Abstract
Intravenous diuretics are the cornerstone of management for patients hospitalized for heart failure. Physiologic data suggest that intermittent high-dose furosemide promotes neuro-hormonal activation, which a slow continuous infusion might remediate. However, the limited clinical data comparing dosing schemes are confounded. This study was a randomized, open-label, single-center trial of twice-daily bolus injection versus continuous infusion furosemide in patients hospitalized with heart failure and volume overload. The primary outcome was change in creatinine from admission to hospital day 3 or discharge. Twenty-one patients were randomized to bolus injection and 20 patients to continuous infusion. Baseline characteristics were balanced between study arms except for gender, with a mean age of 60 ± 15 years, a mean ejection fraction of 35 ± 19%, and a mean creatinine level of 1.9 ± 1.2 mg/dl. The mean doses of furosemide were similar between arms over the first 48 hours (162 ± 48 and 162 ± 52 mg/24 hours). None of the outcomes differed significantly between bolus and continuous dosing from admission to hospital day 3 or discharge (mean change in creatinine −0.02 vs 0.13 mg/dl, p = 0.18; urine output 5,113 vs 4,894 ml, p = 0.78; length of stay 8.8 vs 9.9 days, p = 0.69). All patients survived to discharge. In conclusion, there were no substantial differences between bolus injection and continuous infusion of equal doses of furosemide for the treatment of patients hospitalized with heart failure. Given the high prevalence of heart failure hospitalization and the disparate results of small studies regarding optimal dosing of loop diuretics to treat these patients, larger multicenter blinded studies are needed.
Given the high prevalence of the use of intravenous loop diuretics for patients hospitalized with acute decompensated heart failure (HF)1 and the paucity of data guiding best practices for administration,2– 4 we carried out a randomized pilot study of furosemide by continuous infusion versus twice-daily bolus injection for the treatment of such patients. Our primary hypothesis was that continuous dosing of intravenous furosemide provides gradual diuresis with less neurohormonal activation, which would manifest as less renal dysfunction, compared to bolus dosing in the treatment of acute decompensated HF with volume overload.
Methods
This study was a prospective, open-label, single-center, randomized trial of bolus injection versus continuous infusion of furosemide after hospital admission for HF. Patients were enrolled from Duke University Medical Center from 1999 to 2005. Patients were eligible if they were admitted with a primary diagnosis of acute decompensated HF, could be randomized <24 hours from hospital presentation, and had evidence of volume overload (pulmonary congestion on chest x-ray or pro–B-type natriuretic peptide greater than the upper limit of normal for age). Patients were excluded if they had end-stage renal disease or anticipated need for renal replacement therapy, were not expected to survive hospitalization, or were pregnant. All patients signed informed consent. The study was approved by the Duke institutional review board.
Once enrolled, the dose of intravenous furosemide to be given per 24 hours was decided upon by the attending physician. Patients were then randomized in a 1:1 ratio using a computer-generated scheme to receive the decided upon furosemide dose divided into twice-daily bolus injection or continuous infusion (mixed as a 1:1 ratio in 5% dextrose in water) for ≥48 hours. Subsequent titration of furosemide dose was at the discretion of the attending physician but was guided by a dose-escalation algorithm.
Data were collected at the time of enrollment and then daily through review of the medical record, an interview with the patient, and a physical examination. During the study, patients received daily blood work as part of routine care, including a daily basic metabolic panel as standard care for patients receiving intravenous diuretic therapy. The protocol also recommended the maintenance of serum potassium >4.0 mEq/L and serum magnesium >2.0 mEq/L. Electrolyte repletion was left to the discretion of the physician of record.
The primary outcome measure was change in serum creatinine from admission (hospital day 0) to hospital day 3 or hospital discharge, whichever came first. Creatinine was chosen on the basis of its ease of measurement and strong association with other clinical end points.5,6 Secondary outcome measures included cumulative urine output and other electrolyte changes from admission to hospital day 3 (or hospital discharge), as well as hospital length of stay. Observed survival was assessed through a search of the Duke electronic medical record and the Social Security Death Index. An estimated 42 patients were required to detect a clinically significant difference in the primary outcome of 0.3 mg/dl with power of 80%, assuming a standard deviation of 0.33 mg/dl and a 2-sided p value of 0.05.
