Abstract
Regulation of TGF-β1/Smad3 signaling in fibrogenesis is complex. Previous work by our lab suggests that ERK MAP kinase phosphorylates the linker region (LR) of Smad3 to enhance TGF-β-induced collagen-I accumulation. However the roles of the individual Smad3LR phosphorylation sites (T179, S204, S208 and S213) in the collagen-I response to TGF-β are not clear. To address this issue, we tested the ability of Smad3 constructs expressing wild-type Smad3 or Smad3 with mutated LR phosphorylation sites to reconstitute TGF-β-stimulated COL1A2 promoter activity in Smad3-null or -knockdown cells. Blocking ERK in fibroblasts and renal mesangial cells inhibited both S204 phosphorylation and Smad3-mediated COL1A2 promoter activity. Mutations replacing serine at S204 or S208 in the linker region decreased Smad3-mediated COL1A2 promoter activity, whereas mutating T179 enhanced basal COL1A2 promoter activity and did not prevent TGF-β stimulation. Interestingly, mutation of all four Smad3LR sites (T179, S204, S208 and S213) was not inhibitory, suggesting primacy of the two inhibitory sites. These results suggest that in these mesenchymal cells, phosphorylation of the T179 and possibly S213 sites may act as a brake on the signal, whereas S204 phosphorylation by ERK in some manner releases that brake. Renal epithelial cells (HKC) respond differently from MEF or mesangial cells; blocking ERK neither changed TGF-β-stimulated S204 phosphorylation nor prevented Smad3-mediated COL1A2 promoter activity in HKC. Furthermore, re-expression of wild type-Smad3 or the S204A-Smad3 mutant in Smad3-knockdown HKC reconstituted Smad3-mediated COL1A2 promoter activity. Collectively, these data suggest that Serine-204 phosphorylation in the Smad3LR is a critical event by which ERK enhances Smad3-mediated COL1A2 promoter activity in mesenchymal cells.
Keywords: Fibrosis, Extracellular Matrix, Fibroblast, Epithelial, mesenchymal, ERK MAP kinase
Introduction
TGF-β1 is a critical mediator of extracellular matrix (ECM) accumulation in fibrogenesis [1]. Canonical signaling begins with TGF-β binding the type II receptor (TβRII) to recruit and phosphorylate the type I receptor (TβRI), which in turn recruits and phosphorylates the receptor-specific transcription factors: Smad2 and Smad3. Smad4 is then included to this complex, which translocates into the nucleus to associate with other transcription factors to regulate the transcription of genes, including those encoding extracellular matrix proteins such as type-I collagen (collagen-I). In addition to this canonical TGF-β/Smad pathway, multiple other signaling pathways appear to regulate the myriad and sometimes opposing effects of TGF-β on cells. Our laboratory has characterized additional, non-canonical mediators that modulate TGF-β/Smad3-mediated fibrogenesis. These mediators include PKC-δ [2], PI3K [3], cytoskeletal components [4], HIF-1α [5] and, of critical importance, ERK MAP kinases [6–9].
Smad3 is essential for most renal fibrosis, confirmed by the suppression of fibrogenesis in Smad3-null mice [10]. Smad3, like Smad2 and Smad4, consists of two highly conserved Mad-homology domains (MH1 and MH2) connected by a less-conserved linker region (LR) [11]. TβRI–mediated phosphorylation of the carboxyl-terminal of Smad3 is critical for downstream Smad3 activity. However the Smad3LR also contains several threonine and serine residues (T179, S204, S208 and S213) that can be differentially phosphorylated by the action of MAP kinases (ERK, JNK and p38), cyclin-dependent kinase (CDK) members and glycogen synthase kinase 3 (GSK3) to regulate Smad3 responses in a highly cell context-dependent manner [12–25]. For example, an early study reported that overexpression of constitutively active Ras that activates MAP kinase phosphorylates the LR to prevent the nuclear accumulation of Smad3 and thus block TGF-β signaling [13]. Consistent with that report, CDK-mediated LR phosphorylation can also inhibit Smad3 transcriptional activity to promote aberrant cell cycle progression, generating resistance to the growth-inhibitory effect of TGF-β [16]. However we and others [26, 27] have proposed that the effect of LR phosphorylation on Smad3 responses is highly cell context-dependent. For example, LR phosphorylation by p38 MAPK and ROCK (S204, S208 and S213) and JNK (S208 and S213) may enhance Smad2/3 transcriptional activity [12, 19, 21, 28]. Consistent with these observations, Smad3LR phosphorylation can also promote extracellular matrix production in cultured rat myofibroblasts [15]. Indeed, it has been reported that the Smad3LR includes a transcriptional activation domain that cooperates with the carboxyl-terminal domain of Smad3 to enhance TGF-β-induced transcriptional activation [29, 30]. Previous work from our lab suggests that ERK enhances Smad3LR phosphorylation and collagen-I synthesis in response to TGF-β in human mesangial cells [6, 7]. We proposed that specific LR phosphorylation sites differentially enhance, inhibit or have no effect on Smad3-mediated collagen-I expression. To test this hypothesis, we examined the effect of site-specific Smad3LR mutants on TGF-β-induced COL1A2 promoter activity. Our results indicate that the collagen-I response to TGF-β is regulated through specific phosphorylations in the linker region of Smad3 in a cell phenotype-specific manner.
