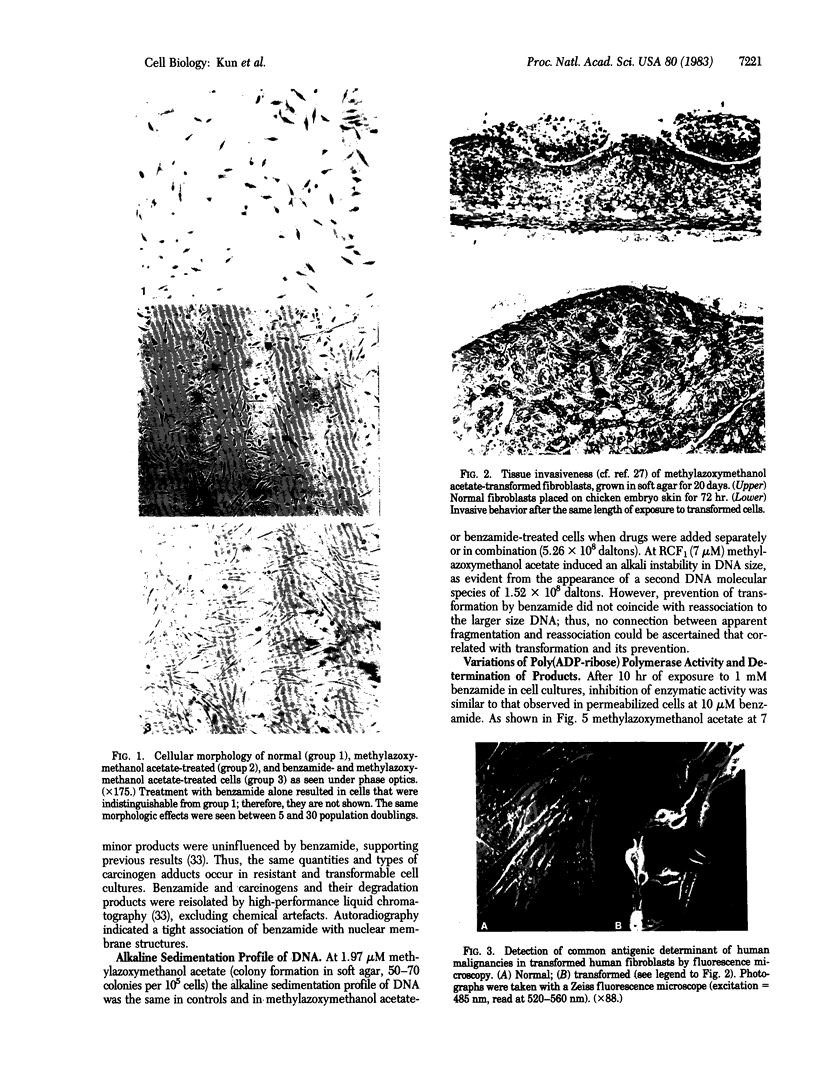
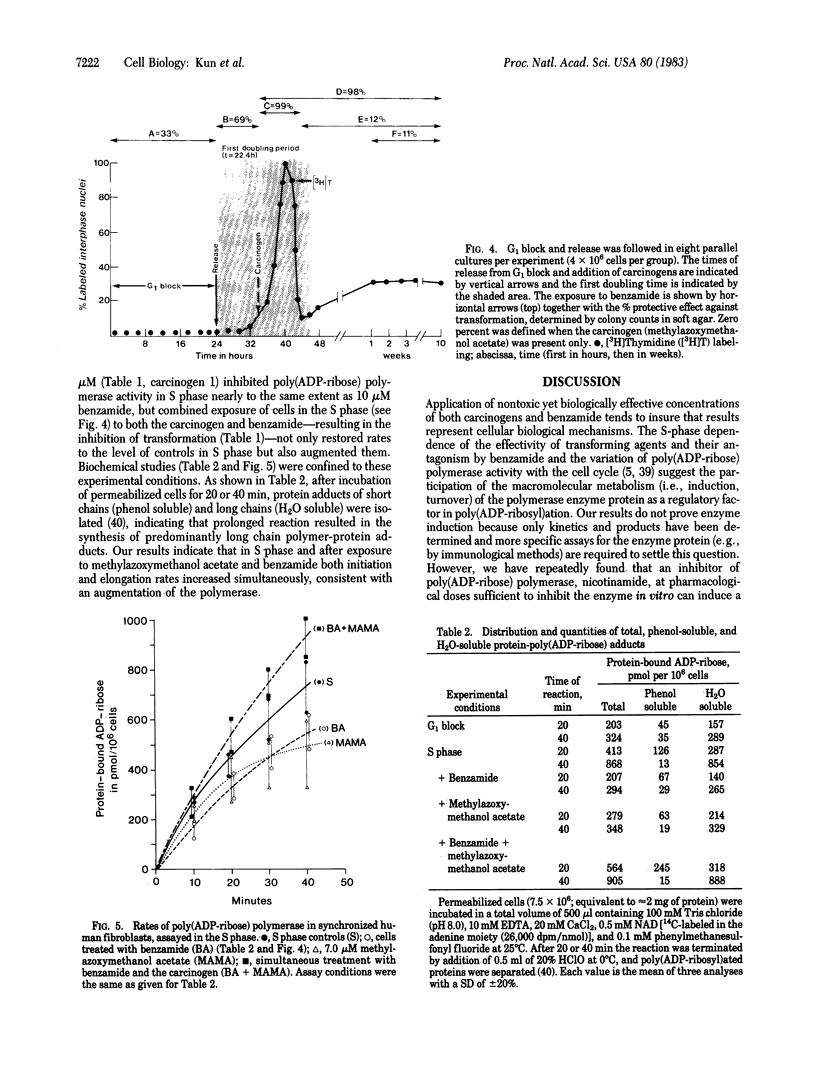
Abstract
Human fibroblasts were subjected to nutritionally induced G1 block, followed by release and subsequent entry into S phase, and exposed to nontoxic concentrations of carcinogens in early S phase. Cell transformation occurred as determined by early morphologic cell alterations, anchorage-independent colony formation, cell invasiveness, and augmentation of Ab 376 human malignancy-specific cell-surface antigenic determinant. Methylazoxymethanol acetate was the most potent transforming agent at doses that were negative in toxicity tests. Benzamide (10 microM intracellular concentration), a specific inhibitor of poly(ADP-ribose) polymerase, prevented transformation in a cell cycle-specific manner, maximal prevention coinciding with early S phase, also characteristic of maximal susceptibility to transformation. Neither an interference of carcinogen deoxyguanosine nucleoside adduct formation nor a chemical reaction between benzamide and carcinogens was detected. Methylazoxymethanol acetate at transforming but nontoxic dose partially inhibited poly(ADP-ribosyl)ation to about the same extent as benzamide. However, simultaneous exposure of cells to both agents in early S phase, resulting in the prevention of transformation, augmented poly(ADP-ribosyl)ation above the controls. Enzymatic activities ran parallel with the formation of DNA-associating polymer-nonhistone protein adducts that are assumed to regulate the physiological function of chromatin at the structural level.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker R. A., Barrows L. R., Shank R. C. Methylation of liver DNA guanine in hydrazine hepatotoxicity: dose-response and kinetic characteristics of 7-methylguanine and O6-methylguanine formation and persistence in rats. Carcinogenesis. 1981;2(11):1181–1188. doi: 10.1093/carcin/2.11.1181. [DOI] [PubMed] [Google Scholar]
- Bertram J. S., Heidelberger C. Cell cycle dependency of oncogenic transformation induced by N-methyl-N'-nitro-N-nitrosoquanidine in culture. Cancer Res. 1974 Mar;34(3):526–537. [PubMed] [Google Scholar]
- Bishop J. M. Enemies within: the genesis of retrovirus oncogenes. Cell. 1981 Jan;23(1):5–6. doi: 10.1016/0092-8674(81)90263-4. [DOI] [PubMed] [Google Scholar]
