Abstract
Objectives
This study aimed to determine the effect of a moderate, tailored exercise program on health-related quality of life, physical function, and arm volume in women receiving treatment for nonmetastatic breast cancer.
Methods
Women who were within 4–12 weeks of surgery for stage I–III breast cancer were randomized to center-based exercise and lymphedema education intervention or patient education. Functional Assessment of Cancer Therapy–Breast Cancer (FACT-B), 6-min walk, and arm volume were performed at 3-month intervals through 18 months. Repeated measures analysis of covariance was used to model the total meters walked over time, FACT-B scores, and arm volume. Models were adjusted for baseline measurement, baseline affected arm volume, number of nodes removed, age, self-reported symptoms, baseline SF-12 mental and physical component scores, visit, and treatment group.
Results
Of the recruited 104 women, 82 completed all 18 months. Mean age (range) was 53.6 (32–82) years; 88% were Caucasian; 45% were employed full time; 44% were overweight; and 28% obese. Approximately, 46% had breast-conserving surgery; 79% had axillary node dissection; 59% received chemotherapy; and 64% received radiation. The intervention resulted in an average increase of 34.3 ml (SD=12.8) versus patient education (p=0.01). Changes in FACT-B scores and arm volumes were not significantly different.
Conclusions
With this early exercise intervention after breast cancer diagnosis, a significant improvement was achieved in physical function, with no decline in health-related quality of life or detrimental effect on arm volume.
Implications for cancer survivors
Starting a supervised exercise regimen that is tailored to an individual’s strength and stamina within 3 months following breast cancer surgery appears safe and may hasten improvements in physical functioning.
Keywords: Breast cancer, Survivorship, Exercise, Physical activity
Introduction
Improvements in early detection and treatment of breast cancer have led to a considerable number of survivors [1]. Long-term maintenance of overall health, function, and health-related quality of life (HRQL) of these women, therefore, is a major concern, since treatment of breast cancer can result in significant and long-term physical and psychological distress [2, 3]. An important goal in survivorship care is to preempt declines in function that occur during treatment. Physical activity and exercise are broad-spectrum interventions that enhance role functioning, physical well-being, and emotional health through improved physical fitness and vitality [4–8]. Although it is known that regular exercise and physical activity can improve health-related quality of life outcomes [3, 9–13], relatively few studies have examined both potential benefits and risks from initiation of exercise regimens shortly after surgery and chemotherapy. There is a longstanding, counterveilling, concern that exercise may have an adverse effect on lymphedema risk which may develop as a progressive and generally irreversible complication of treatment. Recent scientific evidence has indicated that weight training exercises do not appear to increase lymphedema risk under structured conditions [14] and that initiation of a graduated strength training program may improve signs or symptoms associated with preexisting breast cancer-related lymphedema [15]. However, the evidence on the benefits and risks of early exercise following breast cancer surgery derived from randomized-controlled studies spans only a few studies and experimental conditions [14]. We tested the potential benefits and safety of a general exercise and strength training regimen that were designed to minimize the development of lymphedema or arm swelling on HRQL, physical function, and arm volume (the RESTORE study).
Methods
Study design and recruitment
RESTORE was a randomized, controlled, single-blind study of 104 adult women with newly diagnosed stage I–III female breast cancer. Participants were recruited by study staff during their first postoperative visit or by their oncologist during their medical oncology visit, given information about the study, and scheduled for a screening visit to verify eligibility. Eligibility criteria included: (1) having a diagnosis of TNM stage I–III breast cancer with axillary or sentinel lymph node dissection, (2) no previous history of breast cancer, (3) ≥18 years of age, (4) living within 30 mi of the study site to ensure reasonably convenient access to weekly sessions, and (5) able to participate in a moderate exercise program. Those who were excluded were women for whom the safety of physical exercise would be uncertain or contra-indicated: including those who were homebound, dependent upon a walker or wheelchair for mobility, diagnosed or suspected dementia, peripheral artery disease, or unstable angina; had clinically documented cardiac conduction disturbances; or any chronic disease which significantly reduces survival during the study period. In addition, women were excluded if they had been diagnosed with lymphedema by their physician either prior to testing or after reassessment by the physician when noted to have a 200-ml difference in cylindrical water displacement between arms or preoperative versus postoperative during baseline testing. There were no exclusions based upon surgery or level of lymph node involvement.
RESTORE was approved and monitored by the Wake Forest University School of Medicine Institutional Review Board and an informed consent was obtained at the first study visit. Randomization was performed after the obtaining consent and all baseline testing by electronically accessing a randomization database from the study coordinator’s computer. Eligible participants were randomized to either a comprehensive program consisting of tailored exercise, lymphedema prevention, patient and diet education, and counseling or to usual care (patient education) approximately 4–12 weeks post-surgery. Randomization status was known only to the interventionist. Primary outcomes of physical function and health-related quality of life were assessed at 3-month intervals at clinical site visits, with the final visit occurring 18 months post-surgery. For participant convenience, the 15 month assessment were conducted by telephone interview. A secondary outcome assessment in RESTORE was arm volume, which was assessed for each arm by water displacement at each 3-month visit. The RESTORE trial was completed in 2007.
Intervention
Participants randomized to the moderate, tailored exercise intervention began the RESTORE program with a Lymphedema Prevention Module (LPM) delivered by a trained, lymphedema-certified occupational or physical therapist. The LPM included instruction and care for the affected arm and hand to prevent lymphedema, awareness of signs and symptoms of lymphedema, and a lymphedema prevention video-taped tutorial of arm strengthening and lymph flow exercises adapted from American Cancer Society recommendations [16]. Each participant was asked to complete a survey to assess knowledge about lymphedema which was reviewed by the trained therapist. Following the initial LPM, the participants were scheduled to a 1-month follow-up to assess range of motion, strength, and weight resistance. Each participant was given an elastic compression sleeve with instructions to wear it preventively during exercise, heavy arm use, and air travel. Instructions on exercises to promote lymph flow consist of daily breathing techniques and gentle movement of neck and head techniques to improve lymph flow through gentle stroking of the neck, shoulder, arm, and hand. Repeat visits at 3 month (start of center-based exercise intervention) and 9 months (end of center-based exercise intervention) were made to reinforce the lymphedema prevention skills and knowledge. The lymphedema education specialist contacted the participants by telephone 4–6 weeks after surgery to assess the use of the compression sleeve and reassessed participants at 3 months post-surgery.