EXPERIMENTAL PROCEDURES
Materials
Active, recombinant human TGF-β1 (R&D Systems, Minneapolis, MN) was reconstituted as a 4 μg/ml stock solution in 4 mM HCl with 1 mg/ml bovine serum albumin. Antibodies were purchased from the following vendors: anti-phospho-Smad1/2/3 (H-2), anti-phospho-Smad3-S208 from Santa Cruz Biochemistry (Santa Cruz, CA); anti-phospho Smad3 (S423/S425) from Cell Signaling Technology (Beverly, MA); anti-β-actin, anti-phospho-Smad3-T8, -T179, -S204 and -S213 from Abcam (Cambridge, MA). Puromycin was purchased from Sigma. MEK/ERK inhibitors: PD98059 and PD0325901 were purchased from EMD Millipore (Billerica, MA) and Selleckchem (Houston, TX), respectively.
Expression Plasmids
CS2-Smad3 expression constructs (wild-type, T179V, S204A, S208A and EPSM) were a generous gift from F. Liu (Rutgers, Piscataway, NJ). The -378COL1A2-luc construct containing the 378 base pairs of the α2(I) collagen promoter sequence and 58 base pairs of the transcribed sequence, fused to the luciferase reporter gene was constructed as previously described [31]. The pFA-Elk and pFR-Luc plasmids were purchased from Stratagene (La Jolla, CA).
Cell Culture
Mouse embryonic fibroblasts (wild-type and Smad3-null MEF) and human renal tubular epithelial cells (HKC) were generous gifts of E. Böttinger (Mount Sinai, NY) [32] and L. Racusen (John Hopkins Medical School, Baltimore, MD) [33]. Human dermal fibroblasts were supplied by the Skin Disease Research Center, Northwestern University (Chicago, IL). These cells were grown in DMEM/F12 medium supplemented with 10% heat-inactivated fetal bovine serum, glutamine, penicillin-streptomycin, amphotericin B, and HEPES buffer. Human renal mesangial cells were isolated from glomeruli of normal renal cortex as described previously [34] and cultured in DMEM/F12 medium supplemented with 10% heat-inactivated cosmic calf serum obtained from Hyclone (Logan, UT), glutamine, penicillin/streptomycin, sodium pyruvate, HEPES buffer, and 8 μg/ml insulin (Sigma, St. Louis, MO) (used between passages 5 and 8).
Stable Smad3-knockdown HKC
GIPZ clones (empty vector, RHS4349 and human Smad3 shRNA RHS4430-98895790) were obtained from Open Biosystems Products (Huntsville, AL). The constructs were subjected to CaPO4 transfection for lentiviral packaging in HEK293 FT cells (Invitrogen) using psPAX2 and pMD2.G followed by transduction of HKC with the crude viral lysate. Expression of shRNA was visualized at 48 h by expression of GFP that is in tandem with shRNA cassette. Thereafter, infected cells were selected with 2 μg/ml puromycin. Frozen stocks of established cell lines were used for experiments at passages 4–8 after transduction to minimize possible induction of compensatory mechanisms.
