Hypothalamic BDNF is a key element in the regulation of energy balance. Here we investigated gene transfer of BDNF in rodent models of obesity and diabetes. BDNF led to marked weight loss and alleviation of obesity-associated insulin resistance. To facilitate clinical translation and ensure BDNF expression dialed down as weight loss progressed, we developed a molecular autoregulatory system using a single rAAV vector harboring two expression cassettes, one constitutively driving BDNF, the other a specific microRNA targeting the therapeutic gene controlled by a promoter (agouti related peptide) responsive to the BDNF-induced physiological changes. Hence, as body weight declined, microRNA expression was activated inhibiting transgene expression and, in contrast to the progressive weight loss associated with a non-regulated approach, led to a plateau once significant weight loss was achieved. This strategy mimics the body’s endogenous physiological feedback mechanisms thereby resetting the hypothalamic set point to reverse obesity and metabolic syndrome.
Obesity confers significant risk for diabetes, cardiovascular disease, stroke and some cancers1,2. Obesity and the related condition, metabolic syndrome (or syndrome X) are increasing rapidly worldwide with significant morbidity and mortality and socioeconomic burden3. Life style modifications such as exercise and diet as well as approved drugs have limited efficacy. Bariatric surgery can lead to weight loss at the cost of significant morbidity, underscoring the need for a safer and more effective approach.
To identify potential molecular therapeutic candidates, we used an environmental paradigm. In our previous studies, physically and socially more complex housing leads to increased neurogenesis, improved learning and memory and resistance to insults4-6. Moreover, although fed ad libitum on identical diets, such enriched animals gain less weight than standard housing controls with an improved metabolic profile and insulin sensitivity. To further characterize this phenotype we focused on potential regulators in the hypothalamic arcuate nucleus (Arc), a brain region critical to energy balance. Amongst a number of genes screened, we observed a consistent upregulation in BDNF expression at 2, 4 and 9 weeks of enrichment.
BDNF has previously been identified as an important component of the hypothalamic pathway that controls body weight and energy homeostasis7. Obese phenotypes are found in BDNF heterozygous mice8, a conditional knockout model9 and focal hypothalamic deletion in adult mice10. This mature onset obesity is associated with hyperphagia, hyperleptinemia, hyperinsulinemia and hyperglycemia. Moreover in humans similar symptoms are associated with the functional loss of one copy of the BDNF gene11 and with a de novo mutation in the BDNF receptor Ntrk2 gene12. Both peripheral and central administration of BDNF decreases food intake, increases energy expenditure and leads to weight loss13,14. Moreover, acute BDNF ameliorates hyperinsulinemia and hyperglycemia in diabetic db/db mice15. To determine whether BDNF somatic cell gene transfer could replicate the lean body weight and improved metabolic profile associated with an enriched environment, we used rAAV to focally overexpress BDNF in the hypothalamus of normal weight mice on a standard diet (STD). In addition, we looked at the therapeutic potential of this approach in a high fat diet (HFD)-induced obesity (DIO) model. Furthermore, we developed a physiological autoregulatory gene expression system and assessed its efficacy in leptin receptor deficient db/db mice, a genetic model of diabetes and obesity.
Results
Hypothalamic gene transfer of BDNF in normal mice fed with standard diet
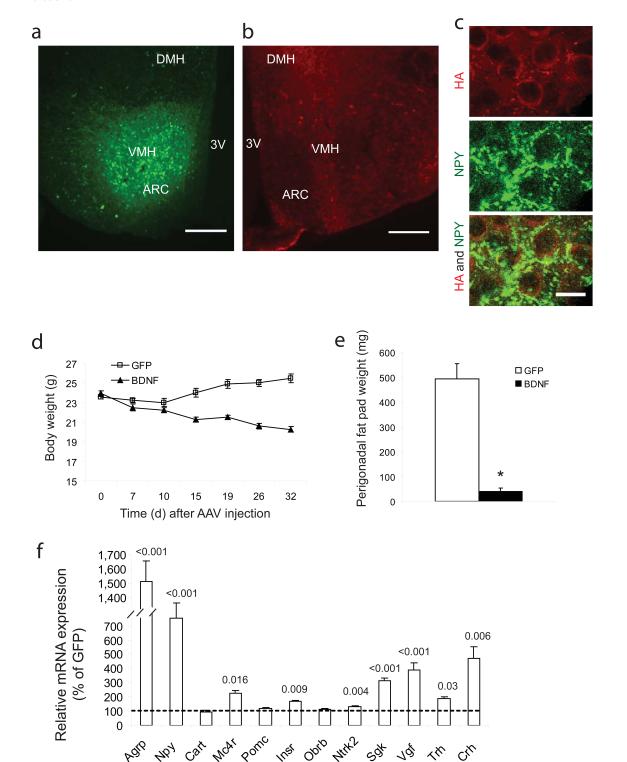
We delivered HA-tagged human BDNF to the hypothalamus bilaterally with GFP as a control using rAAV. In a subset of animals, gene transfer efficacy was determined by HA immunofluorescence and GFP fluorescence for the respective vectors with expression observed in the Arc and ventromedial hypothalamus (VMH) (Fig. 1a, b). Consistent with the use of a constitutive promoter, expression was observed in the majority of neurons in the targeted region including arcuate AgRP/NPY neurons (Fig 1c). An initial surgery-associated weight loss was observed in both groups but GFP mice quickly recovered and then regained weight on their pre-surgery trajectory. In contrast BDNF mice continued to lose weight throughout the course of the experiment (Fig. 1d). By one month post-injection, the weight of BDNF mice had decreased by 3.66 ± 0.27 g while the weight of GFP mice had increased by 1.91 ± 0.37 g. There was no significant change in food consumption (Supplementary Fig 1a). Adiposity was greatly reduced in BDNF mice as indicated by a 92% reduction in the perigonadal fat pad at 50 days(Fig. 1e). BDNF led to a sharp decrease in leptin (12.2 ± 2.6% of GFP, P < 0.001) and insulin (18.0 ± 2.3% of GFP, P < 0.001). Both leptin and insulin levels are known to be correlated to fat mass16. Moreover adiponectin, a major adipokine with a role in regulating insulin sensitivity and inhibiting appetite17, was significantly increased in BDNF mice, whereas cholesterol, triglyceride and IGF-1 were all reduced (Supplementary Table).