Following initiation of the lymphedema prevention module, a center-based tailored exercise component was begun. Each participant was assigned two exercise sessions per week at the Wake Forest University Health and Exercise Science Clinical Research Center (CRC). The sessions were customized to meet baseline levels of strength and function. Each session included a 5-min aerobic warm-up, 30 min of moderate to somewhat hard walking on the rating of perceived exertion (RPE) scale [17], 20 min of upper and lower body strength training using both hand weights and Nautilus plate-adjusted resistance machines, and 10 min of stretching. Participants were instructed to begin walking at a low level and to progress gradually by increasing their intensity. Staff members walked with participants during the walking phase to determine whether exercise prescriptions were appropriate, for feedback on participant’s comfort, and to answer questions regarding the exercise prescription. The participants recorded total walking time, RPE, and number of laps walked on exercise logs, and they were instructed to maintain exercise logs for both center-based and home-based exercise.
Upon completion of baseline strength assessments, an initial weight (50% of established one repetition max) was assigned to each participant. Resistance exercises were started with 50% of established one repetition max for the first 1–2 weeks and weights were increased weekly by approximately 1–2.5 lbs on upper body exercises and 1–5 lbs on lower body exercises. The weight training equipment in the center was specifically designed for gradual progression and the plate-loaded machines included 1 lb increments for all upper body and core muscle groups and 1 lb (leg extension and leg curl) or 5 lb (leg press and calf press) increments for upper and lower leg exercises. Free weights (dumbbells) also provided increments of 1 lb for weights up to 10 and 2.5 lbs for weights above 10 lbs. The muscle groups targeted included muscles in the upper body (chest, upper back, shoulders, and arms) and lower body (upper leg and lower leg) and the core (lower back and abdominals). Once the participants were able to complete 12 repetitions of a specific weight for two consecutive exercise sessions, they were instructed to progress to the next appropriate weight.
Throughout the program, each participant was instructed to exercise at an individually prescribed pace, report any symptoms or problems they might encounter during exercise, and to rest as needed. An individual certified by the American College of Sports Medicine as an exercise specialist and by the American Heart Association for Advanced Cardiac Life Support led the exercise sessions. For the majority of participants, 20–30 min of exercise at a level of 14 to 16 on the RPE scale was well tolerated at the beginning of the exercise program. In order to increase the volume of physical activity, participants who were able to initially walk 30 consecutive minutes were encouraged to increase speed/distance as they progressed in the program, while keeping the duration at 30 min. A small number of participants were instructed to walk in 10-min increments, as 30 continuous minutes was too strenuous. Reasons for lower initial endurance and exercise duration included age and side effects of active treatment (radiation, chemotherapy and reconstructive surgery). As tolerated, these participants were instructed to lengthen the duration of exercise increments until one 30-min continuous walking session was achieved. All participants were able to achieve 30 min of continuous exercise within the first month of participation.
For the first 3 months of the intervention (intensive phase), participants were asked to attend two exercise sessions per week at the CRC. During months 4 through 6, the participants were given the option to transition to home-based exercise, with exercise sessions tapered to once per week at the CRC. Participants were educated on the ASCM-recommended physical activity guidelines (30 min of moderate intensity physical activity on most days of the week), and throughout the intervention, they were encouraged to either exercise at home or at a community center in addition to center-based exercise to meet these guidelines. During months 7 through 12, the participants were not required to attend supervised exercise sessions; however, if they chose to do so, they could continue exercising twice a week at the CRC. If the participant chose to exercise at home, the exercise specialist contacted participants via telephone on a monthly basis during this time to discuss adherence/barrier issues, answer exercise-related questions, and to modify exercise prescriptions as needed.
Usual care
Participants randomized to usual care were given written information about lymphedema awareness and ACS prevention exercises and received a newsletter every quarter that included general tips about nutrition and physical activity. At the end of the study, the usual care group received feedback regarding their functional status and recommendations from a fitness specialist for improving their physical function and strength.
Measures
Clinical data regarding cancer status and treatment were collected by chart review at baseline and updated at 12 months. Demographic information was collected at baseline. Assessed at baseline and 3, 6, 9, 12, and 18 months post-surgery were arm volume by water displacement against a demographic skin mark and the average of two measurements (volume of arm inserted–arm removed). The reliability of water displacement using trained operators and skin marks has been reported found to be very high (an intraclass correlation ≥0.95) [17, 18]. The primary outcome of function was assessed with the 6-min walk and HRQL with the Functional Assessment of Cancer Therapy–Breast Cancer (FACT-B) [19]. The 6-min walk test (6MWT) is a timed-performance test that instructs participants to walk as far as possible in 6 min on an established flat, indoor course. The participants are not provided feedback during the test. The FACT-B is a 44-item measure consisting of subscales assessing physical, social, and emotional well-being, functioning, relationship with doctor, and items specific to quality of life in breast cancer. Both the 6MWT and FACT-B have been widely used as measures of recovery from surgery and validated as sensitive and reliable outcome measures in cancer patients [19, 20]. The FACT-B was also assessed via phone call at the 15-month time-point. The Community Health Activities Model Program for Seniors was used to assess each participant’s physical activity level. Frequency per week in moderate activities was calculated for all exercise-related activities. Secondary self-report assessments included the physical and mental health component scores of the MOS SF-12 general health measure, Ryckman’s physical self-efficacy scale [21], and a symptoms checklist.