Western Blotting Analysis
Following exposure to experimental conditions, cells were washed with ice-cold phosphate-buffered saline (PBS) and then lysed on ice in RIPA buffer (50 mM Tris/HCl, pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS) containing protease and phosphatase inhibitors (1 mM PMSF, 1 mM EDTA, 1 μg/ml of leupeptin, aprotinin, and pepstatin A, 1 mM sodium orthovanadate, 50 mM sodium fluoride, 40 mM 2-glycerophosphate). Lysates were cleared by centrifugation (10,000 × g at 4°C) for 10min and samples of equal protein concentration (determined by protein assay) were resolved by SDS-PAGE on 8% polyacrylamide gels. Proteins were transferred onto Immobilon-P (PVDF) membranes (Millipore, Bedford, MA), blocked 3% BSA in Tris-buffered saline (TBS: 20 mM Tris/HCl, 150 mM NaCl) containing 0.1% Tween-20 (TBS-T). Membranes were probed with antibodies for target proteins overnight at 4°C. Membranes were washed in TBS-T and probed with the appropriate species of secondary-HRP conjugated antibody (prepared in 3% BSA in TBS-T). Immunoreactive bands were visualized using chemiluminescence reagent according to the manufacturer’s instructions (Santa Cruz Biotechnology).
Transient Transfection and Luciferase Assay
Cells were seeded onto 6-well plates (1.0 × 105/well). 18h later the cells were transfected (for 3h) with the indicated plasmids and a β-galactosidase expression vector (transfection efficiency control) in serum-free medium using X-tremeGENE HP DNA Transfection Reagent from Roche (Indianapolis, IN) (3μl: 2μg DNA ratio). Transfected cells were exposed to experimental conditions (described in text) and harvested in reporter lysis buffer (Promega, Madison, WI). Luciferase and β-galactosidase activities were measured as described previously [6, 7]. Each condition was tested in triplicate, and experiments were repeated at least three times. Media from treated cells was also assayed for the activity of lactate dehydrogenase (an enzyme released by dying cells) using the CytoTox96® Non-Radioactive Assay (Promega, Madison, WI).
Statistical analysis
Data is expressed as mean ± SE. GraphPad Prism 6 software was used to analyze statistical differences between experimental groups using analysis of variance and values of P<0.05 by Bonferroni post hoc test were considered significant.
Results
Serine-204 in the Linker Region (LR) of Smad3 is a Primary ERK Target in Fibroblasts and Renal Mesangial Cells
First, to confirm the specificity of the phospho-Smad3 linker region (LR) antibodies, we tested their ability to recognize their targets in wild-type murine embryonic fibroblasts (MEF) and Smad3-null MEF (Fig. S1A, Supplemental data). In response to TGF-β-stimulation, Smad3LR phosphorylations at T179, S204, S208 and S213 were detected in wild-type MEF but not in Smad3-null MEF. The upper (60-kD) of the two bands detected by the anti-phospho-Smad3-T179 antibody corresponds to the analogous phosphorylation site (T220) in the Smad2LR (Fig. S1A). To further verify the specificity of these antibodies, we tested their ability to recognize their targets in either HEK or wild-type MEF transfected with constructs expressing wild-type Smad3, Smad3 with mutated LR phosphorylation sites or empty vector (Fig. S1B, C). The transfected MEFs (but not the HEKs) were then stimulated with TGF-β (1 h) (Fig. S1C). Empty vector-transfected cells did not exhibit LR phosphorylations whereas cells transfected with wild-type Smad3 exhibited strong phosphorylations at T179, S204, S208 and S213 in the Smad3LR (Fig. S1B, C). In contrast, the duration of optimal exposure for developing the blots was so short in Smad construct overexpressing cells that the possible phosphorylation of native Smad3-LR was not detected (Fig. S1B, C, first two lanes). Similar to empty vector-transfected cells, phosphorylations at T179, S204, S208 and S213 were not observed in cells transfected with T179V, S204A, S208A or S213A, respectively. Therefore, each of the phospho-Smad3LR antibodies recognized overexpressed wild-type Smad3 and not their corresponding phosphoacceptor-site Smad3 mutant, further validating the specificity of the phospho-Smad3LR antibodies.
