generate AaeAPO-I from pH 4.0-7.0 in good yield. We also measured the rates of CPO-I formation by the same method (Table S1). At pH 5.0, 4°C, the second-order rate constant for CPO-I formation was 2.3 × 106 M-1s-1, which is three-fold faster than that of AaeAPO. Although AaeAPO and CPO share ~30% sequence similarity, their active site environments, especially the acid-base residues, differ and CPO has a less accessible active site.[9]
Table 1.
Data for oxygen atom transfer between halide ions and AaeAPO-I as a function of pH.
We have found that AaeAPO-I is also highly reactive toward halide ions. The formation of HOBr for the reaction of bromide ion with AaeAPO-I was detected conveniently with the diagnostic indicator, phenol red.[11] The rapid tetra-bromination of phenol red was monitored by the characteristic red shift from 434 nm to 592 nm as shown in Scheme S1 and Figure S2. The oxygenation of bromide by CPO-I was found to be much slower than that of AaeAPO-I at the same pH. (Figure S3) The reaction of chloride ion with AaeAPO-I to afford hypochlorous acid was also found to occur with high efficiency but only under acidic conditions.
The kinetic behavior of halide ion oxygenation by AaeAPO-I was then investigated by double-mixing, stopped-flow spectroscopy. At each selected pH, AaeAPO-I was formed in the first push by mixing ferric enzyme with 3 eq of NaOBr or NaOCl. NaBr or NaCl solution was added in the second push after the peak amount of compound I had been achieved. Time-resolved, diode array spectra clearly showed the transformation of compound I back to the resting ferric state. Kinetic profiles were obtained by monitoring the return of the Soret band of ferric AaeAPO at 417 nm or ferric CPO at 399 nm and fitted to a single exponential equation (Figure S4). The observed pseudo-first order rate constants (kobs) were found to vary linearly with [NaBr] or [NaCl]. The apparent second-order rate constants (krev) were calculated from the slopes and are summarized in Table 1 and Table S1. The pH dependence of log krev is plotted in Figure 2. A slope of -1.0 was obtained over the pH range studied for CPO, suggesting that a single proton is involved in the reaction. However, for AaeAPO, the log krev/pH slope is only -0.3, suggesting that a protonation may not occur in the rate-determining step.
Figure 2.
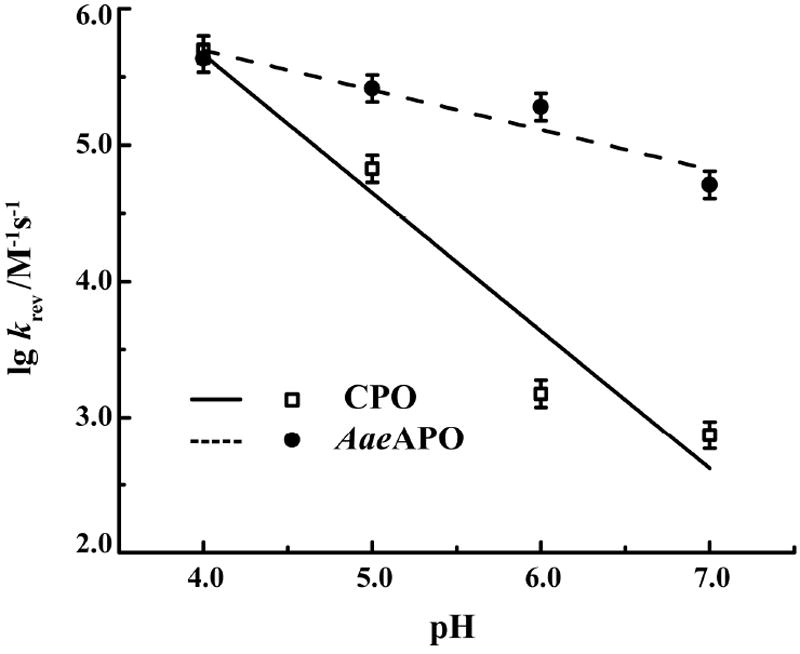
Plots of log krev as a function of pH for oxo-transfer from AaeAPO-I and CPO-I to bromide ion.
