Abstract
Behavioral studies have documented a relative advantage in some aspects of visuospatial cognition in autism although it is not consistently found in higher functioning individuals with autism. The purpose of this functional neuroimaging study was to examine the neural activity in high functioning individuals with autism while they performed a block design task that systematically varied with regard to whether a global pattern was present. Participants were 14 adults with high-functioning autism and 14 age and IQ matched typical controls. The task was to identify a missing block in target figures which had either an obvious global shape or was an arbitrary array of blocks. Behavioral results showed intact, but not superior, performance in our participants with autism. A key group difference was that the participants with autism showed reliably greater activation in occipital and parietal regions in both tasks suggesting an increased reliance of the autism group on posterior brain areas to mediate visuospatial tasks. Thus, increased reliance on relatively posterior brain regions in itself may not guarantee superior performance as seen in the present study.
Keywords: Autism, fMRI, global-local processing, block design task
Individuals with autism spectrum disorders (ASD) have been reported to have intact, sometimes superior (faster and more accurate), performance, relative to typically developing (TD) individuals, in tasks involving visuospatial processing. For instance, children with ASD tend to perform better than TD participants on visual search (O’Riordan et al., 2001; Plaisted, O’Riordan, & Baron-Cohen, 1998a; Plaisted et al., 2003), feature discrimination (O’Riordan & Plaisted, 2001; Plaisted, O’Riordan, & Baron-Cohen, 1998b), and the Embedded Figures Test (EFT; Jolliffe & Baron-Cohen, 1997; Ropar & Mitchell, 2001; Shah & Frith, 1983). In tasks that require the detection of visual elements embedded in larger fields, people with autism have been found to show increased reliance on local details (Frith, 1989; Shah and Frith, 1993; Jolliffe and Baron-Cohen, 1997; Mottron et al, 2003; Lahaie et al, 2006) and hence show intact or superior performance relative to controls. Better performance has also been reported in individuals with autism on the block design task (BDT), first by Shah and Frith (1993) and later by others (Caron et al., 2006; Gilchrist et al., 2001; Goldstein et al., 2001; Happé, 1994; Pellicano et al., 2006; Ropar & Mitchell, 2001). Such advantage in visuospatial processing in autism has been best explained by two theoretical accounts, the Weak Central Coherence (WCC) theory (Frith, 1989; Happé, 2013; Happé and Booth, 2008) and the Enhanced Perceptual Functioning (EPF) account (Mottron et al., 2003; 2006). While WCC addresses visual advantage in autism as a byproduct of a deficit in seeing global form, the EPF attributes it to a default local-oriented processing with increased reliance on details.
BDT, developed by Kohs (1923), requires an individual to replicate a target two-dimensional geometric design by assembling a set of component structures. The components are squares (blocks) that are entirely white, entirely black, or are half-white and half-black (The half and half squares are formed by a division by one of the two diagonals of the square or a bisecting horizontal or vertical boundary, and the colors and organization of the surface may vary in specific experiments). The target figure can always be constructed by some arrangement of the nine possible component blocks. Shah and Frith (1993) found that individuals with autism had no advantage in a control condition in which the geometric design to be constructed was segmented such that each component square of the design was individually identifiable. All the participants had to do to construct the design in this condition was to iteratively select the component blocks and put them in the correct location. However, in the conventional presentation condition, the component blocks of the target design perceptually merge into each other forming global patterns that are larger than individual blocks. In this conventional presentation, the participants with autism completed the block design task faster and with fewer errors than the typically developing individuals.
Of late, several research reports have questioned the idea of a universal superiority for individuals with autism in visuospatial tasks, and have alluded to the existence of possible subgroups within the autism spectrum who may perform better relative to others (see de Jong et al., 2009; Edgin & Pennington, 2005). For instance, better performance on the BDT, relative to other tasks, is not found in individuals with high-functioning autism and Asperger syndrome (Kaland, Mortensen, and Smith, 2007; Mayes & Calhoun, 2003; Ropar & Mitchell, 2001; Ehlers et al., 1997). In a comprehensive study on BDT, Caron et al (2006) examined individuals with autism and controls with and without a visuospatial peak. This study showed that while diminished detrimental influence of perceptual coherence on BDT performance is both sensitive and specific to autism, outstanding BDT performance was seen only in a subgroup of individuals with autism. Thus, it is possible that the autism spectrum has some individuals with superior visuospatial ability and some without. In studies that focused on broader phenotypes, superior performance on BDT was not found in parents of individuals with autism (de Jonge et al., 2009; Fombonne et al., 1997; Piven & Palmer, 1997; Szatmari et al., 1993). There are also reports that visuospatial peaks within the autism spectrum are limited to individuals with a language delay (Mottron et al., 2008). Furthermore, visuospatial peak may be a developmental process as such peaks are seen more in children with autism aged 8 years 11 months than in children aged 5 years 5 months (Joseph et al., 2002). Overall, findings from these studies point to the fact that BDT superiority may be characteristic of some but not all individuals on the autism spectrum (Soulieres et al., 2011; Stewart et al., 2009; Caron et al., 2006).
Although visuospatial processing in autism has been extensively studied using behavioral measures, only a few studies have been conducted using neuroimaging measures. Some previous neuroimaging studies used different versions of the embedded figures task (Damarla et al., 2010; Manjaly et al., 2007; Baron-Cohen et al., 2006; Ring et al., 1999), and two other studies (McGrath et al., 2012; Silk et al., 2006) used a mental rotation task. All of these studies found increased activation in participants with autism in the occipital and superior parietal regions and reduced activation mainly in the frontal regions, suggesting more low level, perceptually oriented processing in autism. A recent fMRI study (Spencer et al., 2012) also used EFT and examined the brain responses in autism and control participants along with unaffected siblings of individuals with autism. This study found atypical activation in temporal and frontal regions in autism and in unaffected siblings. Yet another recent study (Liu et al., 2011) used a perceptual line-counting task and found decreased medial prefrontal activation and decreased connectivity of this region with posterior regions in participants with autism. Overall, most of these studies point to an altered neural response in autism despite intact or superior behavioral performance.
The primary goal of the present study was to use functional magnetic resonance imaging (fMRI) to examine the neural activity in high functioning individuals with autism while they performed a BDT that systematically varied with regard to whether a global pattern was present. There was only one previous fMRI study of BDT in autism (Bolte et al., 2008). This study found reduced activation response in autism in neurons in the visual cortex that respond to shape representation, figure-ground, and gestalt organization. Our study is different from the Bolte et al study in terms of the variations in the stimuli and our focus on the whole brain. The present study also differs from previous neuroimaging studies of visuospatial processing in autism in the following ways. The Silk et al (2006) and Ring et al (1999) studies should be considered as exploratory due to the limited number of participants with autism, 6 and 7 respectively. In addition, our study used BDT unlike the EFT in Damarla et al (2010), Spencer et al (2012), and Ring et al (1999), and unlike the mental rotation task in Silk et al (2006). If individuals with autism have a performance advantage in BDTs, an fMRI study has the potential of revealing the neural basis of that advantage.
