SUMMARY
Hybrid male sterility is a common reproductive isolating barrier between species. Yet, little is known about the actual developmental causes of this phenomenon, especially in naturally hybridizing species. We sought to evaluate the developmental causes of hybrid male sterility, using spadefoot toads as our study system. Plains spadefoot toads (S. bombifrons) and Mexican spadefoot toads (S. multiplicata) hybridize where they co-occur in the southwestern USA. Hybrids are viable, but hybrid males suffer reduced fertility. We compared testes size and developmental stages of sperm cell maturation between hybrid males and males of each species. We found that testes of hybrid males did not differ in mean size from pure-species males. However, hybrids showed a greater range of within-individual variation in testes size than pure-species males. Moreover, although hybrids produced similar numbers of early stage sperm cells, hybrids produced significantly fewer mature spermatozoids than pure-species males. Interestingly, an introgressed individual produced numbers of live sperm comparable to pure-species males, but the majority of these sperm cells were abnormally shaped and non-motile. These results indicate that hybrid incompatibilities in late sperm development serve as a reproductive isolating barrier between species. The nature of this breakdown highlights the possibilities that hybrid males may vary in fertility and that fertility could possibly be recovered in introgressed males.
Keywords: speciation, hybrid fitness, Haldane's rule, postzygotic isolating mechanisms, post-mating prezygotic isolating mechanisms
INTRODUCTION
The origin and maintenance of species depends on the evolution of reproductive isolating mechanisms – traits that preclude gene exchange between species or incipient species (reviewed in Coyne and Orr 2004). One form of isolating mechanism is hybrid male sterility: in many naturally hybridizing species, hybrids are viable, but hybrid males are often fully or partially sterile (Orr 1997; Brothers and Delph 2010; Schilthuizen et al. 2011). Hybrid male fertility can be affected by development at two key levels: gonad development and sperm development (Howard et al. 2009). If gonadal development is disrupted, hybrids may fail to develop testes entirely and therefore be sterile. Alternatively, hybrid males might suffer reduced growth of reproductive tissues, thereby resulting in smaller testes (Hardy et al. 2011; Turner et al. 2012) that produce fewer sperm (Møller 1989).
Even if hybrid males develop testes normally, they may still suffer sterility owing to failed sperm development. Spermatogenesis is a complex, multistep process (Segatelli et al. 2009; Madison-Villar and Michalak 2011). Elucidating at what point in this process sperm development breaks down in hybrids is important for understanding both the fitness consequences of hybridization and the efficacy of hybrid sterility as a barrier to gene flow between species. Whereas failures at the early stages of sperm development could be more likely to result in complete sterility, failures at the last stage may result in at least some sperm production by hybrid males. Moreover, failures in later stages of sperm development could result in sperm that are viable, but of low quality. Because sperm quality and therefore fertilization success depends on motility, morphology (including the ultra-structural organization of the spermatozoids), sperm viability, and sperm density (Rurangwa et al. 2004; Beirao et al. 2009; Dziminski et al. 2009), hybrids may be at least partially infertile if sperm develop, but possess impairments in these traits (Howard et al. 2009). In such cases, hybrid infertility could depend on an interaction between the quality and quantity of sperm produced and the natural environment in which fertilization occurs (Ålund et al. 2013).
Because the developmental causes of hybrid male sterility are not fully understood, we sought to evaluate when during development sperm production fails in hybrids. Using spadefoot toads as our study system, our specific goals were two-fold. We first determined whether hybrid males develop testes, and if so, whether they are morphologically similar to pure-species testes. Second, we evaluated when during spermatogenesis sperm development fails in hybrids, if at all.
MATERIALS AND METHODS
Study System
We used the Mexican spadefoot toad (Spea multiplicata) and its congener, the Plains spadefoot toad (S. bombifrons), as our study species. These two species co-occur and hybridize in the southwestern USA. The frequency of hybridization varies among populations with historical estimates of F1 hybrid frequency as high as 40% (Simovich 1985; Simovich and Sassaman 1986; Pfennig and Simovich 2002) and the frequency of introgressed offspring (i.e., offspring that are products of pairings between hybrids or hybrids and pure-species types) as high as 51% (Pfennig et al. 2012) in populations in southeastern Arizona.