Figure 1.
Hypothalamic gene delivery of BDNF leads to weight loss, changes in serum biomarkers and hypothalamic gene expression in mice fed with standard diet. (a) EGFP fluorescence. Scale bar, 200 μm. (b) Immunoreactivity to HA tag. Scale bar, 200 μm. (c) Colocalization of HA (red) with NPY (green) in Arc. Scale bar, 10 μm. (d) Body weight (n = 10 GFP, n = 14 BDNF, P < 0.0001). (e) Perigonadal white adipose tissue weight (n = 8 GFP, n = 10 BDNF, * P < 0.001). (f) Gene expression in hypothalamus (n = 4 per group, P values are shown on the bars).
Real-time qPCR was used to examine hypothalamic expression of genes involved in energy homeostasis. The orexigenic peptides Agrp and Npy were upregulated 15.11 ± 1.44 fold and 7.55 ± 1.04 fold in BDNF compared to GFP mice respectively (Fig. 1f), consistent with a compensatory response to the weight loss and fat depletion18. Mc4r, proposed to be upstream of BDNF and a major pathway shared by leptin, insulin and other anorexic signals9, was upregulated significantly, while additional anorexigenic molecules Cart and Pomc were not changed (Fig. 1f). The BDNF receptor Ntrk2, was increased indicating positive feedback. Insulin receptor expression was also upregulated while the leptin receptor long form was not changed. Trh and Crh were both increased in BDNF mice.
BDNF gene transfer prevents diet-induced obesity
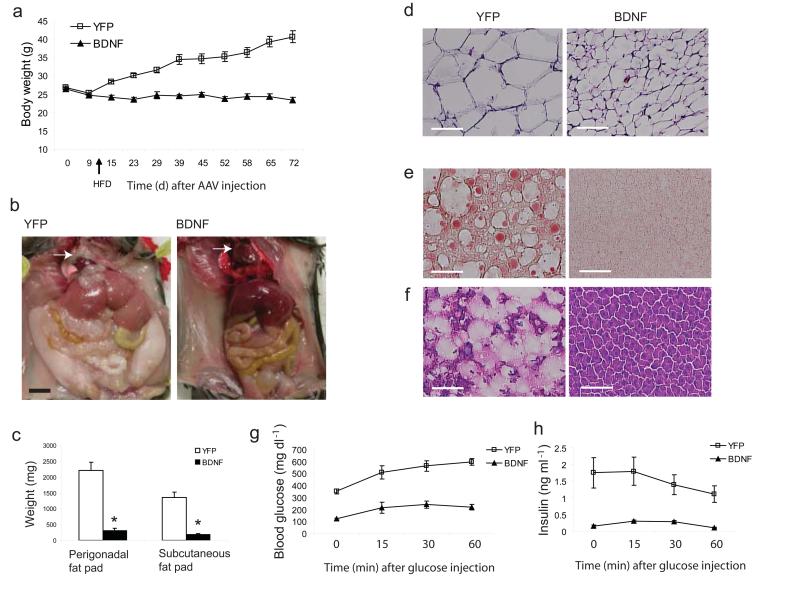
Chronic consumption of a HFD contributes to obesity in experimental animals and humans. We used C57BL/6 mice, a strain prone to DIO to assess the therapeutic efficacy of hypothalamic BDNF gene transfer19. Given the potency of the rAAV-BDNF vector observed in normal mice, we decreased the dose (from 3 × 109 genomic titer per site to 2 × 109) and used older (18 weeks) mice. Ten days post surgery, mice were switched to a 45% HFD. The weight gain of YFP mice accelerated while BDNF mice maintained a stable weight (Fig. 2a). By 72 days, YFP mice had gained 13.78 ± 1.88 g on the HFD, while BDNF mice lost 2.80 ± 0.71 g. with no change in food consumption (Supplementary Fig. 1b). YFP mice developed abdominal obesity with perigonadal fat pads increased by 4.5 fold compared to standard diet controls. In contrast, the BDNF perigonadal pad weight was only 14.2 ± 3.1% of the YFP mice. Subcutaneous fat was also greatly reduced (Fig. 2b, c), and the pericardial fat observed in YFP mice was completely absent in the BDNF mice (Fig. 2b). H&E staining revealed an 85.7 ± 1.1% reduction in adipocyte size (Fig. 2d).
Figure 2.
Hypothalamic gene delivery of BDNF prevents diet-induced obesity. (a) Body weight (n = 9 GFP, n = 10 BDNF, P < 0.0001). Diet was switched from standard chow diet to high fat diet on d 10 after AAV injection. (b) 2 months HFD feeding led to abdominal obesity in YFP mice but not in BDNF mice. Arrow shows pericardial fat absent in BDNF mouse. Scale bar: 1 cm. (c) Fat pad weight 72 d after AAV injection and fed on HFD for 2 months (n = 8 per group, * P < 0.0001). (d) H&E stained WAT section showed the shrink of adipose cell in BDNF mice compared to YFP mice. Scale bar: 300 μm. (e–f) Hepatic steatosis was prevented by BDNF treatment shown by Oil Red-O and H&E staining. Scale bar: 300 μm. (g–h) Glucose tolerance test after overnight fast (n = 4 per group, P < 0.0001 for both glucose and insulin concentration).
DIO was associated with hyperinsulinemia, hyperleptinemia, hyperglycemia and dyslipidemia with BDNF completely preventing this metabolic profile (Supplementary Table). The reduction of circulating leptin was not solely due to the decrease in fat mass. When leptin concentrations were standardized to the perigonadal fat pad weight, BDNF mice still showed a significant reduction (YFP: 7.347 ± 0.612 pg ml−1 g−1, BDNF: 3.735 ± 0.798 pg ml−1 g−1, P = 0.003). In contrast, adiponectin showed a greater than 14 fold increase when its concentration was corrected to fat mass (YFP: 1.624 ± 0.314 ng ml−1 g− 1, BDNF: 24.030 ± 5.540 ng ml−1 g−1, P = 0.005) indicating that BDNF influenced adipocyte autonomous leptin/adiponectin secretion. In addition the insulin insensitivity and glucose intolerance observed in YFP obese mice were greatly improved in BDNF mice (Supplementary Fig. 1c; Fig. 2g, h).