According to previous reports of locally oriented processing in autism (Shah and Frith, 1993; Caron et al., 2006), the detrimental effect of a global pattern (which can obscure the attributes of a particular location in the design) should have less impact on the individuals with autism as compared to TD controls, due to the hypothesized decreased influence of global information for those with autism. Frith (1993) suggested that weak central coherence (WCC) in those with autism is the basis for the superior performance in BDTs, whereas the EPF model (Mottron et al., 2003; 2006) attributes it to enhanced perceptual ability. However, there may be other factors involved, such as an atypical bias toward local processing with a local to global interference (Booth and Happé, 2010; Rinehart et al., 2000) and a preference toward detecting local targets in divided attention conditions (Plaisted et al., 1999). At the neural level, the EPF model (Mottron et al., 2003; 2006) would predict a local overconnectivity, especially in the occipital areas in ASD. Just et al (2012) also point to autonomy in the posterior brain areas in ASD which mediates the recruitment of the visuospatial route to accomplish tasks. Such a pattern of increased use of posterior brain areas has been found in many neuroimaging studies of a variety of tasks in autism (see Samson et al., 2012). The present study examines the neural bases of visuospatial processing in individuals with autism in the context of a block design task.
Method
Participants
The participants were 14 adults with high-functioning autism and 14 typical controls. All participants were male and were matched for age (t(28) = 0.2, ns), Full Scale IQ (t(28) = 0.23, ns), Verbal IQ (t(28) = 1.14, ns), and Performance IQ (t(28) = 0.89, ns) as determined by the Wechsler Adult Intelligence Scale-III (WAIS-III; Wechsler, 1997) (see Table 1 for demographic information).
Table 1.
Demographic information of the participants
| Autism | Control | ||
|---|---|---|---|
| Age (years) | Mean ± SD | 21.5 ± 5.7 | 21.8 ± 4.0 |
| VIQ | Mean ± SD | 107.6 ± 9.0 | 110.9 ± 6.3 |
| PIQ | Mean ± SD | 110.8 ± 8.6 | 108.1 ± 7.1 |
| FSIQ | Mean ± SD | 110.3 ± 8.0 | 110.9 ± 6.5 |
| Handedness | Right: left | 13: 1 | 12: 2 |
| Gender | Male: Female | 14: 0 | 14: 0 |
The diagnosis of autism was established using two structured research diagnostic instruments, the Autism Diagnostic Interview-Revised (ADI-R; Lord et al., 1994) and the Autism Diagnostic Observation Schedule (ADOS-WPS; Lord et al., 2001), supplemented with expert clinical opinion according to the accepted criteria for high-functioning autism (Minshew, 1996). Potential participants with autism were excluded if they had an identifiable cause for their autism (such as fragile-X syndrome, tuberous sclerosis, or fetal cytomegalovirus infection) or if there was evidence of birth asphyxia, head injury, or a seizure disorder. Exclusionary criteria were based on neurologic history and examination and chromosomal analysis or metabolic testing if indicated.
The control participants were community volunteers recruited to match the autism participants on age, IQ, gender, race, and socioeconomic status of family of origin, as measured by the Hollingshead method (Hollingshead, 1957). Potential control participants were screened by questionnaire, telephone, face-to-face interview, and observation during screening psychometric tests. Exclusionary criteria were evaluated through these procedures and included the following: current or past psychiatric and neurologic disorders, birth injury, developmental delay, school problems, acquired brain injury, learning disabilities, substance abuse, and medical disorders with implications for the central nervous system or those requiring regular medication. Potential control participants were also screened to exclude those with a family history (in parents, siblings, and offspring) of autism, developmental cognitive disorders, affective disorders, anxiety disorders, schizophrenia, obsessive compulsive disorder, substance abuse, or other neurologic or psychiatric disorders thought to have a genetic component. Handedness was determined with the Lateral Dominance Examination from the Halstead-Reitan Neuropsychological Test Battery (Reitan & Wolfson, 1985). One participant with autism and two control participants were left-handed. However, the brain activation data from these left-handers were clearly similar to their respective groups; therefore, the data were not separated by handedness.
Materials and Procedure
During fMRI scanning, the participants were presented with a target design on the left side of the screen (See Figure 1 for an example). The pattern in each figure was made of nine blocks that were all black, all white, or half black and half white (divided into two triangles or two rectangles of equal size). On the right side of the screen, a blank matrix of the same size, with a question mark in the location of one of the nine blocks, specifying the block to be identified within the pattern, was shown. At the bottom of the screen were four answer choices. The participant examined the pattern and the blank matrix and then chose which of the four alternatives matched the cued location. In the global condition, the pattern involved a simple and recognizable object such as a house or a sailboat, as shown in Figure 1A. We chose to call this condition “global” as opposed to “gestalt” since there are clear differences between these two terms (see Brosnan et al., 2004), and the term “global” may fit better for our stimuli. In the random array condition (henceforth random), the design was a random configuration of blocks, with the restriction that the target square was clearly defined on all four sides so that no obvious global pattern was present. In order for the target square to be clearly defined, the borders of the squares around it had contrasting color. An example of the random condition is shown in Figure 1B.
Figure 1.
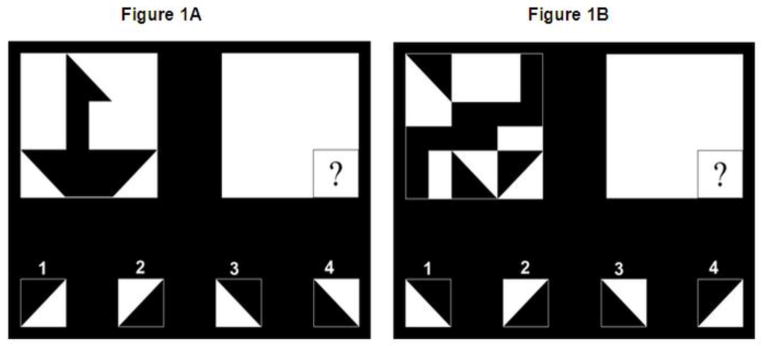
Figure 1A and 1B. Example stimuli from the Global (1A) and the Random (1B) condition. Figures with a target pattern that has an obvious global shape (1A) and a random array target pattern (1B).
At the start of each experimental trial, the pattern on the left side of the screen was presented for 4000 ms (the onset of the first item in each block was time locked to the acquisition of the superior most slice in the prescription). The blank matrix on the right side of the screen appeared 750 ms after the start of the pattern and remained on screen for 3250 ms, disappearing at the same time as the pattern. The answer choices at the bottom of the screen appeared at the same time as the pattern and remained on screen for 7000 ms, thus staying on the screen for 3000 ms after the pattern and blank matrix disappeared. The participant was able to answer any time during the 7000 ms when the answer choices were on the screen. The reaction time data was recorded from the onset of the appearance of the figure on the left side of the screen. The next trial started immediately. Trials of each condition (global or random) were presented in blocks of six. A 12000 ms rest period followed each block during which an asterisk appeared on screen. The blocks alternated between conditions, and counterbalanced across participants. There were three blocks of each condition, for a total of 18 trials in each condition. An asterisk was presented for four 24000-ms fixation periods, evenly distributed among the blocks, with one at the beginning and one after each random condition block. The participants were instructed that the asterisk was “a rest period for you to relax.” On the day of the scan, the participants completed a short practice set of one block of four trials in each condition outside of the scanner to familiarize them with the task.