- Blake R. L., Blake S. L., Loh H. H., Kun E. The effect of nicotinamide and homologs on the activity of inducible enzymes and NAD content of the rat liver. Mol Pharmacol. 1967 Sep;3(5):412–422. [PubMed] [Google Scholar]
- Brash D. E., Hart R. W. Biopsy measurement of DNA damage and repair in vivo: single-strand breaks, alkali-labile bonds, and endonuclease-sensitive sites. Radiat Res. 1982 Jul;91(1):169–180. [PubMed] [Google Scholar]
- Caplan A. I., Rosenberg M. J. Interrelationship between poly (ADP-Rib) synthesis, intracellular NAD levels, and muscle or cartilage differentiation from mesodermal cells of embryonic chick limb. Proc Natl Acad Sci U S A. 1975 May;72(5):1852–1857. doi: 10.1073/pnas.72.5.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Brugge J. S., Erikson R. L. Characterization of a normal avian cell protein related to the avian sarcoma virus transforming gene product. Cell. 1978 Dec;15(4):1363–1369. doi: 10.1016/0092-8674(78)90061-2. [DOI] [PubMed] [Google Scholar]
- Elgin S. C., Weintraub H. Chromosomal proteins and chromatin structure. Annu Rev Biochem. 1975;44:725–774. doi: 10.1146/annurev.bi.44.070175.003453. [DOI] [PubMed] [Google Scholar]
- Essex M., Klein G., Snyder S. P., Harrold J. B. Antibody to feline oncornavirus-associated cell membrane antigen in neonatal cats. Int J Cancer. 1971 Nov 15;8(3):384–390. doi: 10.1002/ijc.2910080305. [DOI] [PubMed] [Google Scholar]
- Jackowski G., Kun E. Age-dependent variation of rates of polyadenosine-diphosphoribose synthesis by cardiocyte nuclei and the lack of correlation of enzymatic activity with macromolecular size distribution of DNA. J Biol Chem. 1981 Apr 25;256(8):3667–3670. [PubMed] [Google Scholar]
- Jackowski G., Kun E. The effect of in vivo treatment with triiodothyronine on the in vitro synthesis of protein-poly(ADP)-ribose adducts by isolated cardiocyte nuclei and the separation of poly(ADP)-ribosylated proteins by phenol extraction and electrophoresis. J Biol Chem. 1983 Oct 25;258(20):12587–12593. [PubMed] [Google Scholar]
- Jackowski G., Kun E. The influence of triiodothyronine on polyadenosine-diphosphoribose polymerase and RNA synthesis in cardiocyte nuclei. J Mol Cell Cardiol. 1982 Sep;14 (Suppl 3):65–70. doi: 10.1016/0022-2828(82)90131-6. [DOI] [PubMed] [Google Scholar]
- Kidwell W. R., Mage M. G. Changes in poly(adenosine diphosphate-ribose) and poly(adenosine diphosphate-ribose) polymerase in synchronous HeLa cells. Biochemistry. 1976 Mar 23;15(6):1213–1217. doi: 10.1021/bi00651a006. [DOI] [PubMed] [Google Scholar]