Hybrids are viable, but they suffer reduced fertility as adults. First-generation (F1) hybrid females produce fewer eggs than either pure-species type (Simovich 1985; Simovich et al. 1991). F1 hybrid males also appear to suffer low fertility, but accounts differ as to whether males are completely sterile (Simovich 1985; Simovich, Sassaman, and Chovnick 1991) or whether hybrid fertility varies among males (Forester 1975). One explanation for these different accounts of male sterility is that the methodology for measuring male fertility varies. Fertilization success of hybrid males in spadefoots has been measured via two different routes: in-vitro fertilization with sperm suspensions obtained from crushed testes of freshly killed animals (Forester 1975) and natural fertilization of paired adults (Simovich 1985; Simovich, Sassaman, and Chovnick 1991). In the former study, hybrid males were found to vary in fertility whereas, in the latter study, the absence of offspring in natural pairings of hybrids was attributed to male sterility.
Although hybrid males are potentially sterile, they exhibit secondary sexual characteristics such as calling behavior to attract females (Pfennig 2000) and nuptial pads: rough thickenings/swellings on the fingers and forearm that develop in reproductively active males for holding females during breeding. The expression of these sexual traits by hybrid males suggests that testicular endocrine function is retained (Takahashi et al. 2005).
Hybrid sterility is a major cost of hybridization, which likely serves as a significant reproductive isolating barrier to gene exchange between S. multiplicata and S. bombifrons (Simovich 1985; Simovich, Sassaman, and Chovnick 1991; Pfennig and Simovich 2002). We therefore used the methods below to determine whether hybrid males develop testes and when during development sperm production fails, if at all.
Testes Preparation and Embedding
To evaluate testes and sperm development in hybrid males versus pure-species males, we collected testes from existing ethanol-preserved whole animal lab specimens. We collected tissues from a total of 32 adult males: 11 S. bombifrons (9 wild caught and 2 lab bred individuals), 10 S. multiplicata (all wild caught), and 11 hybrids (all lab bred). All lab-bred males were the offspring of parents that were wild caught. All wild-caught individuals, including those used to produce lab-bred animals, were obtained from populations in Arizona, New Mexico or Texas, USA between 2004 and 2012 during the summer reproductive seasons. Of the 11 hybrids, 10 were offspring of S. bombifrons females mated to S. multiplicata males and one was the offspring of an S. bombifrons male mated to an S. multiplicata female. Prior to dissection, we measured mass and snout-vent length (SVL) of all males (Table 1).
Table 1.
Testes size and within individual variation in similarly sized males of S. bombifrons, S. multiplicata and their hybrids (n = number of males measured).
| Male type (n) | Mean testes length ± SE | Range of length variation | Mean SVL ± SE | Mean mass ± SE |
|---|---|---|---|---|
| S. bombifrons (11) | 4.56mm ± 0.84 | 2% – 20% | 43.36mm ± 3.64 | 12.54g ± 3.94 |
| S. multiplicata (10) | 4.59mm ± 1.32 | 4% – 30.5% | 44.08mm ± 1.48 | 10.67g ± 2.15 |
| Hybrid (11) | 5.71mm ± 0.92 | 7% – 56% | 42.91mm ± 2.66 | 11.16g ± 2.32 |
Once the testes were dissected out, we took photographs of each testis with a dissecting stereoscope equipped with a Leica camera (4x). The length and width of the isolated testes were measured using ImageJ software (Schneider et al. 2012). For all but one S. bombifrons male and one S. multiplicata male that each had only a single testis, we calculated the mean length for the two testes for each male. We also calculated the percent difference in size of the testes for each male as a measure of within-male variation in size.
The testes were then fixed in Bouin solution for 24h and stored in 70% ethanol. We embedded the fixed testes in plastic (Technovit 7100), and prepared 3 μm thin longitudinal sections with a microtome (Leica JUNG RM 2065). The thin sections were stained with 1% toluidine blue and borax (de Souza Santos and de Oliveira 2008).
We arbitrarily chose sections from one testis per animal for sperm cell counts. Although we did not observe notable variation in sperm cell abundance throughout the sections of testes tissue, we performed cell counts of different developmental sperm cell stages on three randomly chosen sections. We averaged these counts over the three sections for each male to obtain a single measure for each male.
Differentiation stages of sperm cells were determined as described in de Oliveira et al. (2002) and de Souza Santos and de Oliveira (2008). We report the results on the last three developmental stages: elongated spermatids, sperm bundles and spermatozoids, because earlier developmental stages did not show any differences between hybrids and pure-species animals. Counts of all three stages were made in one visual field of a Nikon Eclipse E800 (100x) using ImageJ software (Schneider, Rasband, and Eliceiri 2012).