The stimuli used in the present study is different from the BDT used in Shah and Frith (1993) in a few ways: 1) While their task had segmented and unsegmented designs, our task had global and random patterns without segmentation; 2) The Shah and Frith stimuli had 4 squares/blocks that made up a larger shape, whereas our stimuli consisted of 9 blocks/squares perhaps making our task more complex; and 3) In their task, participants were shown a two-dimensional pattern on a card and were asked to construct the pattern using 4 three-dimensional blocks; whereas our task was computerized and the participants’ task was to identify a missing block in a larger array. We tried to preserve a critical aspect of BDT, which is the ability to break down the visual gestalt into single elements. Block design tasks serve as a measure of general intelligence (Royer et al., 1984), and as indicators of visualization ability (Snow, Kyllonen, & Marshalek, 1984). In Koh’s Block Design as well as in our version, the participant may rely on analytic (the displayed design is mentally segmented into units) or holistic (the design is viewed as a whole and is not differentiated into units) strategies to solve the problem at hand. Thus, the BDT used in our study, in addition to testing global and analytical skills, tests problem-solving in general.
fMRI Procedures
The data were collected using a Siemens Allegra 3.0T scanner (Siemens Inc., Erlangen, Germany) at the Brain Imaging Research Center (BIRC) of Carnegie Mellon University and the University of Pittsburgh. The study was performed with a gradient echo, EPI sequence with TR = 1000 ms, TE = 30 ms and a 60° flip angle. Seventeen oblique-axial slices were acquired; each slice was 5-mm thick with a gap of 1-mm between slices. The acquisition matrix was 64 × 64 with 3.125 mm × 3.125 mm × 5 mm voxels.
fMRI Analyses - Distribution of activation
To compare the participating groups in terms of the distribution of activation, the data were analyzed using SPM2 (Wellcome Department of Cognitive Neurology, London). Images were corrected for slice acquisition timing, motion-corrected, normalized to the Montreal Neurological Institute (MNI) template, resampled to 2×2×2 mm voxels, and smoothed with an 8-mm Gaussian kernel to decrease spatial noise. Statistical analyses were performed on individual and group data by using the general linear model and Gaussian random field theory as implemented in SPM2 (Friston et al., 1995). Group analyses were performed using a random-effects model. Statistical maps were superimposed on normalized T1-weighted images. An uncorrected height threshold of t = 3.36 (P = 0.001) and an extent threshold of twenty-four 8 mm3 voxels was used.
fMRI Analyses - Functional connectivity
The functional connectivity was computed (separately for each participant) as a correlation between the average time course of signal intensity of all the activated voxels in each member of a pair of ROIs. Fifteen functional ROIs were defined to encompass the main clusters of activation in the group activation map for each group in both experimental conditions versus fixation (global and random). Labels for these 13 ROIs [bilateral middle frontal gyrus (MFG), bilateral superior frontal gyrus (SFG), bilateral insula (INS), the supplementary motor area (SMA), bilateral inferior parietal lobule (IPL), bilateral superior parietal lobule (SPL), and bilateral middle occipital gyrus (MOG)] were assigned with reference to the parcellation of the Montreal Neurological Institute (MNI) single subject T1 weighted dataset carried out by Tzourio-Mazoyer and colleagues (Tzourio-Mazoyer et al., 2002). A sphere was defined for each cluster (with a radius ranging from 5 to 12 mm) that best captured the cluster of activation in the map for each group. The ROIs used in the analysis were the union of the two spheres defined for the two groups. The activation time course extracted for each participant over the activated voxels within the ROI originated from the normalized and smoothed images, which were high-pass filtered and had the linear trend removed. Participants who did not show activation in a given functional ROI were excluded from further analyses involving that ROI. The functional connectivity correlation was computed on the images belonging only to the experimental conditions, so it reflects the synchronization between the activation in two areas while the participant is performing the task and not during the baseline condition. Fisher’s r to z transformation was applied to the correlation coefficients for each participant prior to averaging and statistical comparison of the two groups.
Statistical Analyses
For the behavioral data, reaction time and error rate were recorded for each trial of the experiment. We conducted a 2 (Group: autism vs. control) × 2 (Condition: global vs. random) mixed ANOVA on the reaction time data as well as on the accuracy data separately. This analysis provided the main effect of group, main effect of condition, and group by condition interactions. For the reaction time data, we used data from correct trials. For within-group measure of brain activation, we conducted a one-sample t-test and for between-group activation, a two-sample t-test. Moreover, multiple regression analyses were performed using PIQ and VIQ as covariates to predict activation and functional connectivity, which did not reveal any statistically significant relationships.
Results
Group differences in brain activation
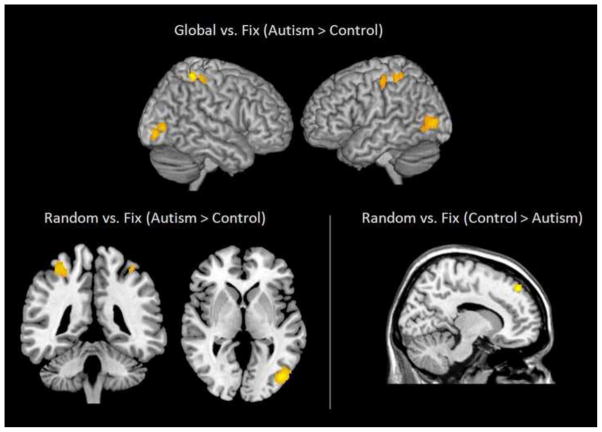
The primary differences in brain activation between the two groups were that the participants with ASD showed reliably more activation (p < 0.001) than the TD participants in bilateral superior parietal and inferior occipital areas while processing global figures, and they showed reliably more activation in the left superior parietal and the right inferior occipital areas while processing random figures (See Figure 2, left panel). Increased activation has been previously found in bilateral superior parietal regions (Han et al., 2004), and in inferior occipital cortex (Fink et al., 1996; Han et al., 2002) when people attended to the local details of a stimulus.
Figure 2.
Between-group comparisons of Global and Random conditions. The participants with autism had greater activation in bilateral inferior occipital and superior parietal regions than the control participants in both conditions. The control participants showed greater activation than participants with autism in medial prefrontal cortex for the Random array condition only (p < 0.001; cluster threshold = 24 voxels).
On the other hand, the participants with autism showed reliably less activation in bilateral superior medial frontal areas as compared to the control group only while processing the random figures (see Figure 2, right). In addition to group differences in activation, functional connectivity between groups was also examined. There was no significant group difference in the mean functional connectivities in any set of inter-lobe functional ROI pairs (e.g., frontal-parietal pairs, frontal-occipital pairs, etc.) nor any set of within-lobe functional ROI pairs (e.g., frontal-frontal pairs, parietal-parietal pairs, etc) in either the global nor in the random condition.
Distribution of activation within groups
Overall, both groups recruited similar occipital and parietal brain regions across the global and random tasks (when contrasted with the fixation baseline), suggesting the use of visual, and spatial processes to accomplish the two tasks. However, the two groups seem to differ in the recruitment of more anterior regions (p < 0.05, familywise error corrected). While control participants showed activation in middle cingulate gyrus, supplementary motor area, and inferior frontal gyrus in both Global and Random conditions, such activation was absent in the autism group (see table 2 and supplementary figure S1 for details).