- Kirsten E., Minaga T., Kun E. Coincidence of subnuclear distribution of poly(ADP-ribose) synthetase and DNA polymerase beta in nuclei of normal and regenerating liver. FEBS Lett. 1982 Mar 8;139(1):117–120. doi: 10.1016/0014-5793(82)80500-0. [DOI] [PubMed] [Google Scholar]
- Klein G., Lenoir G. Translocations involving Ig-locus-carrying chromosomes: a model for genetic transposition in carcinogenesis. Adv Cancer Res. 1982;37:381–387. doi: 10.1016/s0065-230x(08)60887-8. [DOI] [PubMed] [Google Scholar]
- Kun E., Minaga T., Kirsten E., Jackowski G., McLick J., Peller L., Oredsson S. M., Martin L., Pattabiraman N., Milo G. Biochemical basis of the regulatory role of polyadenosine diphosphoribose. Adv Enzyme Regul. 1983;21:177–199. doi: 10.1016/0065-2571(83)90014-6. [DOI] [PubMed] [Google Scholar]
- Lipetz P. D., Galsky A. G., Stephens R. E. Relationship of DNA tertiary and quaternary structure to carcinogenic processes. Adv Cancer Res. 1982;36:165–210. doi: 10.1016/s0065-230x(08)60425-x. [DOI] [PubMed] [Google Scholar]
- Miller E. C. Some current perspectives on chemical carcinogenesis in humans and experimental animals: Presidential Address. Cancer Res. 1978 Jun;38(6):1479–1496. [PubMed] [Google Scholar]
- Miller M. R., Castellot J. J., Jr, Pardee A. B. A permeable animal cell preparation for studying macromolecular synthesis. DNA synthesis and the role of deoxyribonucleotides in S phase initiation. Biochemistry. 1978 Mar 21;17(6):1073–1080. doi: 10.1021/bi00599a021. [DOI] [PubMed] [Google Scholar]
- Milo G. E., DiPaolo J. A. Presensitization of human cells with extrinsic signals to induced chemical carcinogenesis. Int J Cancer. 1980 Dec 15;26(6):805–812. doi: 10.1002/ijc.2910260615. [DOI] [PubMed] [Google Scholar]
- Milo G. E., Jr, DiPaolo J. A. Neoplastic transformation of human diploid cells in vitro after chemical carcinogen treatment. Nature. 1978 Sep 14;275(5676):130–132. doi: 10.1038/275130a0. [DOI] [PubMed] [Google Scholar]
- Milo G. E., Noyes I., Donahoe J., Weisbrode S. Neoplastic transformation of human epithelial cells in vitro after exposure to chemical carcinogens. Cancer Res. 1981 Dec;41(12 Pt 1):5096–5102. [PubMed] [Google Scholar]
- Milo G. E., Oldham J. W., Zimmerman R., Hatch G. G., Weisbrode S. A. Characterization of human cells transformed by chemical and physical carcinogens in vitro. In Vitro. 1981 Aug;17(8):719–729. doi: 10.1007/BF02628409. [DOI] [PubMed] [Google Scholar]