Sperm Viability Assays
To assay sperm viability, we obtained live sperm samples from sexually mature males in our colony at the University of North Carolina at Chapel Hill. We collected samples from four hybrid males, three of which were F1 hybrid. Two of these F1 males were lab-bred offspring of wild-caught S. bombifrons males mated to a wild-caught S. multiplicata females and one was the offspring of a wild-caught S. bombifrons female mated to a wild-caught S. multiplicata male. The third F1 hybrid was wild-caught and had a genotype indicating that it was the offspring of a S. bombifrons female mated to S. multiplicata male. Hybrid genotype was ascertained using a mitochondrial marker and a suite of polymerase chain reaction-restriction fragment length polymorphisms (PCR-RFLPs) as in Pfennig et al. (2012). The fourth hybrid was a wild-caught backcross hybrid, also as indicated by its genotype (Pfennig et al. 2012). Two of the hybrids had clearly visible nuptial pads, indicating they were sexually mature, whereas the other two animals did not. However, these latter males produced mating calls in the lab, indicating that they too were sexually mature. We also collected sperm from six S. multiplicata males and 10 S. bombifrons males. All pure-species males were wild-caught males with nuptial pads.
All males were measured for mass and SVL and then injected subcutaneously with 25 μl of 10 μg/ml GnRH agonist, a hormone that induces breeding by toads in the lab (e.g., Pfennig et al. 2007). Following injection, the males were returned to moist, sand-filled housing boxes for 6 to 8 hours until sperm collection. After this time period, males were removed from the sand and their abdomens gently massaged to induce the release of spermatic urine (Kouba et al. 2009). The spermatic urine was collected on 100 mm × 15 mm petri dishes, transferred to 1.5 ml microcentrifuge tubes, and kept on ice.
To assay the abundance of live sperm cells versus dead sperm cells (i.e., sperm cells that had a compromised cell membrane and were no longer alive) we used Invitrogen's LIVE/DEAD Sperm Viability Kit (L-7011). Before taking a sample of 50 μl of spermatic urine the sample was mixed by pipetting up and down several times. SYBR 14 dye and propidium iodide (PI) were added to the spermatic urine sample with a final concentration of 1 mM and 9.6 mM, respectively, followed by a 10-minute incubation period at room temperature. We next placed 15 μl of this mixture on a microscope slide and covered it with a cover slip. The samples were imaged at 200x using a Leica camera attached to an Epi-fluorescent microscope (excitation wavelength of 520–550 nm, emission wavelength of 610 nm for PI and excitation wavelength of 450–490 nm, emission wavelength of 520–560 nm for SYBR 14). We counted live and dead sperm cells in ten separate, randomly selected visual fields. For each male we obtained a single value of live sperm and a single value of dead sperm by averaging the counts across these visual fields. We also made observations of gross morphological features and motility for the live sperm. Because the introgressed male displayed high numbers of abnormally shaped sperm that were not present in the pure species males or F1 hybrids, we also counted the number of abnormally versus normally shaped sperm in the 10 visual fields for this male.
For all analyses, we used ANOVA followed by post-hoc comparisons where the data met parametric assumptions. Where the data failed to meet parametric assumptions, we used a Wilcoxon test, followed by non-parametric post-hoc comparisons.
RESULTS
Testes Morphology and Size
Hybrid testes were not significantly different in size from either pure-species type (F2,29 = 1.99, P = 0.16; Table 1; Fig. 1). Moreover, intra-individual variation in length between the two gonads was found in most animals regardless of whether they were pure-species types or hybrids. However, hybrids showed a greater range of within-individual variation in testes size (Table 1). Hybrid testes also appeared more variable in pigmentation and morphology than either pure-species type (compare Figs. 1A–D to E–H). In particular, whereas the testes' surface of pure-species males mainly showed a distribution of brown and milky white pigmentation (Fig. 1E–H), hybrid male testes pigmentation ranged from dark black (Fig. 1A) to completely milky-white (Fig. 1C). Two of the 11 hybrid males also showed intra-individual variation in testicular pigmentation, having one strongly pigmented testis and one non-pigmented testis (Fig. 1C). Moreover, the testes of one hybrid were abnormally shaped. The testes of this male had a bloated appearance and seminiferous tubules that were not easily distinguished, possibly because of a greater amount of testicular interstitial tissue containing melanocytes (Fig. 1A).
Figure 1.
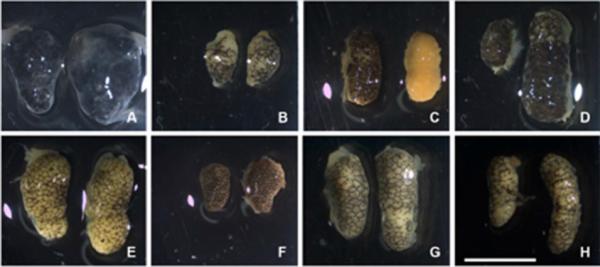
Pure-species and hybrid testes. (A–D): Hybrid testes of four representative individuals showing variation in morphology and pigmentation. (E, F): testes of two representative S. bombifrons males; (G, H): testes of two representative S. multiplicata males. Scale bar = 5mm.