Table 2.
Activation peaks for Autism and Control Groups for Global vs. Fixation and Random vs. Fixation Contrasts at p<0.05, familywise error corrected (FWE) threshold.
| Global vs. Fixation
| |||||||
|---|---|---|---|---|---|---|---|
| Control Group
| |||||||
| Region | Hem | Cluster | x | y | z | t | p (FWE) corr. |
| Inferior Occipital Gyrus | R | 36085 | 36 | −86 | −14 | 6.42 | 0.000 |
| Middle Cingulate Gyrus | L | 5716 | −2 | −42 | 34 | 6.23 | 0.000 |
| Supplementary Motor Area | R | 2119 | 6 | 20 | 46 | 5.49 | 0.004 |
| Superior Temporal Gyrus | R | 428 | 62 | −60 | 18 | 5.40 | 0.006 |
| Inferior Temporal Gyrus | L | 797 | −54 | −18 | −26 | 5.07 | 0.036 |
| Middle Frontal Gyrus | R | 791 | 34 | 0 | 58 | 4.99 | 0.050 |
|
| |||||||
|
Autism Group
| |||||||
| Middle Occipital Gyrus | L | 37282 | −38 | −86 | 4 | 6.59 | 0.000 |
| Precentral Gyrus | R | 2451 | 26 | 2 | 60 | 5.33 | 0.009 |
| Superior Temporal Gyrus | L | 775 | −46 | −60 | 20 | 4.92 | 0.046 |
| Random vs. Fixation
| |||||||
|---|---|---|---|---|---|---|---|
| Control Group
| |||||||
| Region | Hem | Cluster | x | y | z | t | p (FWE) corr. |
| Lingual Gyrus | R | 29830 | 18 | −98 | −12 | 6.63 | 0.000 |
| Supplementary Motor Area | L | 1233 | −2 | 20 | 44 | 5.86 | 0.000 |
| Middle Cingulate Gyrus | L | 1156 | −8 | −22 | 48 | 5.50 | 0.004 |
| Thalamus | R | 1522 | 22 | −26 | 2 | 5.40 | 0.006 |
| Inferior Frontal Gyrus | R | 124 | 54 | 36 | −2 | 5.11 | 0.030 |
|
| |||||||
|
Autism Group
| |||||||
| Middle Occipital Gyrus | L | 26017 | −36 | −86 | 8 | 6.57 | 0.000 |
| Thalamus | L | 347 | −22 | −30 | 2 | 5.15 | 0.017 |
When global and random conditions were contrasted with each other (global vs. random), both autism and control groups showed significantly more occipital and parietal activation, especially in bilateral calcarine sulci and bilateral intraparietal sulcus (IPS) (see Figure 3). In addition, there was also activation in left and right middle frontal gyri (MFG) and bilateral thalami. The random vs. global contrast, on the other hand, revealed activation primarily in midline cortical structures, such as medial prefrontal cortex (MPFC), precuneus, and posterior cingulate, less so in participants with autism than in typical participants.
Figure 3.
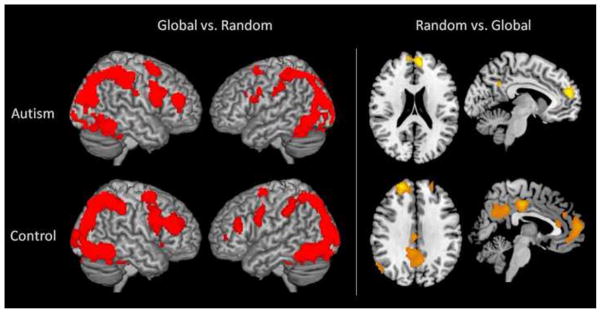
Within-group activation patterns when global and random tasks were compared in both directions (p < 0.001; cluster threshold = 24 voxels). Left panel: similar activation patterns between the two groups when global pattern is contrasted Random array; Right Panel: More activation in the midline cortical structures in controls, relative to autism, when random array is contrasted with global pattern.
Behavioral Results
There was intact ability in the participants with autism for processing both the global and random BDT stimuli. A 2 (Group: autism vs. control) × 2 (Condition: global vs. random) mixed ANOVA on the reaction time data revealed a main effect of condition [F (1, 26) = 31.11, p<.001]. For both groups, the response times were slower in the global condition than in the random condition. However, there was no group difference [F (1, 26) = 1, 18, p = 0.29] nor interaction between group and condition [F (1, 26) = 2.13, p = 0.16]. To account for variability in the RT data, we repeated this analysis by transforming the RT values to its natural logarithm. However, the results of this analysis were the same as the one mentioned above. The error rates yielded a similar pattern, such that for both groups the error rates were higher in the global condition, F (1,26) = 8.99, p < 0.01]; there was a marginally significant main effect of group [F(1,26) = 3.72, p = 0.06] and no interaction between group and condition [F<1]. The behavioral data are presented in Table 3.
Table 3.
Mean response times and error rates (SD: standard deviation).
| Reaction Time (ms) | Error Rate (%) | ||||
|---|---|---|---|---|---|
| Global | Random | Global | Random | ||
| Autism | Mean (SD) | 4077 (621) | 3880 (516) | 10 (0.08) | 5 (0.08) |
| Control | Mean (SD) | 3934 (480) | 3598 (499) | 6 (0.06) | 2 (0.03) |
Since there are indications in the literature that visuospatial abilities in autism may be applicable to only a subgroup of individuals, we examined that possibility in our participant group by looking at the participants’ scores on subtests of the WASI, the test used to measure the intelligence level of the participants. In particular, we examined the raw scores as well as t-scores for the Block Design subtest. The test scores did not reveal the existence of any subgroup in ASD. Nor did it reveal any statistical difference between the ASD and TD groups (see Table 4).
Table 4.
Raw and T scores of the Block design subtest of WASI for both participant groups. Raw score: t(26)=0.78; p > 0.05; T Score: t(26)=1.14; p > 0.05.
| Block Design Subtest | ||||
|---|---|---|---|---|
|
| ||||
| Raw Score | T-Score | |||
|
| ||||
| ASD | TD | ASD | TD | |
|
| ||||
| 52 | 56 | 57 | 57 | |
| 54 | 59 | 64 | 61 | |
| 62 | 52 | 62 | 54 | |
| 56 | 43 | 58 | 49 | |
| 57 | 40 | 58 | 48 | |
| 66 | 45 | 65 | 50 | |
| 25 | 53 | 39 | 56 | |
| 45 | 50 | 51 | 53 | |
| 66 | 56 | 64 | 57 | |
| 37 | 60 | 45 | 61 | |
| 48 | 46 | 52 | 50 | |
| 69 | 49 | 70 | 56 | |
| 46 | 44 | 52 | 52 | |
| 57 | 47 | 58 | 51 | |
|
| ||||
| Mean | 52.5 | 50.2 | 56.7 | 54.2 |
|
| ||||
| S.D. | 12.5 | 6.4 | 8.7 | 4.3 |
|
| ||||
| S.E. | 3.5 | 1.8 | 2.4 | 1.2 |
Discussion
The main findings of this study are these: first, the participants with autism had intact, but not enhanced, behavioral performance on a variation of the BDT. This performance on the task created for the fMRI study was consistent with the lack of a peak on the BDT subtest of the WASI for the participants with autism. Second, similar behavioral performance across the participant groups was accompanied by differences in levels of brain activation. Specifically, the autism group had relatively increased activation in posterior occipital and parietal regions as compared to the TD group, whereas the TD group was also using frontal resources. Third, there were no statistically significant group differences in functional connectivity. Therefore, the difference for the autism group was in the relative use of neural resources in particular regions (less frontal/more posterior) not in the coordination of these regions.