Adherence
Participants in the tailored exercise intervention arm received theory-based behavioral reinforcement consisting of individual sessions with a behavioral interventionist during the intensive phase and an ongoing monthly group troubleshooting session with other participants once the 3-month intensive phase was completed. The purpose of the group session was to discuss barriers and obstacles in implementing an exercise program and overcoming setbacks and relapses once an exercise program had been implemented. Participants were taught to monitor their progress using pedometers worn during the intervention period to record steps on a daily basis. They also recorded distance and time for walking and weight and number of repetitions for strength training. The participants in both groups received incentives for participation, such as t-shirts, notepads, and water bottles with the RESTORE logo. Adherence with the study testing visits was promoted by coordinating the scheduled assessment visit with the participants’ oncologist visits. In addition, transportation was provided for participants who were otherwise unable to attend their scheduled visit. The participants with missed testing visits were contacted to determine the nature of the absence and to reschedule within a 2-week assessment window.
Statistical methods
The goal of the statistical analysis was to estimate and test for differences in FACT-B scores, distance in the 6-min walk, and arm swelling between the intervention groups adjusting for patient characteristics and physical activity measures. Distributions over time were examined in graphical and univariate analyses. Repeated measures analysis of covariance was used to model outcomes over time [22]. Models were adjusted for baseline measurement, baseline affected arm volume, number of nodes removed during surgery, age at diagnosis, number of self-reported symptoms, baseline SF-12 mental and physical components scores, visit timing (linear and quadratic effects), and treatment group. Time was centered to avoid multicollinearity. Interactions of treatment group and time were not significant (all p>0.10); therefore, main effects of group and time were modeled on the post-baseline data. The modeling used a Toeplitz covariance structure to account for the correlation due to repeated measurements. Covariance patterns were allowed to differ by intervention group which provided better model fit. A two-sided p value<0.05 was considered statistically significant. All analyses were performed using SAS v9.1.3 (SAS Institute, Cary, NC).
Results
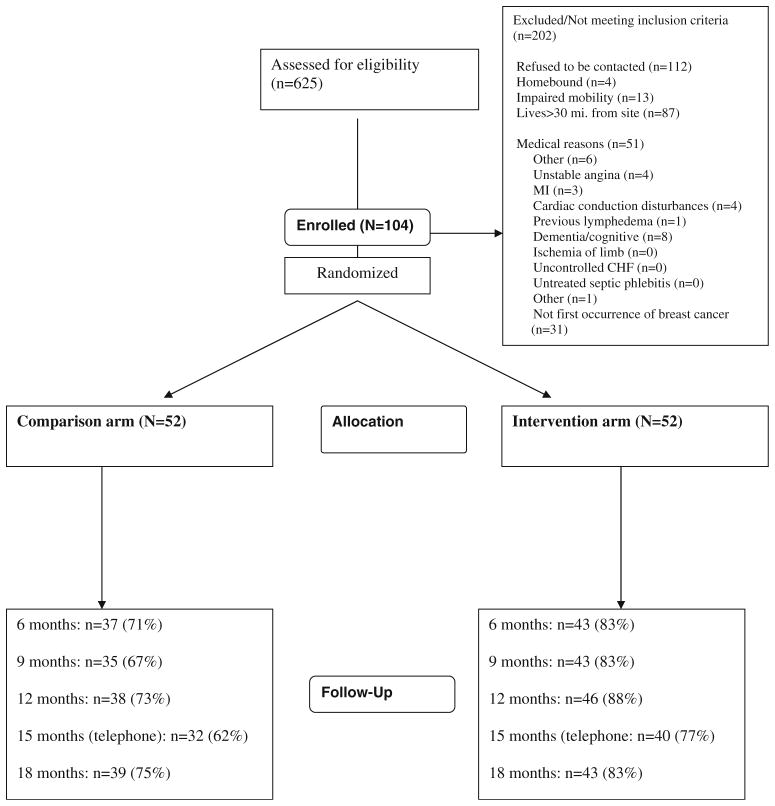
As shown in Fig. 1, 423 women were identified as eligible; 104 (25%) agreed to participate and were randomized to the two study arms. During the study, 82 (79%) of the 104 randomized participants completed the RESTORE 18-month assessment visit. Those not completing the 18-month visit were more proportionately African-American (26% versus 7%; p=0.023) and obese (48% versus 22%; p=0.032); however, there were no differences in outcome measures between completers versus noncompleters (p>0.10). The primary reason stated for not completing the study was ascertained for 21 of 22 noncompleters and included: feeling overwhelmed or a lack of time to participate (38%), lost to follow-up (19%), lack of interest (10%), family issues (10%), death (n=2, 10%), and other reasons (10%). Participants’ characteristics are shown in Table 1. The age ranged from 32 to 82 years, with mean of 53.6 years, and participants were primarily Caucasian (88%). Approximately half (45%) of participants were employed full time. Body mass index (BMI) was ≥25 kg/m2 in 71% of participants, among whom 28% were classified as obese (BMI >30 kg/m2). The types of therapies for treating participants’ breast cancer are described in Table 2 and are balanced between study arms. Approximately half (46%) of participants had breast-conserving surgery; most (79%) had axillary node dissection, 59% received chemotherapy, and 64% received radiation. A total of 39 adverse events were reported to the Data Safety and Monitoring Board and seven were classified as serious. Only two events were deemed by the medical monitor to be study related (pectoral muscle pain and stress fracture in foot).
Fig. 1.
Study flowchart
Table 1.