- Minaga T., Kun E. Probable helical conformation of poly(ADP-ribose). The effect of cations on spectral properties. J Biol Chem. 1983 May 10;258(9):5726–5730. [PubMed] [Google Scholar]
- Minaga T., Kun E. Spectral analysis of the conformation of polyadenosine diphosphoribose. Evidence indicating secondary structure. J Biol Chem. 1983 Jan 25;258(2):725–730. [PubMed] [Google Scholar]
- Minaga T., Marton L. J., Piper W. N., Kun E. Induction of cardiac L-ornithine decarboxylase by nicotinamide and its regulation by putrescine. Eur J Biochem. 1978 Nov 15;91(2):577–585. doi: 10.1111/j.1432-1033.1978.tb12711.x. [DOI] [PubMed] [Google Scholar]
- Minaga T., Romaschin A. D., Kirsten E., Kun E. The in vivo distribution of immunoreactive larger than tetrameric polyadenosine diphosphoribose in histone and non-histone protein fractions of rat liver. J Biol Chem. 1979 Oct 10;254(19):9663–9668. [PubMed] [Google Scholar]
- Murray M. J., Shilo B. Z., Shih C., Cowing D., Hsu H. W., Weinberg R. A. Three different human tumor cell lines contain different oncogenes. Cell. 1981 Aug;25(2):355–361. doi: 10.1016/0092-8674(81)90054-4. [DOI] [PubMed] [Google Scholar]
- Noguchi P. D., Johnson J. B., O'Donnell R., Petricciani J. C. Chick embryonic skin as a rapid organ culture assay for cellular neoplasia. Science. 1978 Mar 3;199(4332):980–983. doi: 10.1126/science.203036. [DOI] [PubMed] [Google Scholar]
- Oldham J. W., Allred L. E., Milo G. E., Kindig O., Capen C. C. The toxicological evaluation of the mycotoxins T-2 and T-2 tetraol using normal human fibroblasts in vitro. Toxicol Appl Pharmacol. 1980 Jan;52(1):159–168. doi: 10.1016/0041-008x(80)90255-0. [DOI] [PubMed] [Google Scholar]
- Poirier G. G., de Murcia G., Jongstra-Bilen J., Niedergang C., Mandel P. Poly(ADP-ribosyl)ation of polynucleosomes causes relaxation of chromatin structure. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3423–3427. doi: 10.1073/pnas.79.11.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnell M. R., Whish W. J. Novel inhibitors of poly(ADP-ribose) synthetase. Biochem J. 1980 Mar 1;185(3):775–777. doi: 10.1042/bj1850775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaschin A. D., Kirsten E., Jackowski G., Kun E. Quantitative isolation of oligo- and polyadenosine-diphosphoribosylated proteins by affinity chromatography from livers of normal and dimethylnitrosamine-treated Syrian hamsters. In vivo and in vitro metabolism of the homopolymer. J Biol Chem. 1981 Aug 10;256(15):7800–7805. [PubMed] [Google Scholar]