Although it is difficult to delineate the specific relationship between behavioral performance and levels of brain activation, an increase in activation has frequently been interpreted as indicating increased use of the processing resources in the relevant brain regions (Aue, Lavelle, & Cacioppo, 2009). Therefore, the increase in activation in the posterior occipital and parietal regions by the autism group could suggest that they were using the resources of these areas to a greater extent than the TD group, but for a similar behavioral result. The increased reliance on posterior brain areas did not translate into superior performance in our participants with autism. This result is consistent with the results of a number of other neuroimaging studies of visuospatial processing in individuals with autism. For example, in a meta-analysis of 26 neuroimaging studies of autism that utilized visual stimuli, Samson et al (2012) found no significant group difference in performance in 69% of the studies. They also found a generally higher task-related activity in posterior brain areas and lower activity in frontal cortex just as in the current study. Previous studies have found posterior parietal cortex (e.g., Donner et al., 2003; Coull et al., 2003) as well as a network of parietal and occipital areas (Nobre et al., 2003) to be extensively involved in visual search, a task that can be accomplished with low-level perceptual processing. The greater activation of posterior regions by the autism group may be reflective of an increased reliance by this group on low-level perceptual processing centers to accomplish this task.
The additional recruitment of posterior processing resources by the autism group occurs in the context of a lack of recruitment of frontal regions. The TD group relied on a frontal-parietal-occipital network to accomplish the task; the autism group had a similar level of coordination with the frontal regions (as indicated by the lack of a difference in functional connectivity) but did not have the expected increased processing in frontal regions. The autism group was using more posterior and less frontal resources but was coordinating the timing of the activation between these areas to a similar extent as indicated by the lack of a difference in functional connectivity between the two groups. In the BDT task, the resources that were recruited were adequate and no difference occurred in behavioral performance. However, the marginally different error rate suggests that the autism group may have been working less not more efficiently than the age and IQ-matched TD controls (thus the increase in activation in the posterior regions) for a similar behavioral result.
Direct comparison of processing global and random figures generated relatively more activation for global figures in both participant groups. This may be because of a) the associated meanings and thought processes generated by the global figure in each picture, e.g., boat, or bird, and/or b) the difficulty in mentally segmenting the global pattern in order to determine the location of the interrogated block in the intact figure. On the other hand, when random stimuli were compared to global ones, the control participants exhibited more activation in the bilateral superior medial frontal areas (MPFC) than the participants with autism. The MPFC has been found to be involved in stimulus-oriented and in stimulus-independent attention, especially in tasks involving navigating around a visually presented shape versus imagining the same shape and navigating around it (Gilbert, Frith, and Burgess, 2005; Gilbert et al., 2007; Burgess, Dumontheil, & Gilbert, 2007). Therefore the MPFC in control participants may be mediating this constant search for meaning and coherence. Activation in MPFC has also been found when participants switch from one way of performing a task to another (Rushworth et al., 2002). In the present study, the control participants may be shifting back and forth between global and local oriented perception of the visual stimuli to identify the missing block. The lack of activation in MPFC in participants with autism is consistent with the findings of a recent fMRI study of a lower-level perceptual line-counting task (Liu et al., 2011) and also with a generally limited level of activation in frontal areas in autism (Samson et al., 2012).
In sum, an atypical pattern of resource allocation (more posterior less frontal) during visuospatial processing may be reflective of the nature of cognitive and neural mechanisms in individuals with autism. The increased activity in posterior areas and intact connectivity we found may be a pattern that is indicative of the stronger role of perceptual processes in autism. Alternately, it may be indicative of a processing network that is reflective of aberrant frontal processing with a resultant reliance on posterior processing resources. Either explanation is consistent with the results of the current study.
Altered brain response with intact behavioral performance, in autism in the present study is consistent with previous studies of visual processing in autism (McGrath et al., 2012; Damarla et al., 2010; Lee et al., 2007; Manjaly et al., 2007; Silk et al., 2006) and generates interesting questions. The lack of superior performance in autism may point to the heterogeneity in autism and the possible existence of individuals within the autism group with differing visuospatial abilities (Soulières et al., 2011; Stewart et al., 2009; Caron et al., 2006). We tested this in our sample with different indices but did not find any visuospatial peaks in autism. For instance, participants in both groups were matched on verbal, performance, and full scale IQs. In addition, our regression analysis using PIQ as a covariate on behavioral and fMRI data did not result in statistically significant relationship. We also examined the block design subtest of WASI and it did not show any significant variation either in ASD or in TD participant groups. It should be noted that such peaks were not seen in some previous studies conducted by our group (Williams et al., 2008; Minshew et al., 2005).
There have been several neuroimaging studies that have provided evidence for decreased frontal-posterior functional connectivity in more complex cognitive tasks in people with autism (e.g. Just et al., 2004, 2007; Kana et al., 2006, 2007, 2009; Koshino et al., 2008; Mason et al., 2008). The findings pertaining to functional connectivity in autism from the current study are different from previous studies in that there are no overall group differences. It is possible that the visual search task in the present study may be one where the proposed enhanced perceptual functioning in autism may manifest (Mottron et al., 2006) resulting in intact connectivity. The BDT used in the present study may be relatively less affected by other higher cognitive components like executive planning and motor control, at least for the participants with autism. It is possible that the default strategy the individuals with autism may use (one that is primarily based on the recruitment of occipital and parietal areas) may work in this task more so than a complex sentence comprehension task or a problem-solving task. Thus, the overall stronger engagement of the visual cortex and parietal areas by participants with autism entailed greater activation and intact coordination. It should be noted that a recent magnetoencephalography study (Khan et al., 2013) of face viewing showed reduced connectivity in local as well as long-range connections in autism.
The EPF account (Mottron et al., 2006) and an updated WCC account (Happé, 2013; Happé & Booth, 2008; Happe & Frith, 2006) attribute visuospatial advantage in autism as due to superiority in local processing and to a bias towards local processing in people with autism. At the brain level, such processing may underlie greater recruitment of relatively posterior areas. Mottron et al (2006) suggest that perception in autism may be different from that in typical individuals in overall superior functioning, involvement, and autonomy of posterior regions like parietal and occipital cortices. The results of the present study, increased parietal and occipital area activation and intact functional connectivity, supports that and can be the result of a more autonomous posterior brain functioning in our participants with autism (Just et al., 2012). The increased recruitment of posterior regions we found in autism can also be the result of reduced top-down feedback as predicted by WCC and the cortical underconnectivity theory (Just et al., 2004; Kana et al., 2006). It has been found that brain activation and connectivity in lower brain regions can be altered by top-down feedback (Friston & Buechel, 2000) and a lack of which may result in abnormal connectivity (Frith, 2004). Hence EPF, WCC, and underconnectivity theory suggest that a posterior-oriented brain functioning may underlie intact or superior visuospatial processing in autism.