Participant characteristics at baseline (N=104)
| Characteristic | Control, N=52 | Intervention, N=52 | pa |
|---|---|---|---|
| Age group | 0.66 | ||
| <50 | 23 (44) | 21 (40) | |
| 50 to <65 | 19 (37) | 23 (44) | |
| 65 to <75 | 7 (13) | 4 (8) | |
| >75 | 3 (6) | 4 (8) | |
| Race/ethnicity | 0.76 | ||
| White | 47 (90) | 45 (87) | |
| African-American | 5 (10) | 7 (13) | |
| Education | |||
| High school graduate or less | 9 (17) | 9 (17) | 0.12 |
| Some college | 19 (37) | 10 (19) | |
| College graduate (4 years)+ | 22 (42) | 31 (60) | |
| N/A | 2 (4) | 2 (4) | |
| Annual family income (US $) | 0.90 | ||
| <20,000/year | 5 (10) | 5 (10) | |
| 20 to <50,000/year | 17 (33) | 14 (27) | |
| 50–100,000/year | 18 (35) | 20 (38) | |
| >100,000/year | 9 (17) | 11 (21) | |
| N/A | 3 (6) | 2 (4) | |
| BMI | 0.91 | ||
| Underweight/normal (<25 kg/m2) | 14 (27) | 16 (31) | |
| Overweight (25–29.9 kg/m2) | 23 (44) | 22 (42) | |
| Obese (>30 kg/m2) | 15 (29) | 14 (27) | |
| Physical self-efficacy (PSE) | 82.4±12.0 [61, 117] | 83.0±13.1 [40, 110] | 0.22 |
| Physical activity, function, and HRQL | |||
| CHAMPS: weekly moderate intensity exercise-related activities | 5.0±4.9, [0, 18] | 3.5±3.5 [0, 15] | 0.17 |
| Total meters walked in 6 min | 538.0±97.2 | 539.2±103.9 | 0.76 |
| SF-12 | |||
| Physical | 45.6±7.6 [28.5, 60.5] | 45.9±8.3 [29.2, 62.3] | 0.87 |
| Mental | 41.9±5.7 [32.8, 57.5] | 41.0±6.2 [27.7, 57.8] | 0.54 |
| FACT-B total score | 103.7±22.1, [36, 143] | 102.6±16.9 [72, 135] | 0.62 |
FACT-B Functional Assessment of Cancer Therapy–Breast Cancer, CHAMPS Community Health Activities Model Program for Seniors, HRQL health-related quality of life, BMI body mass index
p value from t test or Wilcoxon rank-sum for continuous variables or chi-square or Fisher’s exact test for categorical variables
Table 2.
Disease characteristics and cancer treatment (N=104)a
| P-value | |||
|---|---|---|---|
| Stage of breast cancer | 0.52 | ||
| I | 26 (50) | 25 (48) | |
| II | 21 (40) | 19 (37) | |
| III | 4 (8) | 8 (15) | |
| N/A | 1 (2) | 0 | |
| Type of surgery | 0.69 | ||
| Lumpectomy only | 25 (48) | 23 (44) | |
| Mastectomy | 24 (46) | 28 (54) | |
| N/A | 3 (6) | 1 (2) | |
| Type of node dissection | 0.99 | ||
| Sentinel (SND) only | 9 (17) | 10 (19) | |
| Axillary (AND) | 40 (77) | 39 (75) | |
| Neither | 0 | 1 (2) | |
| N/A | 3 (6) | 2 (4) | |
| Positive nodes (#) | 0.50 | ||
| 0 | 30 (58) | 35 (67) | |
| 1–3 | 15 (29) | 10 (19) | |
| 4–9 | 6 (12) | 7 (13) | |
| 10+ | 0 | 0 | |
| N/A | 1 (2) | 0 | |
| Chemotherapy (yes) | 31 (60) | 31 (60) | 0.90 |
| Tamoxifen (yes) | 23 (44) | 26 (50) | 0.50 |
| Radiation therapy (yes) | 36 (69) | 31 (60) | 0.24 |
| Arm volume (mean) | 1,754.3±495.6 | 1,699.5±396.8 | 0.60 |
AND axillary lymph node dissection, SND sentinel lymph node dissection
p value from t test or Wilcoxon rank-sum for continuous variables or chi-square or Fisher’s exact test for categorical variables
Six-min walk and FACT-B
Table 3 includes the results of change in 6-min walk and FACT-B scores, adjusted for model covariates of baseline score, age, and clinical status. At baseline, the level of participation in physical activity (measured by pedometer steps) was positively correlated (p <0.05) with distance on the 6-min walk, r=0.54, and FACT-B total score, r=0.26 (data not shown). Participants in the exercise intervention group had significantly higher total distance (meters) walked for the 6-min walk at 18 months compared to usual care. The adjusted mean distances walked by group were 593.2 m (SE=13.0) and 558.9 m (11.8), respectively (p=0.0098). Mean FACT-B total scores were not statistically different by treatment group at 18 months, 115.8 (SD=1.6) for the treatment group and 114.4 (SD=2.5) for the control group (p=0.57). No significant effect modifiers or interactions with age or BMI were found. There were also no significant differences in the means of FACT-B subscales, adjusted for all model covariates.
Table 3.