Taking advantage of this reversible and kinetically well-behaved oxygen atom transfer reaction (Scheme 1), we determined a set of equilibrium constants, Kequi, from the ratios of the measured forward and reverse rate constants. Since the redox potentials for the couples HOBr/Br- and HOCl/Cl- are known,[12] the corresponding oxygen atom transfer driving force for AaeAPO-I could be calculated at each pH as shown in equations 1 and 2 (n=2, at 4°C).
| (1) |
| (2) |
The derived compound I/ferric enzyme redox potentials for AaeAPO and CPO are summarized in Table 1 and plotted in Figure 3. Fitting those points from pH 3.0 to 7.0 gave linear relationships with a slope of 0.048 for AaeAPO and 0.056 for CPO, close to the theoretical value of 0.055 for the Nernst equation at 4 °C. This similarity supports a Nernst half-reaction involving two electrons and two protons as shown in Scheme 2.
Figure 3.
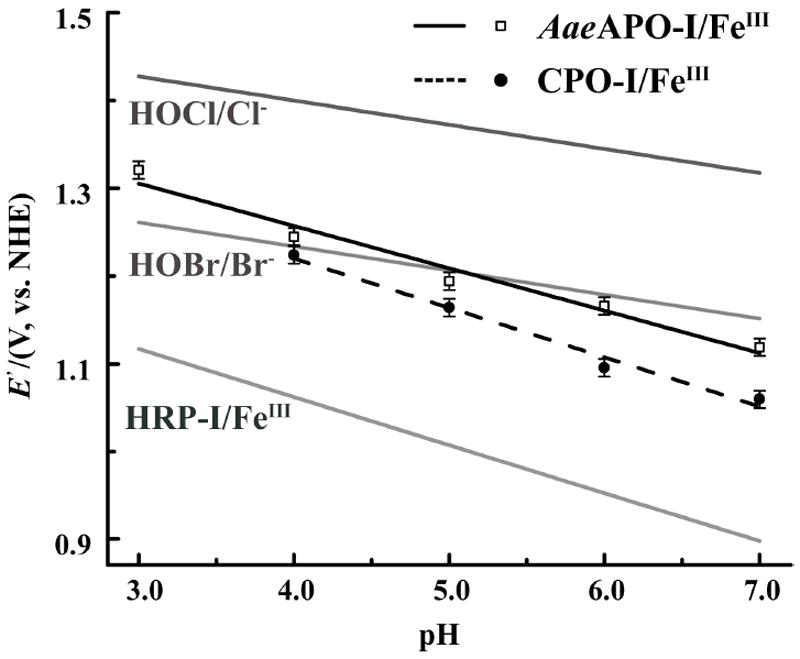
Calculated redox potentials E’(cpd-I/ferric) as a function of pH for AaeAPO-I/FeIII (open squares) and CPO-I/FeIII (closed circles) at 4°C. Nernst equations for HRP-I/FeIII [13], HOBr/Br− and HOCl/Cl− are plotted in gray for comparison.
Scheme 2.
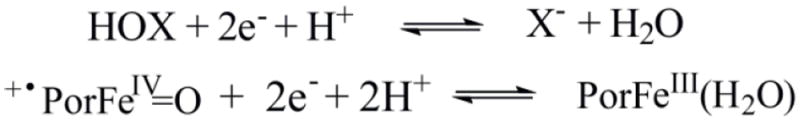
Nernst half-reactions for HOX and +•PorFeIV=O.
As can be seen in Figure 3, the driving force for oxygen atom transfer for AaeAPO-I and CPO-I are similar to that of HOBr and about 200 mV less than that of HOCl. AaeAPO-I and CPO-I are both significantly more oxidizing than HRP-I, while AaeAPO-I has slightly larger redox potentials than those of CPO-I over the entire pH range. Thus, the ordering of the redox potentials parallels the reactivity of these heme proteins. CPO-I reacts slowly with even weak C-H bonds,[4, 14] while HRP-I is barely able to oxidize C-H bonds at all. By contrast, AaeAPO-I is highly reactive toward even very strong C-H bonds, so other active site factors may contribute to the greater facility of C-H hydroxylation than CPO. Similar halide oxidation data for cytochrome P450 is not available. However, by comparing the hydroxylation kinetics of AaeAPO and CYP119 with similar aliphatic substrates,[3, 15] the redox properties of P450-I and AaeAPO-I appear to lie on a similar scale.
What factors contribute to the significantly higher driving force for ferryl oxygen atom transfer by AaeAPO-I and CPO-I reported here as compared to that of HRP-I? The axial ligand for AaeAPO and CPO are both cysteine thiolate anions, while for HRP, it is a neutral, histidine nitrogen. The importance of hydrogen bonding to the cysteine thiolate of P450, CPO and NOS has been noted.[16] According to the Nernst half reaction (Scheme 2), the driving force for the conversion of +•PorFeIV=O to PorFeIII via oxygen atom transfer has two contributions – the electron affinity and the proton affinity of the ferryl species. Although DFT calculations have indicated that the frontier orbitals of a heme-histidine compound I are at lower energy than the corresponding orbitals in heme-thiolate compound I,[17] the strong proton affinity of a thiolate bound compound I may provide a large driving force resulting in a higher net redox potentials and more reactive oxidants.[5k, 18] The intrinsic basicity of the ferryl oxygen in Cys-S-FeIV=O (compound II) in heme-thiolate enzymes has been established.[19] Since Cys-S-FeIII-OH2 is the resting state, Cys-S-FeIII-OH is also basic, thus contributing further to the two-electron, two-proton oxo-transfer redox couples determined here.