Estimated glomerular filtration rate was calculated using the Modification of Diet in Renal Disease (MDRD) equation.7 Torsemide was converted to furosemide equivalents (torsemide 1 mg = furosemide 2 mg). Analysis for all end points was performed on an intention-to-treat basis. Comparisons of changes in outcomes from admission (hospital day 0) to hospital day 3 (or discharge, whichever came first) between the bolus and continuous arms were performed using Wilcoxon’s rank-sum test for continuous variables and the chi-square test for categorical variables. A 2-tailed p value of 0.05 was used to determine significance. Analyses were conducted with SAS version 9.1 (SAS Institute Inc., Cary, North Carolina).
Results
We enrolled and randomized 41 patients. For the overall cohort, the mean age was 60 ± 15 years, the mean ejection fraction was 35 ± 19%, and the mean estimated glomerular filtration rate was 54 ± 30 ml/min/1.73 m2. Mean oral home dose of furosemide equivalent before admission was 99 ± 108 mg/24 hours, with 29% (n = 12) of patients taking no loop diuretics before admission. Baseline characteristics were not statistically different between arms except for gender, which by random sampling produced fewer women in the continuous infusion arm (p = 0.005; Table 1).
Table 1.
Baseline characteristics, stratified by treatment assignment
| Variable | Twice Daily (n = 21) | Continuous (n = 20) |
|---|---|---|
| Age (years) | 58 ± 16 (46, 63, 68) | 61 ± 14 (49, 62, 72) |
| Black | 20 (48%) | 7 (35%) |
| Women | 12 (57%) | 3 (15%) |
| Diabetes mellitus | 15 (71%) | 9 (45%) |
| Hypertension | 18 (86%) | 14 (70%) |
| Atrial fibrillation | 7 (33%) | 9 (45%) |
| Coronary artery disease | 9 (43%) | 11 (55%) |
| Left ventricular ejection fraction (%) | 39 ± 20 (17, 35, 60) | 31 ± 18 (15, 30, 55) |
| Furosemide, home | 14 (67%) | 10 (50%) |
| Torsemide, home | 2 (10%) | 3 (15%) |
| Home oral furosemide equivalent (mg/24 h) | 110 ± 114 (0, 80, 160) | 87 ± 102 (0, 80, 120) |
| Thiazide or thiazide-type diuretic | 1 (5%) | 1 (5%) |
| Spironolactone | 7 (33%) | 8 (40%) |
| Angiotensin-converting enzyme inhibitor or angiotensin receptor blocker | 17 (81%) | 16 (80%) |
| β blocker | 14 (67%) | 17 (85%) |
| Systolic blood pressure (mm Hg) | 127 ± 22 (108, 124, 140) | 112 ± 20 (99, 105, 124) |
| Heart rate (beats/min) | 85 ± 17 (72, 84, 97) | 80 ± 17 (71, 78, 88) |
| Weight (kg) | 104 ± 34 (81, 102, 127) | 96 ± 23 (80, 96, 111) |
| Sodium (mEq) | 141 ± 31 (39, 142, 143) | 139 ± 51 (36, 139, 142) |
| Potassium (mEq) | 3.9 ± 0.5 (3.7, 3.8, 4.1) | 4.2 ± 0.6 (3.8, 4.2, 4.6) |
| Urea nitrogen (mg/dl) | 37 ± 31 (16, 24, 45) | 33 ± 19 (19, 26, 38) |
| Creatinine (mg/dl) | 2.1 ± 1.3 (1.1, 1.5, 2.8) | 1.8 ± 1.0 (1.1, 1.4, 1.9) |
| Glomerular filtration rate (ml/min/1.73 m2) | 48 ± 27 (20, 46, 70) | 59 ± 33 (32, 52, 82) |
| Hemoglobin (g/dl) | 11.1 ± 2.5 (9.2, 11.4, 13.2) | 12.1 ± 2.2 (10.0, 12.2, 13.5) |
Data are expressed as mean ± SD (25th, 50th, and 75th percentiles) or as number (percentage).