Longitudinal regression modeling results for total meters walked and FACT-B total score (N=104)
| Six-min walk | FACT-B | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Characteristic | Beta (SE) | 95% CI | p value | Beta (SE) | 95% CI | p value |
| Intercept | 165.6 (114.3) | (−63.23, 394.45) | – | 49.8 (23.6) | (2.67, 96.95) | – |
| Time (linear)a | 0.76 (0.63) | (−0.50, 2.02) | 0.231 | 0.54 (0.14) | (0.26, 0.83) | <0.001 |
| Time (quadratic)a | −0.61 (0.16) | (−0.93, −0.29) | <0.001 | −0.15 (0.03) | (−0.21, −0.08) | <0.001 |
| Baseline outcome | 0.71 (0.09) | (0.53, 0.90) | <0.001 | 0.58 (0.10) | (0.37, 0.78) | <0.001 |
| Baseline arm volume differenceb | −0.02 (0.02) | (−0.07, 0.02) | 0.304 | −0.01 (0.005) | (−0.02, −0.00) | 0.024 |
| Age group at baseline | 3 df, p=0.201 | 3 df, p=0.896 | ||||
| <50 | 76.6 (35.2) | (6.05, 147.22) | 0.034 | 0.68 (4.80) | (−9.10, 10.45) | 0.889 |
| 50 to <65 | 66.6 (34.0) | (−1.62, 134.81) | 0.056 | 1.56 (4.68) | (−7.98, 11.11) | 0.741 |
| 65 to <75 | 61.7 (35.3) | (−9.04, 132.49) | 0.086 | −1.21 (5.42) | (−12.18, 9.76) | 0.825 |
| >75, RC | – | – | ||||
| Body mass index | 2 df, p=0.678 | 2 df, p=0.735 | ||||
| Low-normal (<25 kg/m2), RC | – | – | – | – | ||
| Overweight (25–29.9 kg/m2) | −13.3 (15.2) | (−43.78, 17.10) | 0.384 | 0.92 (2.80) | (−4.69, 6.52) | 0.745 |
| Obese (>30 kg/m2) | −16.9 (27.5) | (−71.94, 38.14) | 0.541 | −1.78 (4.71) | (−11.22, 7.67) | 0.708 |
| # nodes removed | −0.64 (0.97) | (−2.58, 1.29) | 0.509 | −0.41 (0.16) | (−0.73, −0.09) | 0.012 |
| # of self-reported symptoms | −0.39 (1.18) | (−2.75, 1.97) | 0.744 | 0.22 (0.32) | (−0.42, 0.87) | 0.492 |
| Baseline SF-12 score | ||||||
| Mental | 0.35 (1.40) | (−2.44, 3.14) | 0.803 | 0.23 (0.24) | (−0.26, 0.72) | 0.345 |
| Physical | 0.07 (1.24) | (−2.42, 2.55) | 0.958 | 0.38 (0.22) | (−0.07, 0.82) | 0.097 |
| Intervention group (intervention versus control) | 34.3 (12.8) | (8.61, 60.08) | 0.010 | 1.38 (2.44) | (−3.50, 6.26) | 0.573 |
RC reference category, FACT-B Functional Assessment of Cancer Therapy–Breast Cancer
Time variable was centered at 12 months
Volumes are the average displacement of two trials (volume of arm inserted–arm removed)
Arm volume
In Table 4, the effects of the intervention on swelling of the involved arm, as an indicator of lymphedema, are shown, based on mean change at 18 months compared to baseline. Adjusted mean change in arm volume measured by water displacement in the intervention group was 33.5 ml versus 60.4 in the control group (p=0.54).
Table 4.
Change in involved arm volume (in milliliters) over 18 months
| Group | Unadjusted mean volume change (18 months-BL) mean±SD | Adjusted mean volume change from ANCOVA mean (SE) | ANCOVA p value for group |
|---|---|---|---|
| Control | +57.4±204.4 | 60.4 (32.5) | 0.535 |
| Intervention | +27.3±176.9 | 33.5 (29.0) |
ANCOVA analysis of covariance, SD standard deviation, SE standard error
Adherence
Overall, adherence in the RESTORE trial was very good with participants completing 71.2% of all prescribed exercise sessions with a range of 0–97%. With the majority (61%) of participants attending more than 75% of prescribed sessions and only 13% of participants attending less than 50% of sessions, adherence goals were exceeded and supported the feasibility of an exercise program during treatment for breast cancer. As further evidence of treatment fidelity, mean physical self-efficacy, assessed by asking participants to rate their confidence in general physical abilities including strength, speed, and muscle tone [23] was significantly higher in the intervention group than controls at follow-up (p=0.03) and displays a cumulative increase during the intervention.
Discussion
RESTORE was a randomized, controlled trial testing the effectiveness of a combination of tailored center-based exercise and lymphedema prevention goals started within 4–12 weeks of surgery, versus usual care, to improve physical, social, and psychological well-being in women with early stage breast cancer. The RESTORE intervention was designed to increase physical function and fitness while controlling potential risk for lymphedema secondary to breast cancer treatment and surgery using a graduated and personalized exercise approach.
Over the 18-month study assessment period, women receiving the intervention had increased physical function and showed improvement relative to controls. The magnitude of the treatment effect on gain in physical function of approximately 34 m between groups is modest or about 6% relative to controls. Oftentimes, a 10% improvement in functional tests is considered clinically relevant; however, the degree of improvement depends upon health and capacity for improvement. There was no significant effect of treatment on HRQL or on arm volume. To our knowledge, this is the first randomized trial to test the benefits of a combined exercise and strength training regimen with a lymphedema prevention component upon function and HRQL. The exercise program for RESTORE was developed to ensure participant safety by involving a slow progression from the baseline ability level of each individual to the level necessary for safe return to normal daily activity. Each exercise prescription was individualized to allow a participant to progress at her own pace, depending on her level of function. Walking was chosen as the method of cardiovascular training because of its safety for a broad range of function levels. Strength and range of motion was a major focus in RESTORE as it may become a common patient fear limiting resumption of daily roles. All upper body strength or range of motion activity was slow and controlled and did not require maximal effort.