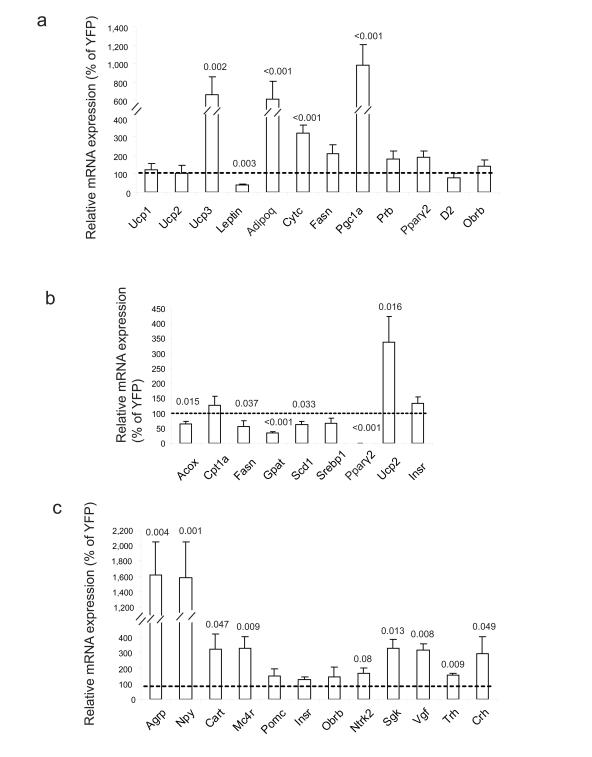
HFD feeding led to liver steatosis in obese YFP mice as characterized by pale macroscopic enlargement (Fig. 2b) and excessive fat accumulation observed in Oil red O (Fig. 2e) and H&E (Fig. 2f) stained sections. BDNF prevented the liver steatosis (Fig. 2e, f) with normalized liver weight (BDNF: 1.18 ± 0.05 g, YFP: 2.61 ± 0.29 g, P = 0.001). We profiled the expression of genes involved in lipid metabolism and mitochondrial activity in both white adipose tissue (WAT, Fig. 3a) and brown adipose tissue (BAT, data not shown)20. In WAT, Pgc1a, a cofactor controlling mitochondrial biogenesis21, was upregulated 9.8 ± 2.2 fold in BDNF mice while the expression of cytochrome c (Cytc) was increased 3.2 ± 0.5 fold compared to the obese control mice. Uncoupling proteins (UCP) are a family of proteins involved in the regulation of lipid oxidation as well as the regulation of energy expenditure22. The expression of Ucp3 was increased 6.8 ± 1.9 fold in BDNF WAT suggesting a possible increase in energy expenditure of WAT. Moreover, Lep was decreased by 62.0 ± 7.9% while Adipoq was increased by 6.1 ± 1.9 fold, consistent with the observed weight loss23. Leptin expression was also decreased by 68.5 ± 14.3% in BDNF BAT while no other genes screened were significantly changed. BDNF treatment significantly suppressed lipogenic gene expression in liver including Fasn by 43.1 ± 17.5%, Gpat by 66.4 ± 4.2% and Scd1 by 37.8 ± 10.9% while effects on lipolytic genes were less marked with a decrease in Acox but no change in Cpt1a although both are involved in fatty acid oxidation24 (Fig. 3b). Pparγ2, an adipocyte-specific PPAR isoform and a type 2-diabetes marker, was decreased by 99.6 ± 0.1% in BDNF liver consistent with the absence of fatty infiltration25. UCP2, a mitochondrial inner-membrane protein that uncouples ATP synthesis and negatively regulates reactive oxygen species production26, was significantly upregulated by 3.39 ± 0.86 fold in BDNF liver, which may serve a protective role when hepatocytes are exposed to metabolic stress such as high-fat feeding. We also profiled hypothalamic gene expression and observed a similar pattern of changes as BDNF mice fed with standard diet except Cart was upregulated ~3 fold in BDNF mice on HFD (Fig. 3c).
Figure 3.
Gene expression profiles of HFD-fed mice. Relative mRNA expression levels of the indicated genes in (a) WAT (b) liver and (c) hypothalamus (n = 6 per group). Bars show the relative expression levels of BDNF mice as the percentage of YFP mice. P values are shown on the bars.
Transgene expression in the hypothalamus was maintained throughout the duration of the experiment with BDNF concentrations of 5541.4 ± 738.4 pg mg−1 in BDNF mice compared to 87.6 ± 11.2 pg mg−1 in YFP mice, P < 0.001. Moreover, histological examination of hypothalamic sections demonstrated lack of cytotoxicity with no cell loss (Nissl staining Supplementary Fig. 2a), no gliosis (glial fibrillary acidic protein GFAP staining, Supplementary Fig. 2b) nor apoptosis (TUNEL assay, Supplementary Fig. 2d).
An autoregulatory BDNF vector in diabetic db/db mice
Gene therapy dose titration in humans is difficult, particularly in a clinical setting where diet is not tightly controlled. We therefore aimed to improve the safety of the approach and develop a vector that could be considered for clinical translation. Ideally, we would like transgene expression tightly coupled to the physiological changes induced by the therapeutic gene. Of the hypothalamic genes profiled in BDNF animals, Agrp was the most robustly upregulated with 15.1 and 16.2 fold increase in mice on STD and HFD, respectively, consistent with the observed weight loss and particularly the decrease in body fat mass27. We amplified two human AGRP promoter fragments of different lengths each containing the hypothalamus-specific exon28. We coupled the AGRP promoter fragments to a luciferase reporter gene and packaged these cassettes into rAAV vectors. We injected these AGRP promoter-driven luciferase vectors into the hypothalamus together with the BDNF vector to induce weight loss, and compared luciferase activity with YFP controls. The 484 bp fragment promoter (AGRP484) showed better inducibility than the 814 bp fragment. The induction of AGRP484 was 2.66 ± 0.57 fold while AGRP814 was 1.32 ± 0.38 fold (n = 6 each group) analyzed when BDNF mice had lost 1.5 ± 0.22 g and YFP mice gained 1.5 ± 0.56 g. We then used AGRP484 to drive a microRNA targeting BDNF (Supplementary Fig. 3), which was inserted into the parent vector with the constitutively expressing BDNF cDNA cassette. In other words we made a vector that expressed both BDNF under a general constitutive promoter and then in addition a microRNA to BDNF (to knockdown expression of the same therapeutic gene) under the control of the AGRP promoter which increases activity as the animals lose weight (Fig. 4a).