Differences in Spermatogenesis of Pure-Species vs. Hybrid Males
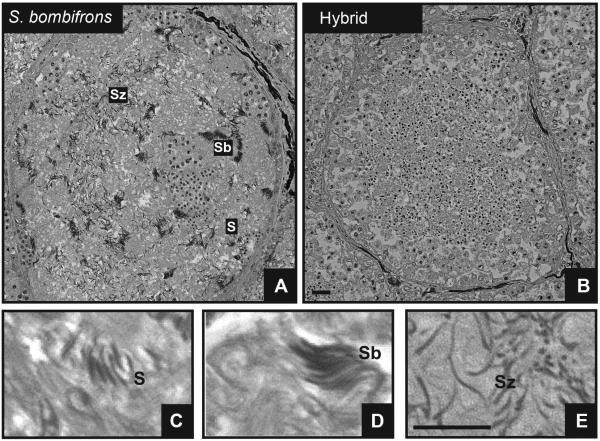
When we examined testes sections, we observed that hybrid male testis tissue generally appeared more homogenous than that of both pure-species males (Fig. 2). Specifically, in pure-species samples the distinct structures of all different developmental sperm cell stages were clearly visible within every seminiferous tubule (Fig. 2A). By contrast, the hybrid samples displayed mainly early developmental sperm cell stages that were evenly distributed throughout the seminiferous tubules; few advanced stages of cell types were observed (Fig. 2B).
Figure 2.
Overview of a typical seminiferous tubule in 3μm thin sections of Technovit embedded testis of (A) a S. bombifrons male and (B) a hybrid male. (C–E): Detailed views of the three last sperm-cell differentiation stages in spermatogenesis: (C) spermatids, (D) sperm bundles, (E) spermatozoids. Note the presence of all different developmental sperm cell stages in the tubule of the pure-species male (A) versus the preponderance of only early developmental sperm cell stages throughout the tubule in the hybrid (B). Scale bars = 100μm.
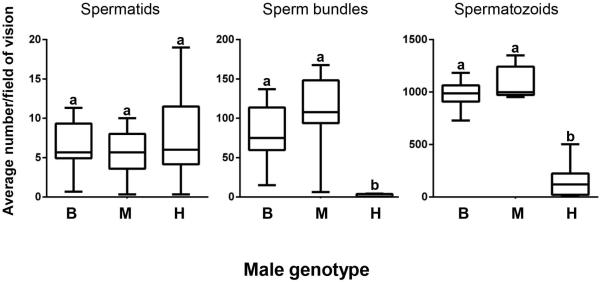
Because hybrids showed similar production of early sperm stages to that of pure species males, we restricted our analysis to the last three stages of sperm development: elongated spermatids (hereafter referred to as spermatids), sperm bundles and spermatozoids (Fig. 2C–E). We found no significant difference in spermatid number among the hybrid and pure-species males (F2,29 = 0.57; P = 0.57; Fig. 4). However, we found a significant effect of male type on sperm bundles (Wilcoxon chi-square approximation = 22.5, df = 2; P < 0.0001; Fig. 4). In particular, although males of the two pure species did not differ in production of sperm bundles (Dunn Z = 1.14, P = 0.77), hybrid males produced significantly fewer sperm bundles than males of both S. bombifrons (Dunn Z = 3.42, P = 0.002) and S. multiplicata (Dunn Z = 4.50, P < 0.001). We also found a significant effect of male type on the number of spermatozoids observed (F2,29 = 133.75; P < 0.0001; Fig. 4). The pure species males did not differ in spermatozoid production (Tukey HSD test, P = 0.34). However, hybrid males produced significantly fewer spermatozoids than males of both S. bombifrons (Tukey HSD test, P < 0.001) and S. multiplicata (Tukey HSD test, P < 0.001).
Figure 4.
Cell counts from 3μm thin sections of sperm cell stages in pure-species versus hybrid males. Different letters indicate groups that are significantly different. B: Spea bombifrons; M: Spea multiplicata; H: hybrid males.
These findings indicate a defect in late spermatogenesis in hybrid males. Interestingly, we found variation among hybrids males' in their ability to produce mature sperm cells (spermatozoids). For four of the 11 hybrids we found few or no spermatozoids, whereas for 7 hybrids, spermatozoid counts ranged from 83–503 per section (Fig. 4).