RESTORE builds upon the related survivorship literature in three important ways. First, it reports clear evidence of the benefit and safety of a moderate intensity exercise program that is feasible to deliver in standard rehabilitation centers. The RESTORE results showing no apparent study-related lymphedema or arm swelling is consistent with a recent report by Schmitz et al. [24] of a trial of weight lifting in breast cancer. In the latter study, participants were enrolled at a mean of 36–46 months since diagnosis, leaving some question whether the timing of exercise resumption must wait several months for lymphedema risk to manifest itself as an exclusion for participation in upper body weight training. Importantly, RESTORE recruited patients directly from the surgeons’ office, and thus, participants began the study at approximately 9 months from diagnosis. Second, RESTORE collected evidence often lacking in the literature on breast cancer and exercise on the internal validity of exercise interventions on a social cognitive level. Third, RESTORE results indicate that although upper body training and walking exercises are safe and beneficial to fitness, the incremental gains in the intervention group did not translate to improvements in a broad measure of health-related quality of life. In secondary exploratory analyses of related psychosocial measures (life satisfaction and positive affect), no intervention effects were likewise found.
Given that the RESTORE intervention was successfully received by participants and resulted in gains in physical function and self-efficacy for physical activity and exercise, lack of translation of improved function to an improvement in HRQL was unexpected. The study was designed with power to detect a clinically significant change of five points in FACT-B scores [25], and based on other reports [26–32], this was a reasonable goal. Possible explanations for the lack of a significant improvement in HRQL might include characteristics of the study population or the main focus of the intervention on resistance training and walking but not coping skills or social support. It is possible that women being treated for breast cancer who were motivated to enroll in RESTORE to promote HRQL also had lifestyles or social support that assisted in hastening the recovery process such as resumption of roles and coping with transitions. In addition, most participants had early stage disease, were white, not obese, and had at least a college education. Sixty percent were receiving adjuvant chemotherapy. Alternatively, perhaps key components of the HRQL measure, such as breast-related symptoms which preferentially improve with exercise [27], were not perceived as a significant problem in these women. It is possible that either a longer period of follow-up or intervention duration is required to observe hypothesized greater declines in HRQL through loss of function in the usual care group versus maintenance or improved function in the intervention group. Alternatively, the FACT-B might not have been sensitive enough to detect change in HRQL in a sample already with a moderate to high level of HRQL at baseline [33]. However, a more general HRQL tool, the SF-12, also did not detect significantly improved well-being. Finally, a secondary post hoc analyses of adherence (assessed as weekly visit attendance) found that the latter did not moderate intervention effects on the study primary outcomes. That is, the differences for intervention versus control did not significantly depend upon level of adherence, adjusting for the other predictors in the modeling. Future studies should include multiple HRQL measures and longer follow-up to provide a clearer picture of the HRQL shifts and their relationship to exercise interventions.
There are several limitations to consider in evaluating the main findings from RESTORE. First, we used a combination of upper body strength training, lymphedema prevention involving arm exercises and prophylactic use of compression sleeves, and walking for increased physical activity. Whether walking alone could have produced the benefit to fitness cannot be determined. Next, the evidence base for the use of a compression sleeve and stretching exercises on lymphedema risk is limited and not established. Also of importance, those in the intervention group had far greater contact with health care and exercise professionals. The increased interaction and socialization with others may have affected the positive outcomes observed in the intervention group. Finally, RESTORE was not designed to test whether our lymphedema prevention education module was effective as a standalone intervention component. Other reports find no adverse effect of exercise on lymphedema incidence or severity in breast cancer survivors without the use of stretching exercises and sleeves [34, 35]. In the RESTORE trial, however, the adjusted increase in arm volume was numerically greater in the control group versus the intervention group (60.4 versus 33.5 ml), and standard error was large, which may have limited our ability to detect a statistically significant difference. Since exercise-induced lymphedema is a concern [36], further exploration is warranted and should be studied in patients who undergo full axillary dissection and/or axillary radiation, as they are at higher risk for lymphedema [35, 37]. In RESTORE, approximately three quarters of patients had sentinel node sampling as the only axillary procedure, for which the associated lymphedema risk is only 5–7% [38].
In summary, the RESTORE trial demonstrated that a multicomponent protocol of tailored exercise and lymphedema prevention instituted within 4–12 weeks of surgery can improve physical function without increasing risk of lymphedema. This is important because exercise has the potential to alleviate fatigue, decrease depression and anxiety, decrease weight gain and cardiovascular risk, and improve well-being [39–41] and major concerns for the growing population of breast cancer survivors [42–46]. Maintaining physical activity and function are now recognized as important positive prognostic factors in breast cancer survivors, independent of diet and body mass index [12, 13]. Since a minority of breast cancer patients attest to healthy eating and exercise behavior [47, 48], it is of paramount importance that we find palatable and flexible healthy diet and exercise options for the great breadth of our patient’s preferences.
Acknowledgments
We would like to acknowledge Electra Paskett, PhD and Nancy Stark for their advice and support regarding the lymphedema education and assessment component of the study, and Cindy Jennings PT of the Martinat Rehabilitation Center, Novant Inc. Winston-Salem, NC and Anne Fleischer, OTR of the Wake Forest Medical Center, Winston-Salem, NC for her assistance with the lymphedema education and prevention instruction. This study was supported by a grant funding from US Army-DAMD17-01-1-0447—Department of Defense.
Footnotes
Previous presentations Information from this study was previously presented at the San Antonio Breast Cancer Symposium in 2007, Cancer Survivorship, Embracing the Future, October, 2006, and the Society of Behavioral Medicine, 2008.
Contributor Information
Roger T. Anderson, Email: rtanders@psu.edu, Department of Public Health Sciences, College of Medicine, Penn State Milton S. Hershey Medical Center, 600 Centerview Drive, Suite 2200, P.O. Box 855, Hershey, PA 17033-0855, USA
Gretchen G. Kimmick, Department of Medicine, Duke University, Durham, NC, USA
Thomas P. McCoy, Department of Biostatistical Sciences, WFUSOM, Winston-Salem, NC, USA
Judith Hopkins, Forsyth Regional Cancer Center, Winston-Salem, NC, USA.
Edward Levine, Department of Surgery, WFUSOM, Winston-Salem, NC, USA.