Figure 4.
Autoregulatory BDNF vector to treat db/db mice. (a) rAAV vectors. The autoregulatory vector contains two expression cassettes, one to express BDNF under a constitutive promoter, the other to express a microRNA targeting the same transgene driven by a promoter responsive to BDNF-induced physiological changes. (b) Body weight of db/db mice (n = 8 YFP, n = 7 BDNF-miR-scr, n = 9 BDNF-miR-Bdnf, P < 0.0001 comparisons between each pair of groups). (c) Mice receiving BDNF-miR-Bdnf remained lean 3 months after AAV injection. Scale bar: 1 cm. (d) Food intake was reduced in BDNF treated mice compared to YFP mice. P values are shown on the bars. (e) Liver weight and fat pad weight were reduced in BDNF-miR-Bdnf mice (n = 8 each group, P < 0.001). (f–g) Glucose tolerance test on mice after overnight fast (n = 6 YFP, n = 5 BDNF-miR-Bdnf, P < 0.05 for glucose concentration, P < 0.0001 for insulin concentration). (h) Biomarkers in serum 1 month after AAV injection (n = 8 YFP, n = 4 BDNF-miR-scr, n = 9 BDNF-miR-Bdnf, * P < 0.001, + P < 0.05, # P = 0.06).
We used a rodent model of type 2 diabetes, db/db mice, and delayed the intervention until they were extremely obese and diabetic to investigate both the therapeutic efficacy as well as autoregulatory efficiency of the dual cassettes vectors. When administered a control YFP virus, db/db mice continued to gain weight. In contrast, when given a BDNF vector together with a scrambled microRNA (BDNF-miR-scr targeting no known genes), their weight dropped precipitiously by 45.3 ± 3.6% in 3 weeks (Fig. 4b). The weights of the animals receiving the BDNF plus the AGRP484-miR-Bdnf dropped significantly but began to level off and stabilized between 3 to 4 weeks after AAV injection with body weight maintained for the entire 11 week duration of the experiment indicating efficient auto-regulation of the BDNF transgene expression (Fig. 4b-c). Both BDNF-miR-Bdnf and BDNF-miR-scr mice showed reduced food intake compared to YFP controls (Fig. 4d) with increased rectal temperature indicating increased energy expenditure (data not shown). Moreover gene therapy with the auto-regulatory BDNF vector alleviated the obesity (Fig. 4e), improved insulin sensitivity and glucose tolerance (Fig. 4f-g), and ameliorated the metabolic disturbances in db/db mice (Fig. 4h). The profile of hypothalamic gene expression showed a similar pattern as observed in wild type mice but with a milder extent of changes that is likely to reflect the more controlled BDNF overexpression (Supplementary Fig. 4a). Indeed, hypothalamic BDNF levels were 2055.6 ± 402.7 pg mg−1 in BDNF-miR-Bdnf mice, an 85% reduction from the 13323.3 ± 3899.8 pg mg−1 concentration in the BDNF-miR-Scr mice, (P = 0.023); and 100.7 ± 13.1 pg mg− 1 in YFP animals. Gene therapy also improved mobility of the extremely obese db/db mice and enhanced their physical activity and exploration behavior as shown in an open field test (Supplementary Fig. 5).
BDNF induced weight loss is reversible by loxP/Cre knockout of transgene
In order to provide a further safeguard of this approach and the potential for a clinical rescue procedure, we used the loxP/Cre recombination system to knockout the transgene if the need ever arose because of adverse events29. We generated an AAV vector with the BDNF transgene flanked by two loxP sites (flox-BDNF), which could be subsequently knocked out by a second viral vector delivering Cre recombinase. The rAAV vector encoding a GFP/Cre fusion protein has been shown to efficiently ablate loxP modified genes in the brain including hypothalamus with low toxicity10, 30. Bilateral injection of Cre vector alone did not influence body weight (data not shown) and no toxicity was observed (Supplementary Fig. 2e, Supplementary Fig. 8a, b).