Although this study revealed important new information about visuospatial processing in autism, there are a few limitations. First, the target figures in this study were presented at the left visual field. While we do not think this would have had a significant impact on the results, future studies may account for this; and second, although we matched the black and white blocks in global and random stimuli, in some stimuli we could not achieve 100% matching due to the constraints of a given shape.
Future studies should examine what would happen if the task demand could be metrically increased. Would there be a point at which the increased activation or use of local processing resources would not be sufficient and would the autism group be able to increase the coordination with frontal areas under this constraint or would we begin to see the decrease in functional connectivity that has been found in more demanding tasks? It might also be informative to seek out individuals with autism who display the reported BDT peak to compare their pattern of processing relative to individuals with autism, such as those in the current study, who did not display this behavioral pattern. In summary, this study has not only looked at processing during a visuospatial task but has provided information about what happens with functional connectivity during a task that the processing resources the group with autism have are adequate for the task and the task can be performed without extensive recruitment of frontal resources. These are two elements that should be systematically varied in future studies.
Supplementary Material
Within-group brain activation for the contrasts Global vs. Fixation and Random vs. Fixation for Autism and Control groups (p < 0.05, FWE corrected).
Highlights.
fMRI study examined visuospatial processing in autism
We used a missing block identification task using visual designs
Similar behavioral performance between autism and control participants
Increased posterior (occipital and parietal) activity in participants with autism
Enhanced posterior brain recruitment may underlie visual processing in autism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aue T, Lavelle LA, Cacioppo JT. Great expectations: What can fMRI research tell us about psychological phenomena? International Journal of Psychophysiology. 2009;73(1):10–16. doi: 10.1016/j.ijpsycho.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Bolte S, Hubl D, Dierks T, Holtmann M, Poistka F. An fMRI-study of locally oriented perception in autism: altered early visual processing of the block design test. Journal of Neural Transmission. 2008;115:545–552. doi: 10.1007/s00702-007-0850-1. [DOI] [PubMed] [Google Scholar]
- Brosnan MJ, Scott FJ, Fox S, Pye J. Gestalt processing in autism: Failure to process perceptual relationships and the implications for contextual understanding. Journal of Child Psychology and Psychiatry. 2004;45:459–469. doi: 10.1111/j.1469-7610.2004.00237.x. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends in Cognitive Science. 2007;11:290–298. doi: 10.1016/j.tics.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Caron MJ, Mottron L, Berthiaume C, Dawson M. Cognitive mechanisms, specificity and neural underpinnings of visuospatial peaks in autism. Brain. 2006;129:1789–1802. doi: 10.1093/brain/awl072. [DOI] [PubMed] [Google Scholar]
- Coull JT, Walsh V, Frith CD, Nobre AC. Distinct neural substrates for visual search amongst spatial versus temporal distractors. Cognitive Brain Research. 2003;17:368–379. doi: 10.1016/s0926-6410(03)00138-1. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Petit L, Maisog JM, Ungerleider LG, Haxby JV. An area specialized for spatial working memory in human frontal cortex. Science. 1998;279:1347–51. doi: 10.1126/science.279.5355.1347. [DOI] [PubMed] [Google Scholar]
- Damarla SR, Keller T, Kana RK, Cherkassky V, Williams D, Minshew N, Just M. Cortical underconnectivity coupled with preserved visuospatial cognition in autism: Evidence from an fMRI study of an embedded figures task. Autism Research. 2010;3:1–7. doi: 10.1002/aur.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge M, Kemner C, Naber F, van Engeland H. Block design reconstruction skills: Not a good candidate for an endophenotypic marker in autism research. European Child & Adolescent Psychiatry. 2009;18(4):197–205. doi: 10.1007/s00787-008-0708-6. [DOI] [PubMed] [Google Scholar]
- Donner TH, Kettermann A, Diesch E, Villringer A, Brandt SA. Parietal activation during visual search in the absence of multiple distractors. NeuroReport. 2003;14:2257–2261. doi: 10.1097/00001756-200312020-00024. [DOI] [PubMed] [Google Scholar]
- Edgin JO, Pennington BF. Spatial Cognition in Autism Spectrum Disorders: Superior, Impaired, Or Just Intact? Journal of Autism and Developmental Disorders. 2005;35:729–745. doi: 10.1007/s10803-005-0020-y. [DOI] [PubMed] [Google Scholar]
- Ehlers S, Nyden A, Gillberg C, Sandberg AD, Dahlgren SO, Hjelmquist E, et al. Asperger syndrome, autism and attention disorders: A comparative study of the cognitive profiles of 120 children. Journal of Child Psychology and Psychiatry. 1997;38:207–217. doi: 10.1111/j.1469-7610.1997.tb01855.x. [DOI] [PubMed] [Google Scholar]
- Fink GR, Halligan PW, Marshall JC, Frith CD, Frackowiak RSJ, Dolan RJ. Where in the brain does visual attention select the forest and the trees? Nature. 1996;382:626–628. doi: 10.1038/382626a0. [DOI] [PubMed] [Google Scholar]
- Fombonne E, Bolton P, Prior J, Jordan H, Rutter M. A family study of autism: Cognitive patterns and levels in parents and siblings. Journal of Child Psychology and Psychiatry. 1997;38:667–683. doi: 10.1111/j.1469-7610.1997.tb01694.x. [DOI] [PubMed] [Google Scholar]
- Freeman BJ, Ritvo ER, Needleman R, Yokota A. The stability of cognitive and linguistic parameters in autism. A five-year prospective study. Journal of American Academy of Child Psychiatry. 1985;24:459–464. doi: 10.1016/s0002-7138(09)60565-3. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C. Attentional modulation of effective connectivity from V2 to V5/MT in humans. Proceedings of the National Academy of Sciences. 2000;97:75912–75916. doi: 10.1073/pnas.97.13.7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KA, Holmes AP, Worsley KJ, Poline JB, Frith C, Frackowiak RSJ. Statistical Parametric Maps in Functional Imaging: A General Linear Approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Frith C. What do imaging studies tell us about the neural basis of autism. In: Bock, Goode, editors. Autism: Neural basis and treatment possibilities. Chichester, U.K: John Wiley & Sons; 2003. pp. 149–176. [PubMed] [Google Scholar]
- Frith C. Is autism a disconnection disorder? Lancet Neurology. 2004;3:577. doi: 10.1016/S1474-4422(04)00875-0. [DOI] [PubMed] [Google Scholar]
- Frith U. Autism: Explaining the enigma. Oxford: Blackwell; 1989. [Google Scholar]