The one-electron redox potential of AaeAPO-I, [Eo(I)], is a particularly important thermodynamic value because it is related to the bond strength [D(O–H)] and the pKa, [pKa (II)] of FeIVO–H in AaeAPO-II (equation 3).[5a, 5b] For cases in which Eo(I) and pKa(II) cannot be measured independently, equation 4 can be derived.[20] Since both Eo(I) and pKa(II) have not been measured independently for any heme-enzyme, the two-electron, two-proton redox potential of AaeAPO-I measured here may be a good first approximation of E0(I). E’(HRP-I/HRP-II) and E’(HRP-II/Ferric) for HRP have been measured and were found to be similar (~0.95 V at pH 6.0).[13, 21] However, this result might be due to the fact that HRP-II is not basic and exists in the FeIV=O form in the functional pH range. The situation is different if we consider that AaeAPO compound II is protonated.[19] For example, if D(O–H) is estimated to be in the range of 100 kcal/mol,[2, 22] the one-electron redox potential, E’(cpd-I/cpd-II), would be 1.4 V vs NHE at pH 7.0, significantly higher than the two-electron E’(cpd-I/ferric) potential of 1.2 V. Accordingly, from equation 5, the reduction potential of AaeAPO-II (E’(cpd-II/ferric)) can be estimated to be ~ 0.8 V. This unsymmetrical partitioning of the two redox steps may be an important factor in facilitating homolytic C-H bond scission by heme-thiolate proteins.
| (3) |
| (4) |
| (5) |
In summary, the results show that chloride and bromide ions are readily oxidized by AaeAPO-I to the corresponding hypohalous acids. The reversibility of this oxo-transfer reaction provides a rare opportunity to place ferryl oxo-transfers by the highly reactive heme-thiolate AaeAPO-I and that of CPO-I on an absolute energy scale. With an estimated BDE for FeIVO–H in AaeAPO-II we are able to obtain redox potentials of three redox couples interconnecting the resting ferric protein with its two oxidized forms, +•Por-FeIV=O and FeIVO–H.
Experimental Section
Reagents
Wild-type extracellular peroxygenase of A. aegerita (isoform II, pI 5.6, 46 kDa) was produced in bioreactors with a soybean-flour suspension as the growth substrate and purified as described previously.[2, 23] Kinetic experiments were performed as we have recently described.[2] Bromination of phenol red was detected by UV/Vis spectroscopy.[7a] At a chosen pH, 2 μl of 10 μM APO or CPO was added to a reaction mixture containing 20 μM of phenol red (sodium salt), 1mM H2O2 and 10 mM NaBr.
The oxidation of ferric enzyme with NaOBr or NaOCl was performed by stopped-flow spectroscopy with the single-mixing mode under the diode-array or single wavelength mode. The first syringe was filled with enzyme in a 100 mM buffer at a chosen pH. The second syringe was filled with the oxidant in slightly basic water solution. Equal volumes of the two reactants were mixed quickly. The halide ion oxidation reactions were performed using the double-mixing mode. Native enzyme was mixed with an equal volume of oxidants (NaOCl or NaOBr) in the first push. After an aging time (optimized for each pH), the sodium halide solution was added in the second push. All concentrations reported are the final concentrations. All the experiments were carried out at 4 °C. The data were analyzed using Kinetic Studio from Hi-Tech.
Supplementary Material
Footnotes
Support of this research by the National Institutes of Health (2R37 GM036298), the European Social Fund (080935557) and the European Union integrated project, Peroxicats (265397) are gratefully acknowledged.
Supporting information for this article is available on the WWW under http://www.angewandte.org.
Contributor Information
Xiaoshi Wang, Department of Chemistry, Princeton University, Princeton, NJ 08544, USA.
Sebastian Peter, Department of Bio- and Environmental Sciences, International Graduate School of Zittau, Zittau, D-02763, Germany.