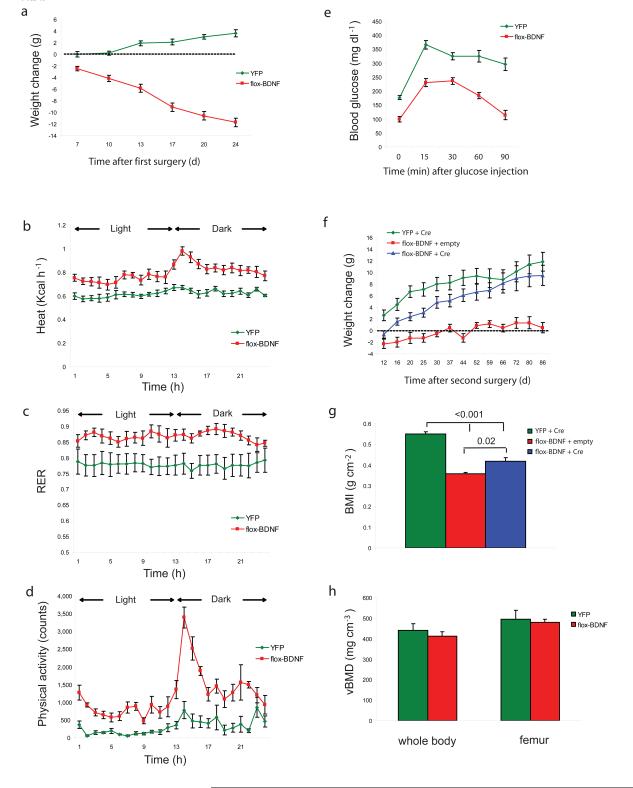
To establish an obesity model with greater clinical relevance, C57BL/6 mice were fed with the HFD for 10 weeks until their body weight reached 40 g. The floxed BDNF vector was injected into the hypothalamus of the obese mice with YFP as a control. Flox-BDNF mice started to lose weight 7 days after injection and by 24 days had lost 29.3 ± 1.9% of their body weight at which time the YFP mice had gained 9.0 ± 1.5% of their baseline weight (Fig. 5a). Food consumption was slightly but significantly reduced in BDNF treated mice (BDNF mice 2.06 ± 0.09 g per mouse d−1, YFP mice 2.44 ± 0.05 g per mouse d−1, P = 0.003). In addition energy expenditure (kilocalories of heat produced) was significantly increased in BDNF mice during both the dark phase and light phase (Fig. 5b). Physical activity was significantly increased in BDNF mice by 4.03 ± 0.28 fold compared to YFP mice in a 24 h period and particularly in the dark phase (Fig. 5d). Notably the respiratory exchange ratio (RER) was increased from 0.78 ± 0.03 in YFP to 0.87 ± 0.01 in BDNF mice (Fig. 5c), suggesting increased carbohydrate oxidation as opposed to lipid oxidation although both groups were fed with HFD. Obesity associated glucose intolerance was alleviated by BDNF treatment 3 weeks post-rAAV injection (Fig. 5e). We then randomized flox-BDNF mice to receive a second viral vector injection to the same site as the first surgery, receiving either GFP/Cre or empty viral vector as a control. All YFP mice received the GFP/Cre viral vector in the second surgery. GFP/Cre vector injection significantly suppressed BDNF mRNA and protein expression by 63.9 ± 9.0% and 71.6 ± 1.9% respectively (P = 0.002). Hypothalamic immunohistochemistry showed widespread GFP/Cre expression with less residual HA immunoreactivity consistent with the ~72% protein knockdown. Moreover, HA positive cells (transduced by flox-BDNF) and GFP positive cells (transduced by GFP/Cre) were located in the same area but no colocalization was observed consistent with efficient Cre recombinase activity in co-transduced cells (Supplementary Fig 8c). After the second surgery, YFP mice continued to gain weight while flox-BDNF mice receiving empty vector in the second surgery continued to lose weight although at a lower rate and eventually became stable. Flox-BDNF mice receiving Cre virus reversed the progressive weight loss, and commenced to regain weight gradually although their weight remained significantly lower than the YFP obese mice (Fig. 5f). At the end of the study approximately 4 months after the first surgery, both groups of BDNF mice showed significantly lower body mass index than the YFP controls (Fig. 5g). Since body weight influences bone density and is considered as a risk factor for fracture31, we measured the bone mineral density of BDNF mice after significant weight loss and found no difference in either the whole body skeleton (excluding skull) or femur only (Fig. 5h), indicating a lack of adverse effect on bones following BDNF induced weight loss.
Figure 5.
BDNF induced weight loss is reversible by Cre-loxP knockout of transgene. (a) Body weight of mice after first AAV injection (n = 10 YFP, n = 24 flox-BDNF, P < 0.01). (b–d) Effect of BDNF treatment on energy expenditure. Energy expenditure (Heat), respiratory exchange ratio (RER) and physical activity were significantly increased in BDNF mice (n = 5 YFP, n = 6 flox-BDNF, P < 0.05) during both light and dark cycles. (e) Glucose tolerance test on mice after overnight fast (n = 7 YFP, n = 9 flox-BDNF, 3 weeks after first AAV injection P < 0.05). (f) Weight change of mice since second AAV injection (n = 10 YFP + Cre, n = 7 flox-BDNF + empty vector, n = 14 flox-BDNF + Cre, P < 0.05 flox-BDNF + Cre vs flox-BDNF + empty). (g) Body mass index of mice 4 months after the first surgery (n = 5 YFP + Cre, n = 4 flox-BDNF + empty vector, n = 6 flox-BDNF + Cre, P values are shown on the bars). (h) Bone mineral density of whole body (excluding skull) and right femur were measured by microCT scan (n = 3 YFP, n = 4 flox-BDNF).
Discussion
Clinical gene transfer should ideally include some regulatory control of therapeutic gene expression particularly when constitutive expression of the transgene may be deleterious32. Several pharmacological gene regulation technologies have been developed. The Tet regulatory system based on the use of small molecules such as tetracycline or doxycycline is the most widely used33,34. However, problems include basal leakiness of the system and potential immunogenicity of the foreign proteins in addition to the need to administer a pharmacological agent with its own attendant risks. A dimerizer-regulated approach such as the rapamycin system allows tight control in vivo and is less likely to be immunogenic because the key components of this system are derived from human proteins. However, the size limitation of the cloned sequences in rAAV requires splitting the regulatory system into two separate vectors35,36,37. The use of two separate vectors is inefficient due to the need of double-infection of the host cell. In addition, it remains a question whether the inducer drugs will be appropriate for the clinic.
Therefore, we constructed an autoregulatory system to control therapeutic gene expression mimicking the body’s natural feedback systems. Here we demonstrate the efficacy of such an approach using BDNF as the therapeutic gene, with weight loss and fat depletion as the physiological readout, and an AGRP promoter-driven microRNA cassette as the regulatory agent. All the components of this system can be packaged into a single rAAV vector for efficient delivery. In order to evaluate the regulation efficacy in vivo we used a very high dose of the vector in db/db mice. The unchecked overexpression of BDNF led to drastic weight loss, decrease of adiposity and improvement in serum metabolic parameters. However, the weight of mice receiving BDNF coupled with scrambled microRNA continued to drop (over 45% by 3 weeks after AAV injection) and showed no sign of stabilization, ultimately requiring euthanization. Moreover the BDNF-treated mice with severe weight loss and fat depletion, both wild type mice on standard diet as well as the db/db mice, had a hyperactive phenotype and in addition their immunocompetency was compromised (data not shown). In contrast, the body weight of mice receiving an identical dose of the autoregulatory vector, in which the constitutive BDNF cassette was coupled with a physiologically responsive inhibitory microRNA construct, had a more gradual weight loss without any behavioral hyperactivity. The weight loss of these mice reached ~20% by 3 weeks and then plateaued throughout the entire 11 week duration of the experiment. This significant alleviation of obesity was associated with loss of liver steatosis, improvement in insulin sensitivity and glucose tolerance, and reversal of hyperleptinemia and lipid dyslipidemia. The approach we describe here could be potentially generalized to other molecular intervention studies in which expression of any given functional transgene is self-regulated by a microRNA driven by promoters activated by the physiological changes induced by the transgene of interest.