- Gilbert SJ, Frith CD, Burgess PW. Involvement of rostral prefrontal cortex in selection between stimulus-oriented and stimulus-independent thought. European Journal of Neuroscience. 2005;21:1423–31. doi: 10.1111/j.1460-9568.2005.03981.x. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Williamson IDM, Dumontheil I, Simons JS, Frith CD, Burgess PW. Distinct regions of medial rostral prefrontal cortex supporting social and non-social functions. Social Cognitive and Affective Neuroscience. 2007;2:217–26. doi: 10.1093/scan/nsm014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist A, Green J, Cox A, Burton D, Rutter M, Le Couteur A. Development and current functioning in adolescents with Asperger syndrome: A comparative study. Journal of Child Psychology and Psychiatry. 2001;42:227–240. [PubMed] [Google Scholar]
- Goldstein G, Beers SR, Siegel DJ, Minshew NJ. A comparison of WAIS-R profiles in adults with high-functioning autism or differing subtypes of learning disability. Applied Neuropsychology. 2001;8:148–154. doi: 10.1207/S15324826AN0803_3. [DOI] [PubMed] [Google Scholar]
- Han S, Jiang Y, Gu H. Neural substrates differentiating global/local processing of bilateral visual inputs. Human Brain Mapping. 2004;22:321–328. doi: 10.1002/hbm.20044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Weaver J, Murray S, Yund EW, Woods DL. Hemispheric asymmetry in global/local processing: Effects of stimulus position and spatial frequency. NeuroImage. 2002;17:1290–1299. doi: 10.1006/nimg.2002.1255. [DOI] [PubMed] [Google Scholar]
- Happé FGE. Weak Central Coherence. In: Volkmar FE, editor. The Encyclopedia of Autism Spectrum Disorders. NY: Springer; 2013. pp. 3344–3346. [Google Scholar]
- Happé FGE. An Advanced Test of Theory of Mind: Understanding of Story Characters’ Thoughts and Feelings by Able Autistic, Mentally Handicapped and Normal Children and Adults. Journal of Autism and Developmental Disorders. 1994;24:129–154. doi: 10.1007/BF02172093. [DOI] [PubMed] [Google Scholar]
- Happé FGE, Booth RDL. The Power of the Positive: revisiting weak coherence in autism spectrum disorders. The Quarterly Journal of Experimental Psychology. 2008;61(1):50–63. doi: 10.1080/17470210701508731. [DOI] [PubMed] [Google Scholar]
- Happé FGE, Frith U. The weak coherence account: Detail-focused cognitive style in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2006;36:5–25. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Petit L, Ungerleider LG, Courtney SM. Distinguishing the functional roles of multiple regions in distributed neural systems for visual working memory. Neuroimage. 2000;11:380–91. doi: 10.1006/nimg.2000.0592. [DOI] [PubMed] [Google Scholar]
- Hill EL, Frith U. Understanding autism: Insights from mind and brain. Philosophical transactions of the Royal Society B: Biological Sciences. 2003;358:281–289. doi: 10.1098/rstb.2002.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead A. Unpublished manuscript. New Haven, CT: Yale University; 1957. Four-factor index of social status. [Google Scholar]
- Jolliffe T, Baron-Cohen S. Are people with autism and Asperger syndrome faster than normal on the Embedded Figures Test? Journal of Child Psychology & Psychiatry. 1997;38:527–534. doi: 10.1111/j.1469-7610.1997.tb01539.x. [DOI] [PubMed] [Google Scholar]
- Joseph RM, Tager-Flusberg H, Lord C. Cognitive profiles and social communicative functioning in children with autism spectrum disorder. J Child Psychol Psychiatry. 2002;43:807–21. doi: 10.1111/1469-7610.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: Evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: Evidence from an fMRI study of an executive function task and corpus callosum morphometry. Cerebral Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Keller TA, Malave VL, Kana RK, Varma S. Autism as a neural systems disorder: a theory of frontal-posterior underconnectivity. Neuroscience and Biobehavioral Reviews. 2012;36(4):1292–313. doi: 10.1016/j.neubiorev.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaland N, Mortensen EL, Smith L. Disembedding performance in children and adolescents with Asperger syndrome or high-functioning autism. Autism. 2007;11:81–92. doi: 10.1177/1362361307070988. [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: Thinking in pictures with decreased functional connectivity. Brain. 2006;129:2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Minshew NJ, Just MA. Inhibitory control in high functioning autism: Decreased activation and underconnectivity in inhibition networks. Biological Psychiatry. 2007;62:198–206. doi: 10.1016/j.biopsych.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Atypical frontal-posterior synchronization of Theory of Mind regions in autism during mental state attribution. Social Neuroscience. 2009;4:135–152. doi: 10.1080/17470910802198510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Gramfort A, Shetty NR, Kitzbilchler MG, Ganesan S, Kenet T, et al. Local and Long-range Functional Connectivity is reduced in concert in Autism Spectrum Disorders. PNAS. 2013;110(8):3107–3112. doi: 10.1073/pnas.1214533110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshino H, Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. fMRI investigation of working memory for faces in autism: Visual coding and underconnectivity with frontal areas. Cerebral Cortex. 2008;18:389–300. doi: 10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohs SC. Intelligence measurement: A psychological and statistical study based upon the block-design tests. New York: MacMillan; 1923. pp. 39–63. [Google Scholar]
- Lahaie A, Mottron L, Arguin M, Berthiaume C, Jemel B, et al. Face perception in high-functioning autistic adults: Evidence for superior processing of face parts, not for a configural face-processing deficit. Neuropsychology. 2006;20:30–41. doi: 10.1037/0894-4105.20.1.30. [DOI] [PubMed] [Google Scholar]
- Liu Y, Cherkassky VL, Minshew NJ, Just MA. Autonomy of Lower-level Perception from Global Processing in Autism: Evidence from brain activation and functional connectivity. Neuropsychologia. 2011;49:2105–2111. doi: 10.1016/j.neuropsychologia.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule. Los Angeles: Western Psychological Services; 2001. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Manjaly ZM, Bruning N, Neufang S, Stephan KE, Brieber S, Marshall JC, Kamp-Becker I, Remschmidt H, Herpertz-Dahlmann B, Konrad K, Fink GR. Neurophysiological correlates of relatively enhanced local visual search in autistic adolescents. Neuroimage. 2007;35:283–291. doi: 10.1016/j.neuroimage.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RA, Williams DL, Kana RK, Minshew N, Just MA. Theory of Mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia. 2008;46:269–280. doi: 10.1016/j.neuropsychologia.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes SD, Calhoun SL. Analysis of WISC-II, Stanford-Binet: IV, and academic achievement test scores in children with autism. Journal of Autism and Developmental Disorders. 2003;33:329–341. doi: 10.1023/a:1024462719081. [DOI] [PubMed] [Google Scholar]
- McGrath J, Johnson K, Ecker C, O’Hanlon E, Gill M, Gallagher L, Garavan H. Atypical Visuospatial Processing in Autism: Insights from functional connectivity analysis. Autism Research. 2012 doi: 10.1002/aur.1245. [DOI] [PubMed] [Google Scholar]
- Minshew NJ. Autism. In: Berg B, editor. Principles of child neurology. New York: McGraw-Hill; 1996. pp. 1713–1729. [Google Scholar]
- Minshew NJ, Turner CA, Goldstein G. The application of short forms of the Wechsler Intelligence Scales in adults and children with high functioning autism. Journal of Autism and Developmental Disorders. 2005;35:45–52. doi: 10.1007/s10803-004-1030-x. [DOI] [PubMed] [Google Scholar]