- Romaschin A. D., Kun E. Decrease of hepatic mono and oligo adenosine diphosphoribose content and augmentation of [14C]ribose incorporation during induction of growth by bovine growth hormone in hypophysectomized rats. Biochem Biophys Res Commun. 1981 Oct 15;102(3):952–957. doi: 10.1016/0006-291x(81)91630-2. [DOI] [PubMed] [Google Scholar]
- Scarpelli D. G., Rao M. S., Subbarao V. Augmentation of carcinogenesis by N-nitrosobis(2-oxopropyl)amine administered during S phase of the cell cycle in regenerating hamster pancreas. Cancer Res. 1983 Feb;43(2):611–616. [PubMed] [Google Scholar]
- Seeger R. C., Rosenblatt H. M., Imai K., Ferrone S. Common antigenic determinants on human melanoma, glioma, neuroblastoma, and sarcoma cells defined with monoclonal antibodies. Cancer Res. 1981 Jul;41(7):2714–2717. [PubMed] [Google Scholar]
- Takahashi S., Ohnishi T., Denda A., Konishi Y. Enhancing effect of 3-aminobenzamide on induction of gamma-glutamyl transpeptidase positive foci in rat liver. Chem Biol Interact. 1982 Apr;39(3):363–368. doi: 10.1016/0009-2797(82)90052-7. [DOI] [PubMed] [Google Scholar]
- Tanuma S., Kanai Y. Poly(ADP-ribosyl)ation of chromosomal proteins in the HeLa S3 cell cycle. J Biol Chem. 1982 Jun 10;257(11):6565–6570. [PubMed] [Google Scholar]
- Tejwani R., Jeffrey A. M., Milo G. E. Benzo[a]pyrene diol epoxide DNA adduct formation in transformable and non-transformable human foreskin fibroblast cells in vitro. Carcinogenesis. 1982;3(7):727–732. doi: 10.1093/carcin/3.7.727. [DOI] [PubMed] [Google Scholar]
- Tobey R. A., Ley K. D. Isoleucine-mediated regulation of genome repliction in various mammalian cell lines. Cancer Res. 1971 Jan;31(1):46–51. [PubMed] [Google Scholar]
- Urban J. L., Burton R. C., Holland J. M., Kripke M. L., Schreiber H. Mechanisms of syngeneic tumor rejection. Susceptibility of host-selected progressor variants to various immunological effector cells. J Exp Med. 1982 Feb 1;155(2):557–573. doi: 10.1084/jem.155.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring M. J. DNA modification and cancer. Annu Rev Biochem. 1981;50:159–192. doi: 10.1146/annurev.bi.50.070181.001111. [DOI] [PubMed] [Google Scholar]