Our data in two obesity and diabetes models show the potency of hypothalamic gene transfer of BDNF. Both suppression of food intake and heightening of energy expenditure contribute to the weight loss of db/db mice receiving BDNF although the increase in energy expenditure of both basal metabolism and spontaneous activity appears more important since BDNF overexpressing wild type mice fed with either standard diet or HFD (while weight remained normal) did not change food intake. Our data is consistent with previous reports which have shown that BDNF has a more potent effect on appetite in obese mice. For example, chronic administration of BDNF protein significantly suppressed food intake in diet induced obesity mice but not those fed on a standard diet38. In addition, acute or chronic administration of BDNF protein led to a marked reduction in food intake in genetic obesity models including yellow agouti mice38 and db/db mice15. This selective effect on appetite suppression in obese animals might best be explained by the basal pattern of anorexic and/or orexigenic signaling in the hypothalamus prior to BDNF treatment. The orexigenic peptides Agrp and Npy mRNA levels were approximately 6 fold and 3 fold higher in db/db mice than wild-type mice, respectively, while the anorexic peptide Pomc was 30% lower (Supplemental Fig. 4b). The hyperphagia associated with these obese models (genetic or diet induced obesity) appears to reveal the appetite-suppressing effect of BDNF gene therapy that is not apparent in euphagic animals with normal food intake. We also showed that genes involved in energy expenditure such as UCPs were significantly upregulated in the liver and WAT of BDNF mice. Moreover the comprehensive analysis of gene expression in hypothalamus, liver and fat in this study provides further insights into the potential mechanisms underlying the hypothalamic BDNF regulation of energy balance. For example hypothalamic Crh expression was consistently increased in all the models we used and accompanied with the increase in physical activity suggests a potential pathway of BDNF regulation of the HPA axis, food intake and energy expenditure39,40. In addition WAT appears to be a primary peripheral organ responsive to hypothalamic BDNF with not only massive reduction of adipocyte size but also significant changes in the expression profile of adipokines and genes involved in lipid metabolism and mitochondrion activity. The WAT reduction was primarily due to a reduction in adipocyte size without significant impact on cellular viability as shown by lack of adipocyte apoptosis (data not shown) and reversible weight gain when the BDNF transgene was knocked out by the Cre virus.
In summary we have developed several strategies to achieve potent and safe gene therapy for obesity and related metabolic syndromes using AAV-BDNF vectors including dose adjustment, an autoregulatory negative feedback system using RNA interference coupled to transgene-induced physiological changes, and finally a definitive knockout via delivery of a second “rescue” vector. Long term observation of mice receiving these therapeutic vectors in both DIO and diabetic genetic models showed improved general health, metabolic parameters and physical activity with no adverse impact on bone density or disturbance in circadian rhythm or home cage activity. The combination of these strategies will further strengthen the safety of this gene therapy approach and provide potent therapeutics for morbid obesity.
Methods
Animals
We used male 8 week-old C57Bl/6 mice (from Charles River) and male 4 month-old db/db mice (from Jackson Lab). All use of animals was approved by and in accordance with the Ohio State University Animal Care and Use Committee.
rAAV vector construction and packaging
The rAAV plasmid contains a vector expression cassette consisting of the CMV enhancer and chicken β-actin (CBA) promoter, woodchuck post-transcriptional regulatory element (WPRE) and bovine growth hormone poly-A flanked by AAV inverted terminal repeats. Human BDNF cDNA was fused to HA tag at the 5’ terminal and then inserted into the multiple cloning sites between the CBA promoter and WPRE sequence. EGFP or destabilized YFP were cloned into the rAAV plasmid as controls. rAAV serotype 1 vectors were packaged and purified as described elsewhere 5,6.
AAV mediated BDNF overexpression in mice kept on standard diet
We randomly assigned 23 C57Bl/6 mice, male, 8 weeks of age, to receive AAV-BDNF (n = 13) or AAV-GFP (n = 10). Mice were anaesthetized with a single dose of ketamine/xylazine (100 mg kg−1 and 20 mg kg−1; i.p.) and secured via ear bars and incisor bar on a Kopf stereotaxic frame. A mid-line incision was made through the scalp to reveal the skull and two small holes were drilled into the skull with a dental drill above the injection sites (−1.2 AP, ± 0.5 ML, −6.2 DV, mm from bregma). rAAV vectors (3×109 genomic particles per site) were injected bilaterally into the hypothalamus at a rate of 0.1 μl min−1 using a 10 μl Hamilton syringe attached to Micro4 Micro Syringe Pump Controller (World Precision Instruments Inc., Sarasota, USA). At the end of infusion, the syringe was slowly raised from the brain and the scalp was sultured. Animals were placed back into a clean cage and carefully monitored post-surgery until recovery from anaesthesia. We fed mice with standard chow diet (STD, 11% fat, caloric density 3.4 kcal g−1).