Follow-up was 100% at discharge. There were no protocol violations, with all patients receiving furosemide dosing as dictated by study assignment starting <24 hours after enrollment and continuing during the 48 hours after randomization. The mean doses of furosemide were similar between arms over the first 48 hours (Table 2).
Table 2.
In-hospital outcome measures, stratified by treatment assignment
| Variable | Twice Daily (n = 21) | Continuous (n = 20) | p Value |
|---|---|---|---|
| Furosemide dose (mg/24 h) | 162 ± 48 160 (140 to 200) |
162 ± 52 160 (120 to 240) |
1.00 |
| Δ creatinine (mg/dl) | −0.02 ± 0.39 0.1 (−0.1 to 0.2) |
0.13 ± 0.34 0.05 (−0.05 to 0.35) |
0.18 |
| Urine output (ml) | 5,113 ± 2,258 4,675 (3,230 to 6,250) |
4,894 ± 2,205 4,448 (3,625 to 4,650) |
0.64 |
| Δ weight (kg) | −1.64 ± 2.34 −2.55 (−3.30 to 0.25) |
−2.66 ± 2.44 −2.10 (−4.85 to ± 1.25) |
0.27 |
| Δ serum potassium (mEq/L) | −0.07 ± 0.57 −0.2 (−0.5 to 0.3) |
0.22 ± 0.67 0.3 (−0.2 to 0.7) |
0.08 |
| Rates of hypokalemia (<3.5 mEq/L) | 5 (24%) | 2 (10%) | 0.44 |
| Δ serum sodium (mEq/L) | −2.19 ± 3.25 −2 (−5 to 0) |
−1.15 ± 3.12 −1 (−4 to 1) |
0.30 |
| Δ systolic blood pressure (mm Hg) | −5.6 ± 21.2 −3 (−15 to 7) |
2.9 ± 15.0 0 (−2 to 4) |
0.11 |
| Length of stay (days) | 8.86 ± 3.82 7 (5.5 to 11) |
9.85 ± 11.72 7 (4.5 to 9.5) |
0.69 |
Data are expressed as mean ± SD and as median (interquartile range). Measured from admission (hospital day 0) until hospital day 3 or discharge (whichever came first), except for length of stay.
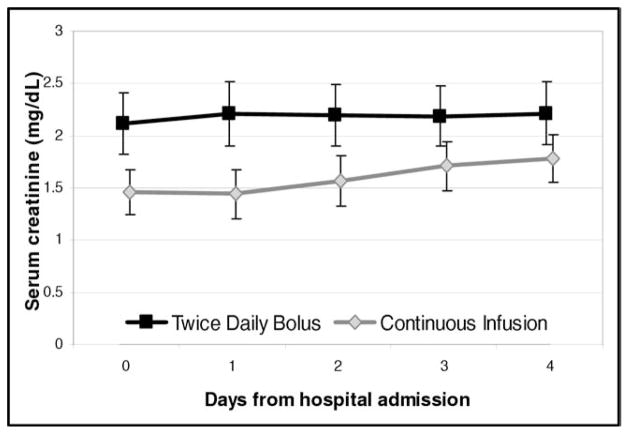
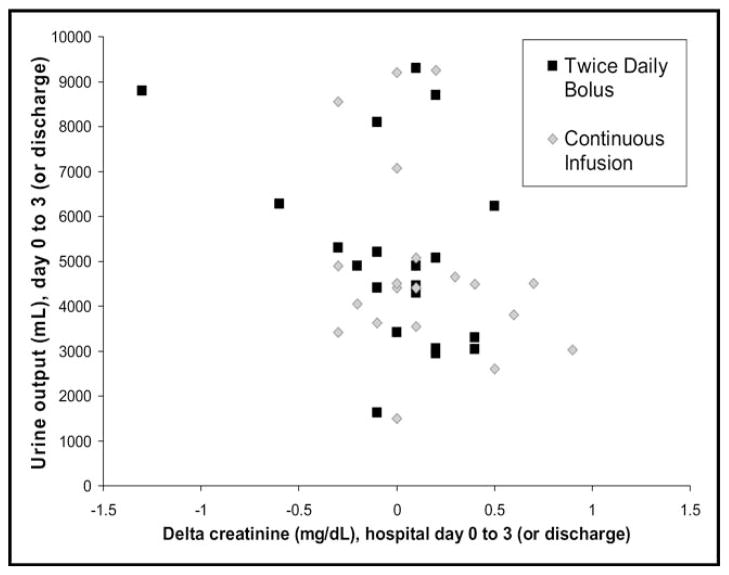
None of the outcomes showed a statistically significant difference between bolus and continuous dosing from admission to hospital day 3 or discharge (Table 2). The primary outcome of change in serum creatinine showed a nonsignificant trend toward improvement in the bolus dosing arm (Figure 1). The vector plot of urine output to change in creatinine showed no significant relation between these 2 variables (Figure 2). Decreases in serum potassium, serum sodium, and systolic blood pressure showed nonsignificant trends in favor or continuous infusion. All patients survived to discharge; 1 death occurred in the continuous arm within 90 days.
Figure 1.
Creatinine over time, stratified by treatment assignment.
Figure 2.