Gary Miller, Department of Health & Exercise Science, Wake Forest University (WFU), Winston-Salem, NC, USA.
Paul Ribisl, Department of Health & Exercise Science, Wake Forest University (WFU), Winston-Salem, NC, USA.
Shannon L. Mihalko, Department of Health & Exercise Science, Wake Forest University (WFU), Winston-Salem, NC, USA
References
- 1.Burstein HJ, Winer EP. Primary care for survivors of breast cancer. N Engl J Med. 2000;343:1086–94. doi: 10.1056/NEJM200010123431506. [DOI] [PubMed] [Google Scholar]
- 2.Kim CJ, Kang DH, Smith BA, et al. Cardiopulmonary responses and adherence to exercise in women newly diagnosed with breast cancer undergoing adjuvant therapy. Cancer Nurs. 2006;29:156–65. doi: 10.1097/00002820-200603000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Hewitt ME, Greenfield S, Stovall E, et al. In: From cancer patient to cancer survivor—lost in transition: an American Society of Clinical Oncology and Institute of Medicine Symposium. Hewitt ME, Ganz PA, editors. Washington: The National Academies Press; 2006. [Google Scholar]
- 4.Petruzzello SJ, Landers DM, Hatfield BD, et al. A meta-analysis on the anxiety-reducing effects of acute and chronic exercise. Outcomes and mechanisms. Sports Med. 1991;11:143–82. doi: 10.2165/00007256-199111030-00002. [DOI] [PubMed] [Google Scholar]
- 5.North TC, McCullagh P, Tran ZV. Effect of exercise on depression. Exerc Sport Sci Rev. 1990;18:379–415. [PubMed] [Google Scholar]
- 6.Byrne A, Byrne DG. The effect of exercise on depression, anxiety and other mood states: a review. J Psychosom Res. 1993;37:565–74. doi: 10.1016/0022-3999(93)90050-p. [DOI] [PubMed] [Google Scholar]
- 7.Folkins CH, Sime WE. Physical fitness training and mental health. Am Psychol. 1981;36:373–89. doi: 10.1037//0003-066x.36.4.373. [DOI] [PubMed] [Google Scholar]
- 8.Sonstroem RJ. Exercise and self-esteem. Exerc Sport Sci Rev. 1984;12:123–55. [PubMed] [Google Scholar]
- 9.Pinto BM, Trunzo JJ. Health behaviors during and after a cancer diagnosis. Cancer. 2005;104:2614–23. doi: 10.1002/cncr.21248. [DOI] [PubMed] [Google Scholar]
- 10.Demark-Wahnefried W, Pinto BM, Gritz ER. Promoting health and physical function among cancer survivors: potential for prevention and questions that remain. J Clin Oncol. 2006;24:5125–31. doi: 10.1200/JCO.2006.06.6175. [DOI] [PubMed] [Google Scholar]
- 11.Demark-Wahnefried W. Cancer survival: time to get moving? Data accumulate suggesting a link between physical activity and cancer survival. J Clin Oncol. 2006;24:3517–8. doi: 10.1200/JCO.2006.06.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holmes MD, Chen WY, Feskanich D, et al. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 13.Holick CN, Newcomb PA, Trentham-Dietz A, et al. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:379–86. doi: 10.1158/1055-9965.EPI-07-0771. [DOI] [PubMed] [Google Scholar]
- 14.Cavanaugh KM. Effects of early exercise on the development lymphedeam in patients with breast cancer treated with axillary lymph node dissection. J Oncol Pract. 2011;7(2):89–93. doi: 10.1200/JOP.2010.000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmitz KH, Holtzman J, Courneya KS, et al. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2005;14:1588–95. doi: 10.1158/1055-9965.EPI-04-0703. [DOI] [PubMed] [Google Scholar]
- 16.Rockson SG, Miller LT, Senie R, et al. American Cancer Society Lymphedema Workshop. Workgroup III: Diagnosis and management of lymphedema. Cancer. 1998;83:2882–5. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2882::aid-cncr45>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Brady MJCDF, Mo F, Bonomi AE, et al. Reliability and validity of the functional assessment of cancer therapy-breast quality-of-life instrument. J Clin Oncol. 1997;15:974. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 18.Megens AM, Harris SR, Kim-Sing C, McKenzie DC. Measurement of upper extremity volume in women after axillary dissection for breast cancer. Arch Phys Med Rehabil. 2001;82:1639–44. doi: 10.1053/apmr.2001.26822. [DOI] [PubMed] [Google Scholar]
- 19.Sander AP, Hajer NM, Hemenway K, Miller AC. Upper-extremity volume measurements in women with lymphedema: a comparison of measurements obtained via water displacement with geometrically determined volume. Phys Ther. 2002;82:1201–12. [PubMed] [Google Scholar]
- 20.Moriello C, Mayo NE, Feldman L, et al. Validating the six-minute walk test as a measure of recovery after elective colon resection surgery. Arch Phys Med Rehabil. 2008;89:1083–9. doi: 10.1016/j.apmr.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 21.Ryckman RM, Robbins MA, Thornton B, Cantrell P. Development and validation of a physical self-efficacy scale. J Personal Soc Psychol. 1982;42(5):891–900. [Google Scholar]
- 22.Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. Hoboken: Wiley; 2004. [Google Scholar]
- 23.McAuley E, Mihalko S. Measuring exercise-related self-efficacy. In: Duda JL, editor. Advances in sport and exercise psychology measurement. Morgantown: Fitness Information Technology; 1998. [Google Scholar]
- 24.Schmitz KH, Ahmed R, Troxel A, et al. Weight lifting for women at risk for breast cancer-related lymphedema: a randomized trial. JAMA. 2010;304(24):2699–705. doi: 10.1001/jama.2010.1837. [DOI] [PubMed] [Google Scholar]