High fat diet induced obesity model
We randomly assigned 24 C57Bl/6 mice, male, 18 weeks of age, to receive AAV-BDNF (n = 13) or AAV-YFP (n = 11). rAAV vectors (2×109 genomic particles per site) were injected bilaterally into the hypothalamus as described above. We switched the diet to high fat diet (HFD, 45% fat, caloric density 4.73 kcal g−1, Research Diets, Inc.) on day 10 after AAV injection and fed the mice with HFD until the end of the study (72 d after injection).
microRNA vector construction and AAV vector production
We used microRNA to target BDNF. We cloned two targeting sequences in the BDNF coding region into the Block-iT PolII miR RNAi expression vector (pcDNA6.2-Gw/miR, Invitrogen). In in vitro experiments, both miR constructs inhibited BDNF expression when co-transfected with a BDNF expression plasmid confirmed by qPCR and BDNF ELISA. We chose the miR-BDNF921 (mature miR seq: AAGTGTACAAGTCCGCGTCCT) for in vivo experiments. This miR-Bdnf and a scrambled miR (miR-scr, with the scrambled sequence targeting no known gene, Invitrogen) were subcloned to the AAV plasmid driven by CBA promoter as described above.
Auto-regulatory system
We amplified two AGRP promoter fragments from human genomic DNA by PCR: AGRP 484 (484 bp, −133/+351) and AGRP 814 (814 bp, −463/+351). We inserted the AGRP promoter fragments to AAV vectors to drive luciferase report gene expression. In order to verify the induction of AGPR promoter by BDNF-induced physiological change, we injected the combination of viral vectors to the ARC of 4 groups of mice: YFP + AGRP484-luc, YFP + AGRP814-luc, BDNF + AGRP484-luc, BDNF + AGRP814-luc. Equal amount of each viral vector (1.5×109 genomic titer) was injected bilaterally. We sacrificed the mice and dissected the hypothalamus 3 weeks after AAV injection. We measured the luciferase activity in the hypothalamic lysate using Bright-Glo Luciferase Assay (Promega) and calibrated the luminescence to the protein concentration. We chose AGRP484 to develop auto-regulatory system. We generated vectors containing two cassettes, one cassette expresses BDNF driven by the CBA promoter as described above, the other cassette expresses microRNA (miR-Bdnf or miR-scr) driven by AGRP484. Both transgene and the microRNA cassettes were packaged to a single viral vector (BDNF-miR-scr, BDNF-miR-Bdnf).
db/db mice
We randomly assigned 30 db/db mice to receive AAV-YFP, AAV-BDNF-miR-scr, AAV-BDNF-miR-Bdnf, 10 mice per group. rAAV vectors (3.4×1010 genomic particles per site) were injected bilaterally into the hypothalamus as described above. We fed the db/db mice with standard chow diet throughout the experiment. We sacrificed the mice receiving AAV-BDNF-miR-scr one month after injection due to their severe weight loss. We recorded the body weight and food consumptions periodically until the end of the experiment (79 days after injection).
Knockdown transgene expression using Cre/loxP recombination
We generated diet induced obesity model by feeding mice with high fat diet for 10 weeks until the body weight reached 40 g. The obese mice were randomly assigned to receive AAV flox-BDNF or AAV-YFP. We injected AAV vectors bilaterally to the hypothalamus as described above (1.0×1010 genomic particles per site). We monitored body weight every 5–7 days and recorded the food intake. One month after first surgery, we split the flox-BDNF mice to two groups to receive AAV-GFP/Cre or empty AAV as control. All YFP mice were injected with AAV-GFP/Cre. The second surgery was performed with the same procedure as the first surgery. We kept the mice on high fat diet till the end of the study (4 months after the first surgery).
Statistical analysis
Values are expressed as mean ± s.e.m. For body weight, insulin tolerance and glucose tolerance, we determined the overall significance using one-way repeated measures. We used one-way ANOVA to analyze serum biomarker measurements, liver weight and adipose tissue weight. We used multivariate ANOVA to analyze quantitative RT-PCR data.
Supplementary Material
Acknowledgements
We thank Adam Martin for technical assistance and Drs Deborah Young, Trish Lawlor and Dahna Fong for providing the HA-tagged BDNF cDNA and helpful discussions. The project was supported in part by the NIH NS44576. E.D.L. is supported by New Zealand Foundation for Research, Science and Technology.
References
- 1.McMillan DC, Sattar N, McArdle CS. ABC of obesity. Obesity and cancer. BMJ. 2006;333:1109–1111. doi: 10.1136/bmj.39042.565035.BE1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batsis JA, Nieto-Martinez RE, Lopez-Jimenez F. Metabolic syndrome: from global epidemiology to individualized medicine. Clin. Pharmacol. Ther. 2007;82:509–524. doi: 10.1038/sj.clpt.6100355. [DOI] [PubMed] [Google Scholar]
- 3.Anghel SI, Wahli W. Fat poetry: a kingdom for PPARgamma. Cell Res. 2007;17:486–511. doi: 10.1038/cr.2007.48. [DOI] [PubMed] [Google Scholar]
- 4.During MJ, Cao L. VEGF, a mediator of the effect of experience on hippocampal neurogenesis. Curr. Alzheimer Res. 2006;3:29–33. doi: 10.2174/156720506775697133. [DOI] [PubMed] [Google Scholar]
- 5.Young D, Lawlor PA, Leone P, Dragunow M, During MJ. Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nat. Med. 1999;5:448–453. doi: 10.1038/7449. [DOI] [PubMed] [Google Scholar]
- 6.Cao L, et al. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat. Genet. 2004;36:827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- 7.Wisse BE, Schwartz MW. The skinny on neurotrophins. Nat. Neurosci. 2003;6:655–656. doi: 10.1038/nn0703-655. [DOI] [PubMed] [Google Scholar]
- 8.Lyons WE, et al. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc. Natl. Acad. Sci.USA. 1999;96:15239–15244. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu B, et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat. Neurosci. 2003;6:736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Unger TJ, Calderon GA, Bradley LC, Sena-Esteves M, Rios M. Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J. Neurosci. 2007;27:14265–14274. doi: 10.1523/JNEUROSCI.3308-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray J, et al. Hyperphagia, severe obesity, impaired cognitive function, and hyperactivity associated with functional loss of one copy of the brain-derived neurotrophic factor (BDNF) gene. Diabetes. 2006;55:3366–3371. doi: 10.2337/db06-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeo GS, et al. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat. Neurosci. 2004;7:1187–1189. doi: 10.1038/nn1336. [DOI] [PubMed] [Google Scholar]