Vector end point of change in creatinine and urine output from admission (hospital day 0) to hospital day 3 (or discharge if before day 3).
Discussion
In this pilot trial randomizing 41 patients hospitalized with HF to either twice-daily bolus injection or continuous infusion of furosemide, we found no significant differences between the different dosing regimens. Although not significant, most measured outcomes trended in favor of the bolus dosing, a finding contrary to our initial hypothesis.
These findings add to the limited data that currently exist to guide intravenous loop diuretic dosing in acute decompensated HF. Despite theoretical advantages of a continuous infusion (i.e., constant urine output, more gradual changes in intravascular volume, less neurohormonal activation, less vasoconstriction, and lower peak drug concentrations with fewer and less severe side effects), the totality of existing human data do not appear to show significant clinical advantages of continuous infusion. A frequently cited Cochrane review from 2005 combining 8 randomized trials comparing continuous infusion to bolus injection of loop diuretics in 254 patients with HF concluded that continuous infusion is more effective and has fewer adverse effects than intermittent doses, including decreased mortality.4 However, these differences were entirely driven by the inclusion of a single study of 107 patients for whom the “continuous infusion” arm lasted only 30 minutes twice a day and was confounded by the concomitant infusion of hypertonic saline.8 Excluding the hypertonic saline study, meta-analysis of the other 7 studies (of which the largest included 40 patients) did not show significant differences in urine output, rates of hypokalemia, or changes in serum creatinine.4,9 Studies of bolus versus continuous dosing of intravenous loop diuretics in patients hospitalized for non-HF causes have also been small and heterogenous and have shown mixed results.10–12
Intermittent dosing offers increased physical freedom for patients, decreased carrier fluid infusion, and ease of preparation and administration. If there are no clinical advantages to continuous infusion of loop diuretics, bolus dosing should be the default choice. However, safety side effects (i.e., hypokalemia and hypotension) may potentially be improved in the continuous infusion strategy. This warrants further examination in larger scale studies, particularly for high-risk patients.