Dr. René Ullrich, Department of Bio- and Environmental Sciences, International Graduate School of Zittau, Zittau, D-02763, Germany
Prof. Martibn Hofrichter, Department of Bio- and Environmental Sciences, International Graduate School of Zittau, Zittau, D-02763, Germany
Prof. John T. Groves, Department of Chemistry, Princeton University, Princeton, NJ 08544, USA, Fax: (+1) 609-258-0348, jtgroves@princeton.edu
References
- 1.a) Hofrichter M, Ullrich R, Pecyna MJ, Liers C, Lundell T. Appl Microbiol Biotechnol. 2010;87:871–897. doi: 10.1007/s00253-010-2633-0. [DOI] [PubMed] [Google Scholar]; b) Peter S, Kinne M, Wang X, Ullrich R, Kayser G, Groves JT, Hofrichter M. FEBS J. 2011;278:3667–3675. doi: 10.1111/j.1742-4658.2011.08285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Ullrich R, Hofrichter M. FEBS Lett. 2005;579:6247–6250. doi: 10.1016/j.febslet.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Peter S, Kinne M, Hofrichter M, Groves JT. J Am Chem Soc. 2012;134:12897–12900. doi: 10.1021/ja3049223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rittle J, Green MT. Science. 2010;330:933–937. doi: 10.1126/science.1193478. [DOI] [PubMed] [Google Scholar]
- 4.Zhang R, Nagraj N, Lansakara DSP, Hager LP, Newcomb M. Org Lett. 2006;8:2731–2734. doi: 10.1021/ol060762k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Mayer JM. Acc Chem Res. 1998;31:441–450. [Google Scholar]; b) Bordwell FG, Cheng JP, Ji GZ, Satish AV, Zhang X. J Am Chem Soc. 1991;113:9790–9795. [Google Scholar]; c) Concepcion JJ, Jurss JW, Brennaman MK, Hoertz PG, Patrocinio AOT, Iha NYM, Templeton JL, Meyer TJ. Acc Chem Res. 2009;42:1954–1965. doi: 10.1021/ar9001526. [DOI] [PubMed] [Google Scholar]; d) Meyer TJ, Huynh MHV, Thorp HH. Angew Chem. 2007;119:5378–5399. [Google Scholar]; Angew Chem Int Ed. 2007;46:5284–5304. doi: 10.1002/anie.200600917. [DOI] [PubMed] [Google Scholar]; e) Mayer JM. Ann Rev Phys Chem. 2004;55:363–390. doi: 10.1146/annurev.physchem.55.091602.094446. [DOI] [PubMed] [Google Scholar]; f) Cukier RI, Nocera DG. Ann Rev Phys Chem. 1998;49:337–369. doi: 10.1146/annurev.physchem.49.1.337. [DOI] [PubMed] [Google Scholar]; g) Borovik AS. Chem Soc Rev. 2011;40:1870–1874. doi: 10.1039/c0cs00165a. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Warren JJ, Tronic TA, Mayer JM. Chem Rev. 2010;110:6961–7001. doi: 10.1021/cr100085k. [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Gunay A, Theopold KH. Chem Rev. 2010;110:1060–1081. doi: 10.1021/cr900269x. [DOI] [PubMed] [Google Scholar]; j) Waidmann CR, Miller AJM, Ng CWA, Scheuermann ML, Porter TR, Tronic TA, Mayer JM. Energy Env Sci. 2012;5:7771–7780. [Google Scholar]; k) Lai WZ, Li CS, Chen H, Shaik S. Angew Chem. 2012;124:5652–5676. doi: 10.1002/anie.201108398. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2012;51:5556–5578. doi: 10.1002/anie.201108398. [DOI] [PubMed] [Google Scholar]
- 6.Jin N, Bourassa JL, Tizio SC, Groves JT. Angew Chem. 2000;112:4007–4009. doi: 10.1002/1521-3773(20001103)39:21<3849::AID-ANIE3849>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2000;39:3849–3851. doi: 10.1002/1521-3773(20001103)39:21<3849::AID-ANIE3849>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 7.a) Lahaye D, Groves JT. J Inorg Biochem. 2007;101:1786–1797. doi: 10.1016/j.jinorgbio.2007.07.017. [DOI] [PubMed] [Google Scholar]; b) Umile TP, Wang D, Groves JT. Inorg Chem. 2011;50:10353–10362. doi: 10.1021/ic201430v. [DOI] [PubMed] [Google Scholar]; c) Umile TP, Groves JT. Angew Chem. 2011;123:721–724. doi: 10.1002/anie.201004482. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:695–698. doi: 10.1002/anie.201004482. [DOI] [PubMed] [Google Scholar]