Sperm Viability and Sperm Morphology
When we evaluated numbers of live versus dead sperm, we found that S. multiplicata and S. bombifrons males both had significantly more live than dead sperm cells in the collected spermatic urine (Wilcoxon signed-rank = 10.5, P = 0.03, and Wilcoxon chi-square approximation = 27.5, P = 0.002, for S. multiplicata and S. bombifrons respectively). For all the F1 hybrids, we were unable to find any spermatozoids in the collected “spermatic” urine. These results contrasted with our observation of spermatozoids in the testes sections of some hybrid males (Fig. 4).
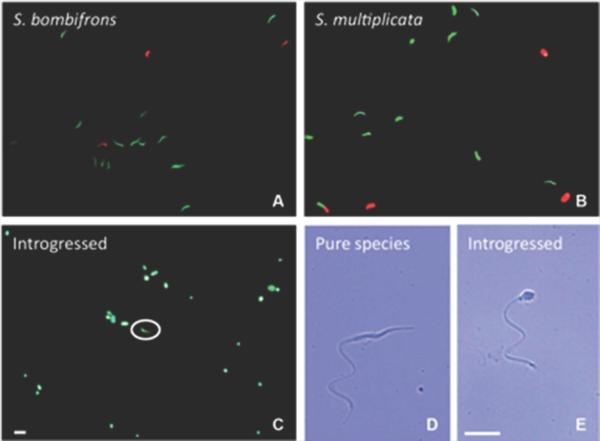
Unlike the F1 hybrids, the backcross hybrid produced a spermatozoid count that was within the range of the pure-species males (Fig. 5). Indeed, we did not find any dead sperm cells in the backcross hybrid's spermatic urine. However, 95% of the spermatozoids were abnormally shaped with shortened, round heads (Fig. 6C, E). In particular, in sperm counts of the introgressed male, we found an average of 4.4 normal sperms cells per visual field compared with an average of 84 abnormally shaped sperm per visual field.
Figure 5.
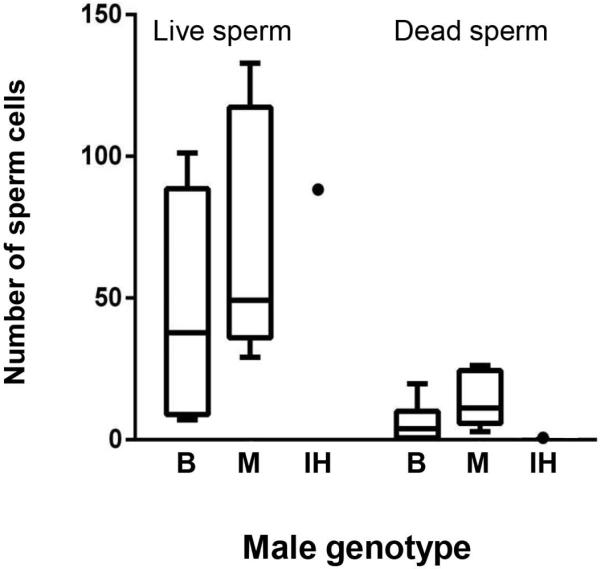
Counts of live and dead sperm cells from spermatic urine of pure-species males and an introgressed hybrid. Both types of pure-species males produced more live than dead sperm. Note that the introgressed male has live sperm cell numbers comparable to the pure-species individuals. B: Spea bombifrons; M: Spea multiplicata; IH: Introgressed hybrid.
Figure 6.
Sperm viability and morphology. Epifluorescence images of live/dead staining of sperm (green: live cells; red: dead cells) in spermatic urine of S. bombifrons (A), S. multiplicata (B) and an introgressed individual (C). Typical (D) and abnormal (E) spermatozoid morphology are shown with bright field images at a higher magnification (400X). The white circle in (C) indicates a normally shaped spermatozoid (as in D) in the sample collected from the introgressed male. Both scale bars = 100 μm.
By contrast, sperm cells from both types of pure-species males generally had elongated, filiform heads (Fig. 6D). Sperm of the introgressed and the pure-species males were similar in their flagellum shape: all had a single flagellum without any accessory structures (Fig. 6D, E). Nevertheless, we observed no movement in the motility of the malformed spermatozoids in the backcross hybrid. Although we did not quantify sperm motility, we observed that the normally shaped sperm cells in the introgressed male showed motility that appeared similar to that of the pure-species sperm.
DISCUSSION
Hybrid testes were not smaller than testes of pure-species males. However, hybrid testes displayed higher variation in coloration and shape (Fig. 1). These results indicate that hybrid sterility does not necessarily arise because gonads fail to develop or are smaller. Instead, our results indicate that hybrid sterility may result from failures at the final stages of sperm development.