- 13.Bariohay B, Lebrun B, Moyse E, Jean A. Brain-derived neurotrophic factor plays a role as an anorexigenic factor in the dorsal vagal complex. Endocrinology. 2005;146:5612–5620. doi: 10.1210/en.2005-0419. [DOI] [PubMed] [Google Scholar]
- 14.Pelleymounter MA, Cullen MJ, Wellman CL. Characteristics of BDNF-induced weight loss. Exp. Neurol. 1995;131:229–238. doi: 10.1016/0014-4886(95)90045-4. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa T, et al. Brain-derived neurotrophic factor (BDNF) regulates glucose and energy metabolism in diabetic mice. Diabetes Metab. Res. Rev. 2002;18:185–191. doi: 10.1002/dmrr.290. [DOI] [PubMed] [Google Scholar]
- 16.Frederich RC, et al. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat. Med. 1995;1:1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 17.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 18.Dhillo WS. Appetite regulation: an overview. Thyroid. 2007;17:433–445. doi: 10.1089/thy.2007.0018. [DOI] [PubMed] [Google Scholar]
- 19.Townsend KL, Lorenzi MM, Widmaier EP. High-fat diet-induced changes in body mass and hypothalamic gene expression in wild-type and leptin-deficient mice. Endocrine. 2008;33:176–188. doi: 10.1007/s12020-008-9070-1. [DOI] [PubMed] [Google Scholar]
- 20.Dali-Youcef N, et al. Adipose tissue-specific inactivation of the retinoblastoma protein protects against diabesity because of increased energy expenditure. Proc. Natl. Acad. Sci.USA. 2007;104:10703–10708. doi: 10.1073/pnas.0611568104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mootha VK, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 22.Ricquier D. Respiration uncoupling and metabolism in the control of energy expenditure. Proc. Nutr. Soc. 2005;64:47–52. doi: 10.1079/pns2004408. [DOI] [PubMed] [Google Scholar]
- 23.Qi Y, et al. Adiponectin acts in the brain to decrease body weight. Nat. Med. 2004;10:524–529. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi N, Qi Y, Patel HR, Ahima RS. A novel aminosterol reverses diabetes and fatty liver disease in obese mice. J. Hepatol. 2004;41:391–398. doi: 10.1016/j.jhep.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Vidal-Puig A, et al. Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. J. Clin. Invest. 1996;97:2553–2561. doi: 10.1172/JCI118703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arsenijevic D, et al. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat. Genet. 2000;26:435–439. doi: 10.1038/82565. [DOI] [PubMed] [Google Scholar]
- 27.Bertile F, Oudart H, Criscuolo F, Maho YL, Raclot T. Hypothalamic gene expression in long-term fasted rats: relationship with body fat. Biochem. Biophys. Res. Commun. 2003;303:1106–1113. doi: 10.1016/s0006-291x(03)00481-9. [DOI] [PubMed] [Google Scholar]
- 28.Brown AM, Mayfield DK, Volaufova J, Argyropoulos G. The gene structure and minimal promoter of the human agouti related protein. Gene. 2001;277:231–238. doi: 10.1016/s0378-1119(01)00705-3. [DOI] [PubMed] [Google Scholar]
- 29.Li XG, et al. Viral-mediated temporally controlled dopamine production in a rat model of Parkinson disease. Mol. Ther. 2006;13:160–166. doi: 10.1016/j.ymthe.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Kaspar BK, et al. Adeno-associated virus effectively mediates conditional gene modification in the brain. Proc. Natl. Acad. Sci.USA. 2002;99:2320–2325. doi: 10.1073/pnas.042678699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reid IR. Relationships between fat and bone. Osteoporos. Int. 2008;19:595–606. doi: 10.1007/s00198-007-0492-z. [DOI] [PubMed] [Google Scholar]
- 32.Le Bec C, Douar AM. Gene therapy progress and prospects--vectorology: design and production of expression cassettes in AAV vectors. Gene Ther. 2006;13:805–813. doi: 10.1038/sj.gt.3302724. [DOI] [PubMed] [Google Scholar]
- 33.Stieger K, et al. Long-term doxycycline-regulated transgene expression in the retina of nonhuman primates following subretinal injection of recombinant AAV vectors. Mol. Ther. 2006;13:967–975. doi: 10.1016/j.ymthe.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Jiang L, et al. Tight regulation from a single tet-off rAAV vector as demonstrated by flow cytometry and quantitative, real-time PCR. Gene Ther. 2004;11:1057–1067. doi: 10.1038/sj.gt.3302245. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen M, et al. Rapamycin-regulated control of antiangiogenic tumor therapy following rAAV-mediated gene transfer. Mol. Ther. 2007;15:912–920. doi: 10.1038/mt.sj.6300079. [DOI] [PubMed] [Google Scholar]
- 36.Ye X, et al. Regulated delivery of therapeutic proteins after in vivo somatic cell gene transfer. Science. 1999;283:88–91. doi: 10.1126/science.283.5398.88. [DOI] [PubMed] [Google Scholar]
- 37.Lebherz C, et al. Long-term inducible gene expression in the eye via adeno-associated virus gene transfer in nonhuman primates. Hum. Gene Ther. 2005;16:178–186. doi: 10.1089/hum.2005.16.178. [DOI] [PubMed] [Google Scholar]
- 38.Nakagawa T, et al. Anti-obesity and anti-diabetic effects of brain-derived neurotrophic factor in rodent models of leptin resistance. Int. J. Obes. Relat. Metab. Disord. 2003;27:557–565. doi: 10.1038/sj.ijo.0802265. [DOI] [PubMed] [Google Scholar]
- 39.Naert G, Ixart G, Tapia-Arancibia L, Givalois L. Continuous i.c.v. infusion of brain-derived neurotrophic factor modifies hypothalamic-pituitary-adrenal axis activity, locomotor activity and body temperature rhythms in adult male rats. Neuroscience. 2006;139:779–789. doi: 10.1016/j.neuroscience.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 40.Wang C, Bomberg E, Billington C, Levine A, Kotz CM. Brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus reduces energy intake. Am. J. Physiol. 2007;293:R1003–1012. doi: 10.1152/ajpregu.00011.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.