- 8.Bell SR. PhD thesis. Princeton University (USA); 2010. [Google Scholar]
- 9.a) Pecyna MJ, Ullrich R, Bittner B, Clemens A, Scheibner K, Schubert R, Hofrichter M. Appl Microbiol Biotechnol. 2009;84:885–897. doi: 10.1007/s00253-009-2000-1. [DOI] [PubMed] [Google Scholar]; b) Sundaramoorthy M, Terner J, Poulos TL. Chem Biol. 1998;5:461–473. doi: 10.1016/s1074-5521(98)90003-5. [DOI] [PubMed] [Google Scholar]
- 10.Bard AJ, Parsons R, Jordan J. Standard Potentials in Aqueous Solution. Marcel Dekker, Inc.; New York: 1985. pp. 67–92. [Google Scholar]
- 11.a) Walker JV, Morey M, Carlsson H, Davidson A, Stucky GD, Butler A. J Am Chem Soc. 1997;119:6921–6922. [Google Scholar]; b) Totaro RM, Williams AM, Apella MC, Blesa MA, Baran EJ. J Chem Soc Dalton Trans. 2000:4403–4406. [Google Scholar]
- 12.Holm RH, Donahue JP. Polyhedron. 1993;12:57–589. [Google Scholar]
- 13.Farhangrazi ZS, Fossett ME, Powers LS, Ellis WR., Jr Biochemistry. 1995;34:2866–2871. doi: 10.1021/bi00009a017. [DOI] [PubMed] [Google Scholar]
- 14.Zaks A, Dodds DR. J Am Chem Soc. 1995;117:10419–10424. [Google Scholar]
- 15.a) Su Z, Horner JH, Newcomb M. ChemBioChem. 2012;13:2061–2064. doi: 10.1002/cbic.201200387. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Davydov R, Dawson JH, Perera R, Hoffman BM. Biochemistry. 2013;52:667–671. doi: 10.1021/bi301527c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.a) Mak PJ, Yang YT, Im S, Waskell LA, Kincaid JR. Angew Chem. 2012;124:10549–10553. doi: 10.1002/anie.201205912. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2012;51:10403–10407. doi: 10.1002/anie.201205912. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lang J, Santolini J, Couture M. Biochemistry. 2011;50:10069–10081. doi: 10.1021/bi200965e. [DOI] [PubMed] [Google Scholar]; c) Galinato MGI, Spolitak T, Ballou DP, Lehnert N. Biochemistry. 2011;50:1053–1069. doi: 10.1021/bi101911y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.a) Kumar D, De Visser SP, Sharma K, Derat E, Shaik S. J Biol Inorg Chem. 2005;10:181–189. doi: 10.1007/s00775-004-0622-4. [DOI] [PubMed] [Google Scholar]; b) Kumar D, Sastry GN, de Visser SP. J Phys Chem B. 2012;116:718–730. doi: 10.1021/jp2113522. [DOI] [PubMed] [Google Scholar]
- 18.a) Dey A, Jiang Y, Ortiz de Montellano R, Hodgson KO, Hedman B, Solomon EI. J Am Chem Soc. 2009;131:7869–7878. doi: 10.1021/ja901868q. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Takahashi A, Yamaki D, Ikemura K, Kurahashi T, Ogura T, Hada M, Fujii H. Inorg Chem. 2012;51:7296–7305. doi: 10.1021/ic3006597. [DOI] [PubMed] [Google Scholar]; c) Hughes TF, Friesner RA. J Chem Theo Comp. 2012;8:442–459. doi: 10.1021/ct2006693. [DOI] [PubMed] [Google Scholar]; d) Isobe H, Yamaguchi K, Okumura M, Shimada J. J Phys Chem B. 2012;116:4713–4730. doi: 10.1021/jp211184y. [DOI] [PubMed] [Google Scholar]
- 19.Green MT, Dawson JH, Gray HB. Science. 2004;304:1653–1656. doi: 10.1126/science.1096897. [DOI] [PubMed] [Google Scholar]
- 20.Wang D, Zhang M, Buhlmann P, Que L. J Am Chem Soc. 2010;132:7638–7644. doi: 10.1021/ja909923w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayashi Y, Yamazaki I. J Biol Chem. 1979;254:9101–9106. [PubMed] [Google Scholar]
- 22.Bell SR, Groves JT. J Am Chem Soc. 2009;131:9640–9641. doi: 10.1021/ja903394s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ullrich R, Nuske J, Scheibner K, Spantzel J, Hofrichter M. Appl Environ Microbiol. 2004;70:4575–4581. doi: 10.1128/AEM.70.8.4575-4581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