Our study had a number of limitations that should be recognized. The study was small and thus was not powered to detect relatively small but potentially important differences in creatinine, nor was it powered to detect large differences in hard clinical end points. The study was not designed to test noninferiority, and thus the absence of statistical findings of superiority does not necessarily imply equivalence. The study was not blinded, which may introduce bias, although we used objective outcomes measures that should have been relatively unaffected by knowledge of treatment assignment. The study was conducted at a single academic center among a relatively young population of patients with HF, such that the results may not generalize to other settings or populations. Additionally, decisions regarding amount of diuretic and the use of other HF medications may be different at other institutions. Enrollment occurred intermittently over many years, and thus the cohort studied may not represent contemporary treatment of patients with HF, although treatment options for acute decompensated HF have changed little over the past decade. By chance, randomization did not adequately balance baseline covariates between the 2 treatment arms, potentially allowing for confounding of the results.
Existing studies, including the pilot study presented here, have had sample sizes inadequate to definitively establish recommendations for loop diuretic dosing for the care of patients hospitalized for acute decompensated HF. The National Heart, Lung, and Blood Institute’s Heart Failure Clinical Trials Network is completing a randomized, double-blind trial of continuous versus bolus intravenous furosemide in patients hospitalized with HF, which will provide a significant improvement over existing data regarding optimal dosing regimens for loop diuretics.13 Until then, our data do not support one furosemide dosing approach over another.
Acknowledgments
We thank Mona Fiuzat, PharmD, for her assistance is finalizing this report.
References
- 1.Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Allen LA, O’Connor CM. Management of acute decompensated heart failure. Can Med Assoc J. 2007;176:797–805. doi: 10.1503/cmaj.051620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah MR, Stevenson LW. Searching for evidence: refractory questions in advanced heart failure. J Card Fail. 2004;10:210–218. doi: 10.1016/j.cardfail.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Salvador DR, Rey NR, Ramos GC, Punzalan FE. Continuous infusion versus bolus injection of loop diuretics in congestive heart failure. Cochrane Database Syst Rev. 2005:CD003178. doi: 10.1002/14651858.CD003178.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen LA, Hernandez AF, O’Connor CM, Felker GM. End points for clinical trials in acute heart failure syndromes. J Am Coll Cardiol. 2009;53:2248–2258. doi: 10.1016/j.jacc.2008.12.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 7.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 8.Licata G, Di Pasquale P, Parrinello G, Cardinale A, Scandurra A, Follone G, Argano C, Tuttolomondo A, Paterna S. Effects of high-dose furosemide and small-volume hypertonic saline solution infusion in comparison with a high dose of furosemide as bolus in refractory congestive heart failure: long-term effects. Am Heart J. 2003;145:459–466. doi: 10.1067/mhj.2003.166. [DOI] [PubMed] [Google Scholar]
- 9.Dormans TP, van Meyel JJ, Gerlag PG, Tan Y, Russel FG, Smits P. Diuretic efficacy of high dose furosemide in severe heart failure: bolus injection versus continuous infusion. J Am Coll Cardiol. 1996;28:376–382. doi: 10.1016/0735-1097(96)00161-1. [DOI] [PubMed] [Google Scholar]
- 10.Schuller D, Lynch JP, Fine D. Protocol-guided diuretic management: comparison of furosemide by continuous infusion and intermittent bolus. Crit Care Med. 1997;25:1969–1975. doi: 10.1097/00003246-199712000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Gulbis BE, Spencer AP. Efficacy and safety of a furosemide continuous infusion following cardiac surgery. Ann Pharmacother. 2006;40:1797–1803. doi: 10.1345/aph.1G693. [DOI] [PubMed] [Google Scholar]
- 12.Ostermann M, Alvarez G, Sharpe MD, Martin CM. Frusemide administration in critically ill patients by continuous compared to bolus therapy. Nephron Clin Pract. 2007;107:c70–c76. doi: 10.1159/000108641. [DOI] [PubMed] [Google Scholar]
- 13.Felker GM, O’Connor CM, Braunwald E. Loop diuretics in acute decompensated heart failure: necessary? Evil? A necessary evil? Circ Heart Fail. 2009;2:56–62. doi: 10.1161/CIRCHEARTFAILURE.108.821785. [DOI] [PMC free article] [PubMed] [Google Scholar]