In particular, histological sections of testes revealed that the seminiferous tubules of S. multiplicata and S. bombifrons males contained a high abundance of sperm bundles and spermatozoids. By contrast, hybrid males had significantly fewer mature sperm cells or sperm bundles. Indeed, hybrid testes contained mainly earlier developmental sperm cell stages throughout the seminiferous tubules (Figs. 2–4), suggesting that development of sperm generally fails to progress beyond the spermatid stage. Similarly, an arrest of sperm differentiation in late spermatogenesis was suggested to cause hybrid male sterility in Xenopus (Malone et al. 2007).
Our comparisons of live sperm between the pure-species and hybrid males were consistent with these findings. Although both S. bombifrons and S. multiplicata males produced significantly more live than dead sperm cells, the F1 hybrids produced no observable sperm in spermatic urine samples. It could be argued that the F1 hybrid males failed to produce sperm in response to our hormone treatment, because the hormonal receptor (and therefore the ability of GnRH agonist to induce sperm production) is dysfunctional in hybrids (Malone, Chrzanowski, and Michalak 2007). However, such an explanation does not fully account for the absence of later sperm stages in the testes tissue (Figs. 2 and 3).
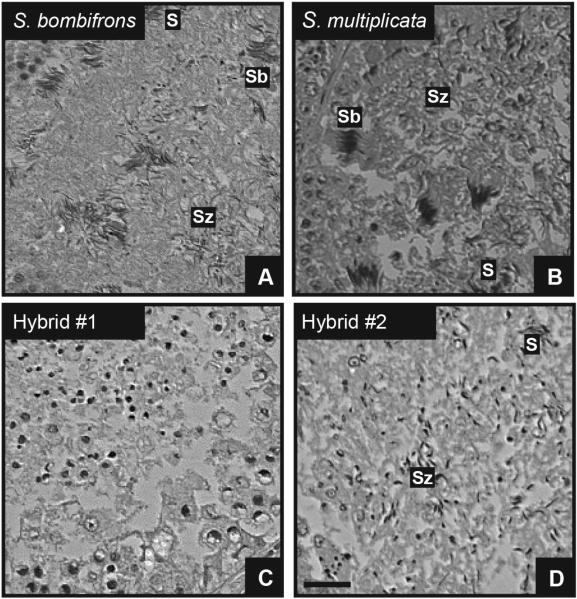
Figure 3.
3μm thin sections of Technovit embedded testis of pure-species and hybrid males showing seminiferous tubules of S. bombifrons (A), S. multiplicata (B) and hybrid individuals (C, D) with last three developmental cell stages indicated as in Fig. 2. Note the lack of later developmental stages of sperm cells in (C) and the absence of sperm bundles in (D). S: Spermatids; Sb: sperm bundles; Sz: spermatozoids. Scale bar = 100μm.
The absence of spermatozoids in the spermatic urine of F1 hybrids contrasts somewhat with the observations of mature spermatozoids (albeit in significantly reduced numbers) in sections of hybrid male testes. It is possible that hybrid males produce so few sperm that they are essentially absent from spermatic urine. Alternatively, because hybrid males vary in spermatozoid production (Fig. 4), the few males we sampled for live sperm might have been those that produced few to no spermatozoids. Regardless, our results could explain the variability in male hybrid fertilization success described in spadefoot toads (Forester 1975; Simovich 1985; Simovich, Sassaman, and Chovnick 1991). Crushing of testes might release enough sperm to enable fertilization in lab studies (as in Forester 1975), but males might be functionally sterile in more natural situations (as in Simovich 1985; Simovich, Sassaman, and Chovnick 1991) if sperm numbers are low. Generally, a better understanding of hybrid male fertility requires evaluating how dysfunctions in testes and sperm development, if any, contribute to reduced fertilization success in the wild (Ålund et al. 2013). Such studies are critical to evaluating how hybrid infertility acts as a reproductive isolating barrier between species (Albrechtova et al. 2012; Ålund et al. 2013).
By contrast to the F1 hybrid males, the live sperm cell count in the introgressed male was comparable to the counts of the pure-species males (Fig. 5). However, we found that 95% of the spermatozoids were abnormally shaped with shortened, round heads (Fig. 6). Other anuran species display a low percentage of round-head spermatozoids in their ejaculate and it is thought that they might be immature sperm cells (Lipke et al. 2009). However, the absence of such sperm in our pure-species samples suggests that the abnormally shaped sperm we observed is a manifestation of hybrid infertility. Indeed, abnormalities of sperm morphology have been observed in other hybrid systems (Malone, Chrzanowski, and Michalak 2007; Hardy, Lougheed, and Markow 2011; White et al. 2012; Ålund et al. 2013), and they might result from various causes including defective chromatin packing and insufficient sperm head elongation during late spermatogenesis (Madison-Villar and Michalak 2011). More critically, this abnormal sperm morphology is often associated with infertility (Pilder et al. 1997), which suggests that, although backcross males may produce sperm, they may still be partially or fully sterile.