- 25.Cella DF, Tulsky DS, Gray G, et al. The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–9. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 26.Rogers LQ, Hopkins-Price P, Vicari S, et al. A randomized trial to increase physical activity in breast cancer survivors. Med Sci Sports Exerc. 2009;41:935–46. doi: 10.1249/MSS.0b013e31818e0e1b. [DOI] [PubMed] [Google Scholar]
- 27.Bicego D, Brown K, Ruddick M, et al. Effects of exercise on quality of life in women living with breast cancer: a systematic review. Breast J. 2009;15:45–51. doi: 10.1111/j.1524-4741.2008.00670.x. [DOI] [PubMed] [Google Scholar]
- 28.Courneya KS, Mackey JR, Bell GJ, et al. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21:1660–8. doi: 10.1200/JCO.2003.04.093. [DOI] [PubMed] [Google Scholar]
- 29.Sandel SL, Judge JO, Landry N, et al. Dance and movement program improves quality-of-life measures in breast cancer survivors. Cancer Nurs. 2005;28:301–9. doi: 10.1097/00002820-200507000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Segal R, Evans W, Johnson D, et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. J Clin Oncol. 2001;19:657–65. doi: 10.1200/JCO.2001.19.3.657. [DOI] [PubMed] [Google Scholar]
- 31.Campbell A, Mutrie N, White F, et al. A pilot study of a supervised group exercise programme as a rehabilitation treatment for women with breast cancer receiving adjuvant treatment. Eur J Oncol Nurs. 2005;9:56–63. doi: 10.1016/j.ejon.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 32.McKenzie DC, Kalda AL. Effect of upper extremity exercise on secondary lymphedema in breast cancer patients: a pilot study. J Clin Oncol. 2003;21:463–6. doi: 10.1200/JCO.2003.04.069. [DOI] [PubMed] [Google Scholar]
- 33.Cadmus LA, Salovey P, Yu H, et al. Exercise and quality of life during and after treatment for breast cancer: results of two randomized controlled trials. Psychooncology. 2009;18:343–52. doi: 10.1002/pon.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Demark-Wahnefried W, Clipp EC, Lipkus IM, et al. Main outcomes of the FRESH START trial: a sequentially tailored, diet and exercise mailed print intervention among breast and prostate cancer survivors. J Clin Oncol. 2007;25:2709–18. doi: 10.1200/JCO.2007.10.7094. [DOI] [PubMed] [Google Scholar]
- 35.Schmitz KH, Ahmed RL, Troxel A, et al. Weight lifting in women with breast-cancer-related lymphedema. N Engl J Med. 2009;361:664–73. doi: 10.1056/NEJMoa0810118. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed RL, Thomas W, Yee D, et al. Randomized controlled trial of weight training and lymphedema in breast cancer survivors. J Clin Oncol. 2006;24:2765–72. doi: 10.1200/JCO.2005.03.6749. [DOI] [PubMed] [Google Scholar]
- 37.Fleissig A, Fallowfield LJ, Langridge CI, et al. Post-operative arm morbidity and quality of life. Results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Res Treat. 2006;95:279–93. doi: 10.1007/s10549-005-9025-7. [DOI] [PubMed] [Google Scholar]
- 38.Tsai RJ, Dennis LK, Lynch CF, et al. The risk of developing arm lymphedema among breast cancer survivors: a meta-analysis of treatment factors. Ann Surg Oncol. 2009;16:1959–72. doi: 10.1245/s10434-009-0452-2. [DOI] [PubMed] [Google Scholar]
- 39.Torres Lacomba M, Yuste Sanchez MJ, Zapico Goni A, et al. Effectiveness of early physiotherapy to prevent lymphoedema after surgery for breast cancer: randomised, single blinded, clinical trial. BMJ. 2010;340:5396. doi: 10.1136/bmj.b5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mock V, Dow KH, Meares CJ, et al. Effects of exercise on fatigue, physical functioning, and emotional distress during radiation therapy for breast cancer. Oncology Nursing Forum. 1997;24:991. [PubMed] [Google Scholar]
- 41.Alfano CM, Day JM, Katz ML, et al. Exercise and dietary change after diagnosis and cancer-related symptoms in long-term survivors of breast cancer: CALGB 79804. Psycho-Oncology. 2009;18:128–33. doi: 10.1002/pon.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brosse AL, Sheets ES, Lett HS, et al. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med. 2002;32:741–60. doi: 10.2165/00007256-200232120-00001. [DOI] [PubMed] [Google Scholar]
- 43.Bower JE, Ganz PA, Desmond KA, et al. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18:743–53. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 44.Burgess C, Cornelius V, Love S, et al. Depression and anxiety in women with early breast cancer: five year observational cohort study. BMJ. 2005;330:702. doi: 10.1136/bmj.38343.670868.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Irwin ML, McTiernan A, Baumgartner RN, et al. Changes in body fat and weight after a breast cancer diagnosis: influence of demographic, prognostic, and lifestyle factors. J Clin Oncol. 2005;23:774–82. doi: 10.1200/JCO.2005.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ganz PA, Kwan L, Stanton AL, et al. Quality of life at the end of primary treatment of breast cancer: first results from the moving beyond cancer randomized trial. J Natl Cancer Inst. 2004;96:376. doi: 10.1093/jnci/djh060. [DOI] [PubMed] [Google Scholar]
- 47.Irwin ML, Crumley D, McTiernan A, et al. Physical activity levels before and after a diagnosis of breast carcinoma—the health, eating, activity, and lifestyle (HEAL) study. Cancer. 2003;97:1746. doi: 10.1002/cncr.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pinto BM, Maruyama NC, Clark MM, et al. Motivation to modify lifestyle risk behaviors in women treated for breast cancer. Mayo Clin Proc. 2002;77:122–9. doi: 10.4065/77.2.122. [DOI] [PubMed] [Google Scholar]