- Mottron L, Burack J. Enhanced perceptual functioning in the development of autism. In: Burack, Charman, Yirmiya, Zelazo, editors. The development of autism: Perspectives from theory and research. Mahwah, NJ: Erlbaum; 2001. pp. 131–148. [Google Scholar]
- Mottron L, Burack JA, Iarocci G, Belleville S, Enns JT. Locally oriented perception with intact global processing among adolescents with high-functioning autism: Evidence from multiple paradigms. Journal of Child Psychology and Psychiatry. 2003;44:904–913. doi: 10.1111/1469-7610.00174. [DOI] [PubMed] [Google Scholar]
- Mottron L, Dawson M, Soulières I, Hubert B, Burack JA. Enhanced perceptual functioning in autism: An update, and eight principle of autistic perception. Journal of Autism and Developmental Disorders. 2006;36:27–43. doi: 10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- Mottron L, Peretz I, Ménard E. Local and global processing of music in high-functioning persons with autism. Journal of Child Psychology and Psychiatry. 2000;41:1057–1068. [PubMed] [Google Scholar]
- Mottron L, Soulieres I, Simard-Meileur AA, Dawson M. Peaks of ability as a subtyping tool for autism. International Meeting for Autism Research; London, England. 2008. [Google Scholar]
- Nobre AT, Coull JT, Walsh V, Frith CD. Brain Activations during Visual Search: Contributions of Search Efficiency versus Feature Binding. Neuroimage. 2003;18:91–103. doi: 10.1006/nimg.2002.1329. [DOI] [PubMed] [Google Scholar]
- O’Riordan MA, Plaisted KC, Driver J, Baron-Cohen S. Superior visual search in autism. Journal of Experimental Psychology: Human Perception and Performance. 2001;27:719–730. doi: 10.1037//0096-1523.27.3.719. [DOI] [PubMed] [Google Scholar]
- O’Riordan M, Plaisted K. Enhanced discrimination in autism. The Quarterly Journal of Experimental Psychology. 2001;54:961–79. doi: 10.1080/713756000. [DOI] [PubMed] [Google Scholar]
- Pellicano E, Gibson L, Mayberry M, Durkin K, Badcock DR. Abnormal global processing along the dorsal visual pathway in autism: A possible mechanism for weak central coherence. Neuropsychologia. 2005;43:1044–1053. doi: 10.1016/j.neuropsychologia.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philosophical Transactions of the Royal Society B: Biological Sciences. 2005;360:781–95. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piven J, Palmer P. Cognitive deficits in parents from multiple-incidence autism families. Journal of Child Psychology and Psychiatry. 1997;41:491–502. doi: 10.1111/j.1469-7610.1997.tb01618.x. [DOI] [PubMed] [Google Scholar]
- Plaisted K, O’Riordan M, Baron-Cohen S. Enhanced discrimination of novel, highly similar stimuli by adults with autism during a perceptual learning task. Journal of Child Psychology and Psychiatry. 1998a;39:765–775. [PubMed] [Google Scholar]
- Plaisted K, O’Riordan M, Baron-Cohen S. Enhanced discrimination of novel, highly similar stimuli by adults with autism during a perceptual learning task. Journal of Child Psychology and Psychiatry. 1998b;39:765–775. [PubMed] [Google Scholar]
- Plaisted K, Saksida L, Alcantara J, Weisblatt E. Towards an understanding of the mechanisms of weak central coherence effects: Experiments in visual configural learning and auditory perception. Philosophical Transactions of the Royal Society of London, Series B. 2003;358:375–386. doi: 10.1098/rstb.2002.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaisted K, Swettenham J, Rees L. Children with autism show local precedence in a divided attention task and global precedence in a selective attention task. Journal of Child Psychology and Psychiatry. 1999;40:733–742. [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and clinical interpretation. Tucson, AZ: Neuropsychology Press; 1985. [Google Scholar]
- Rinehart NJ, Bradshaw JL, Moss SA, Brereton AV, Tonge BJ. Atypical interference of local detail on global processing in high-functioning autism and Asperger’s disorder. Journal of Child Psychology and Psychiatry. 2000;41:769–778. [PubMed] [Google Scholar]
- Ring H, Baron-Cohen S, Williams S, Wheelwright S, Bullmore E, Brammer M, Andrew C. Cerebral correlates of preserved cognitive skills in autism. A functional MRI study of Embedded Figures task performance. Brain. 1999;122:1305–1315. doi: 10.1093/brain/122.7.1305. [DOI] [PubMed] [Google Scholar]
- Ropar D, Mitchell P. Susceptibility to illusions and performance on visuospatial tasks in individuals with autism. Journal of child Psychology and Psychiatry. 2001;42:539–549. [PubMed] [Google Scholar]
- Samson F, Mottron L, Soulieres I, Zeffiro TA. Enhanced Visual Functioning in Autism: an ALE meta-analysis. Human Brain Mapping. 2012;33:1553–1581. doi: 10.1002/hbm.21307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Frith U. An islet of ability in autistic children: a research note. Journal of Child Psychology and Psychiatry. 1983;24:613–620. doi: 10.1111/j.1469-7610.1983.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Shah A, Frith U. Why do autistic individuals show superior performance on the block design task? Journal of Child Psychology and Psychiatry. 1993;34:1351–1364. doi: 10.1111/j.1469-7610.1993.tb02095.x. [DOI] [PubMed] [Google Scholar]
- Snowling M, Frith U. Comprehension in “hyperlexic” readers. Journal of Experimental Child Psychology. 1986;42:392–415. doi: 10.1016/0022-0965(86)90033-0. [DOI] [PubMed] [Google Scholar]
- Silk TJ, Rinehart N, Bradshaw JL, Tonge B, Egan G, O’Boyle MW, Cunnington R. Visuospatial processing and the function of prefrontal-parietal networks in autism spectrum disorders: a functional MRI study. American Journal of Psychiatry. 2006;163:1440–1443. doi: 10.1176/ajp.2006.163.8.1440. [DOI] [PubMed] [Google Scholar]
- Soulières I, Zeffiro TA, Girard ML, Mottron L. Enhanced mental image mapping in autism. Neuropsychologia. 2011;49(5):848–857. doi: 10.1016/j.neuropsychologia.2011.01.027. [DOI] [PubMed] [Google Scholar]
- Spencer MD, Holt RJ, Chura LR, Calder AJ, Suckling J, Bullmore ET, Baron-Cohen S. Atypical Activation during the Embedded Figures Task as a functional Magnetic Resonance Imaging Endophenotype of Autism. Brain. 2012;135:3469–3480. doi: 10.1093/brain/aws229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart ME, Watson J, Allcock A, Yaqoob T. Autistic traits predict performance on the block design. Autism. 2009;13(2):133–142. doi: 10.1177/1362361308098515. [DOI] [PubMed] [Google Scholar]
- Szatmari P, Jones MB, Tuff L, Bartolucci G, Fisman S, Mahoney W. Lack of cognitive impairment in first-degree relatives of children with pervasive developmental disorders. Journal of American Academy of Child and Adolescent Psychiatry. 1993;32:1264–1273. doi: 10.1097/00004583-199311000-00022. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Williams DL, Goldstein G, Kojkowski N, Minshew NJ. Do Individuals with High-functioning Autism have the IQ profine associated with non-verbal learning disability? Research on Autism Spectrum Disorders. 2008;2(2):353–361. doi: 10.1016/j.rasd.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Within-group brain activation for the contrasts Global vs. Fixation and Random vs. Fixation for Autism and Control groups (p < 0.05, FWE corrected).