Generally, understanding how hybrid male infertility arises in natural hybridizing species is critical for determining the potential for gene exchange between species. If hybrid males produce sufficient numbers of functional sperm cells, then hybrid males could contribute to gene exchange between species. In spadefoots, female hybrids are fertile (albeit with reduced fecundity compared to pure-species females) and can interbreed with pure-species males to produce introgressed offspring (Simovich 1985; Simovich and Sassaman 1986; Pfennig and Simovich 2002; Pfennig et al. 2012). Our results suggest that the resulting introgressed male offspring can produce sperm (albeit with abnormal morphology; Figs. 5 and 6), and, in some cases, may be partially fertile. However, whether or not they are partially fertile will depend not only on successful sperm development but also the natural context in which fertilization occurs. Spadefoot toads are external fertilizers: males release sperm over eggs as females release eggs. The degree to which the abnormal morphology of the sperm affects hybrid fertilization ability and, therefore the potential for gene flow between species, remains a problem to be addressed.
Taken together, our results indicate that hybrid incompatibilities in the last stages of sperm development can serve as a reproductive isolating barrier between species. Yet, the nature of this breakdown also highlights the potential for hybrid males to vary in fertility and for fertility to be recovered in introgressed males. Evaluating these possibilities is critical to fully understand the role of gonadal and gametic development in the origin and maintenance of species.
ACKNOWLEDGEMENTS
We are grateful to Jeff Dangl and Bill Kier for use of their lab facilities, and to Tony Perdue for help with microscopy. We also thank Terry Law, Petra Epple and Farid El Kasmi for their lab assistance and comments on the project, and Emily Schmidt and Sofia De La Serna Buzon for comments on the manuscript. A New Innovator Award from the Office of The Director, National Institutes of Health (1 DP2 OD004436-01) to KSP funded the work.
REFERENCES
- Albrechtova J, Albrecht T, Baird SJE, Macholan M, Rudolfsen G, Munclinger P, Tucker PK, Pialek J. Sperm-related phenotypes implicated in both maintenance and breakdown of a natural species barrier in the house mouse. Proceedings Of The Royal Society B-Biological Sciences. 2012;279(1748):4803–4810. doi: 10.1098/rspb.2012.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ålund M, Immler S, Rice AM, Qvarnstrom A. Low fertility of wild hybrid male flycatchers despite recent divergence. Biology Letters. 2013;9(3):20130169. doi: 10.1098/rsbl.2013.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beirao J, Soares F, Herraez MP, Dinis MT, Cabrita E. Sperm quality evaluation in Solea senegalensis during the reproductive season at cellular level. Theriogenology. 2009;72(9):1251–61. doi: 10.1016/j.theriogenology.2009.07.021. [DOI] [PubMed] [Google Scholar]
- Brothers AN, Delph LF. Haldane's rule is extended to plants with sex chromosomes. Evolution. 2010;64(12):3643–3648. doi: 10.1111/j.1558-5646.2010.01095.x. [DOI] [PubMed] [Google Scholar]
- Coyne JA, Orr HA. Speciation. Sinauer; Sunderland, MA: 2004. [Google Scholar]
- de Oliveira C, Zanetoni C, Zieri R. Morphological observations on the testes of Physalaemus cuvieri (Amphibia, Anura) Revista chilena de anatomía. 2002;20:263–268. [Google Scholar]
- de Souza Santos LR, de Oliveira C. Histological aspects and structural characteristics of the testes of Dendropsophus minutus (Anura, Hylidae) Micron. 2008;39(8):1266–1270. doi: 10.1016/j.micron.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Dziminski MA, Roberts JD, Beveridge M, Simmons LW. Sperm competitiveness in frogs: slow and steady wins the race. Proceedings Of The Royal Society B-Biological Sciences. 2009;276(1675):3955–3961. doi: 10.1098/rspb.2009.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forester DC. Laboratory evidence for potential gene flow between two species of spadefoot toads, Scaphiopus bombifrons and Scaphiopus hammondii. Herpetologica. 1975;31:282–286. [Google Scholar]
- Hardy RW, Lougheed A, Markow TA. Reproductive tract and spermatid abnormalities of hybrid males from reciprocal crosses between Drosophila mojavensis and D. arizonae. Fly (Austin) 2011;5(2):76–80. doi: 10.4161/fly.5.2.15571. [DOI] [PubMed] [Google Scholar]
- Howard DJ, Palumbi SR, Birge LM, Manier MK. Sperm and speciation. In: Birkhead TR, Hosken DJ, Pitnick S, editors. Sperm biology: an evolutionary perspective. 2009. [Google Scholar]
- Kouba AJ, Vance CK, Willis EL. Artificial fertilization for amphibian conservation: current knowledge and future considerations. Theriogenology. 2009;71(1):214–27. doi: 10.1016/j.theriogenology.2008.09.055. [DOI] [PubMed] [Google Scholar]
- Madison-Villar MJ, Michalak P. Misexpression of testicular microRNA in sterile Xenopus hybrids points to tetrapod-specific microRNAs associated with male fertility. Journal Of Molecular Evolution. 2011;73(5–6):316–24. doi: 10.1007/s00239-011-9478-8. [DOI] [PubMed] [Google Scholar]
- Malone JH, Chrzanowski TH, Michalak P. Sterility and gene expression in hybrid males of Xenopus laevis and X. muelleri. Plos One. 2007;2(8):e781. doi: 10.1371/journal.pone.0000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller AP. Ejaculate quality, testes size and sperm production in mammals. Functional Ecology. 1989;3(1):91–96. [Google Scholar]
- Orr HA. Haldane's rule. Annual Review of Ecology and Systematics. 1997;28:195–218. doi: 10.1146/annurev.ecolsys.28.1.85. [DOI] [PubMed] [Google Scholar]
- Pfennig KS. Female spadefoot toads compromise on mate quality to ensure conspecific matings. Behavioral Ecology. 2000;11:220–227. [Google Scholar]
- Pfennig KS, Allenby A, Martin RA, Monroy A, Jones CD. A suite of molecular markers for identifying species, detecting introgression and describing population structure in spadefoot toads (Spea spp.) Molecular Ecology Resources. 2012;12(5):909–17. doi: 10.1111/j.1755-0998.2012.03150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfennig KS, Chunco AJ, Lackey ACR. Ecological selection and hybrid fitness: hybrids succeed on parental resources. Evolutionary Ecology Research. 2007;9:341–354. [Google Scholar]
- Pfennig KS, Simovich MA. Differential selection to avoid hybridization in two toad species. Evolution. 2002;56:1840–1848. doi: 10.1111/j.0014-3820.2002.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Pilder SH, OldsClarke P, Orth JM, Jester WE, Dugan L. Hst7: A male sterility mutation perturbing sperm motility, flagellar assembly, and mitochondrial sheath differentiation. Journal of Andrology. 1997;18(6):663–671. [PubMed] [Google Scholar]
- Rurangwa E, Kime DE, Ollevier F, Nash JP. The measurement of sperm motility and factors affecting sperm quality in cultured fish. Aquaculture. 2004;234(1–4):1–28. [Google Scholar]
- Schilthuizen M, Giesbers MCWG, Beukeboom LW. Haldane's rule in the 21st century. Heredity. 2011;107(2):95–102. doi: 10.1038/hdy.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nature Methods. 2012;9(7):671–5. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segatelli TM, Batlouni SR, Franca LR. Duration of spermatogenesis in the bullfrog (Lithobates catesbeianus) Theriogenology. 2009;72(7):894–901. doi: 10.1016/j.theriogenology.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Simovich MA. Ph.D. Riverside; University of California: 1985. Analysis of a hybrid zone between the spadefoot toads Scaphiopus multiplicatus and Scaphiopus bombifrons. [Google Scholar]
- Simovich MA, Sassaman CA. Four independent electrophoretic markers in spadefoot toads. Journal of Heredity. 1986;77:410–414. doi: 10.1093/oxfordjournals.jhered.a110271. [DOI] [PubMed] [Google Scholar]
- Simovich MA, Sassaman CA, Chovnick A. Post-mating selection of hybrid toads (Scaphiopus multiplicatus and Scaphiopus bombifrons) Proceedings of the San Diego Society of Natural History. 1991;1991:1–6. [Google Scholar]
- Takahashi H, Nagai T, Goto A. Hybrid male sterility between the fresh- and brackish-water types of ninespine stickleback Pungitius pungitius (Pisces, Gasterosteidae) Zoological Science. 2005;22(1):35–40. doi: 10.2108/zsj.22.35. [DOI] [PubMed] [Google Scholar]
- Turner LM, Schwahn DJ, Harr B. Reduced male fertility is common but highly variable in form and severity in a natural house mouse hybrid zone. Evolution. 2012;66(2):443–58. doi: 10.1111/j.1558-5646.2011.01445.x. [DOI] [PubMed] [Google Scholar]
- White MA, Stubbings M, Dumont BL, Payseur BA. Genetics and evolution of hybrid male sterility in house mice. Genetics. 2012;191(3):917–34. doi: 10.1534/genetics.112.140251. [DOI] [PMC free article] [PubMed] [Google Scholar]