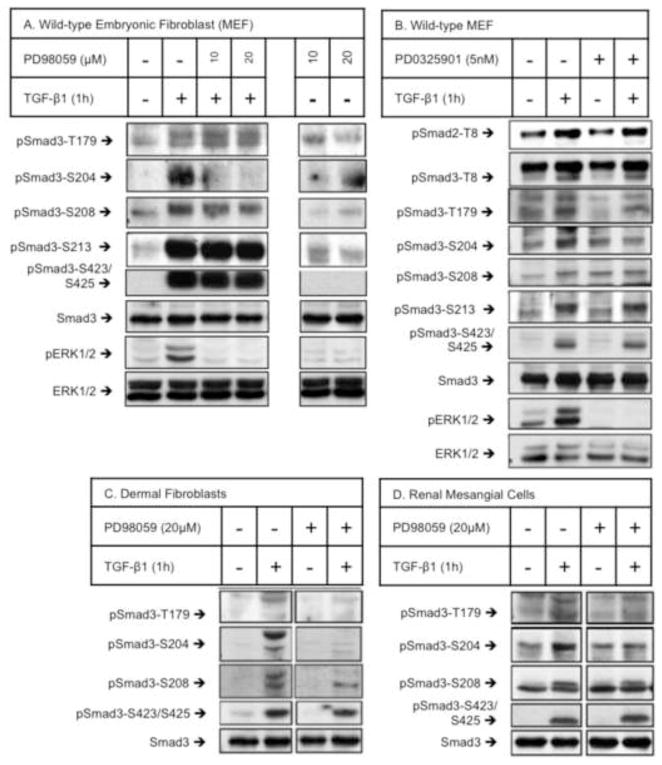
We then evaluated how several signaling molecules, which previously have been implicated in Smad signaling, affect specific Smad3LR phosphorylation. TGF-β-stimulated wild-type MEF (Fig. 1A, B), dermal fibroblasts (Fig. 1C) and renal mesangial cells (Fig. 1D) exhibited increased phosphorylation at all four Smad3LR sites: T179, S204, S208 and S213. Threonine-8 (T8) in the MH1 domain of Smad3 was also phosphorylated in TGF-β-treated MEFs (Fig. 1B). Chemical inhibitors of MEK/ERK (PD98059 and PD0325901) were used to evaluate a role for the ERK MAP kinases on these phosphorylations. PD98059 specifically blocked TGF-β-stimulated S204 phosphorylation without changing the phosphorylation of the remaining LR sites (T179, S208 or S213) (Fig. 1A). Consistent with these results, another MEK/ERK inhibitor, PD0325901 also blocked TGF-β-induced S204 phosphorylation without affecting the phosphorylation of the remaining sites (T8, T179, S208, S213 or S423/S425) (Fig. 1B). The ability of PD98059 and PD0325901 to block TGF-β-stimulated ERK phosphorylation was verified (Fig. 1A, B). The MEK/ERK inhibitor, PD98059 also reduced TGF-β-induced S204 phosphorylation without changing the phosphorylation of T179, S208 nor S423/S425 in both human renal mesangial cells (Fig. 1C) and human dermal fibroblasts (Fig. 1D). In contrast, S204 phosphorylation was not inhibited by inhibition of p38 (SB202190) or JNK (SP600125) (data not shown). These results indicate that S204 phosphorylation is ERK-dependent in fibroblasts and renal mesangial cells. In light of previous studies reporting that TGF-β-stimulated S204 phosphorylation is mediated by GSK3 in multiple cell types including MEF [23, 24] we also tested the effect of the GSK3 inhibitor (SB216763) on TGF-β-stimulated Smad3LR phosphorylations. GSK3 inhibition blocked TGF-β-induced S204 phosphorylation without changing the phosphorylation of the remaining sites (T179, S208, S213 nor S423/S425) in MEF (Fig. S1E, Supplemental data).
Figure 1. Serine-204 in the Smad3LR is a primary ERK target in fibroblasts and renal mesangial cells.
Wild-type murine embryonic fibroblasts (MEF) (A, B), human dermal fibroblasts (C) and human renal mesangial cells were pre-treated (1 h) with vehicle (DMSO), PD98059 (10 and 20 μM) or PD0325901 (5 nM) prior to vehicle- or TGF-β1 (2 ng/ml) stimulation (1 h). Smad3 phosphorylations at T8, T179, S204, S208, S213 and S423/S425 were analyzed by western blotting using site-specific phospho-Smad3LR antibodies. The upper of the two bands detected by the anti-phospho-Smad3-T8 and anti-phospho-Smad3-T179 antibodies represents cross-reaction with the T8 and T220 sites in the Smad2LR, respectively. Shown here are representative experiments of four independent experiments.
ERK MAP Kinases are required for Optimal Smad3-mediated TGF-β-induced COL1A2 Promoter Activity in Fibroblasts and Renal Mesangial Cells
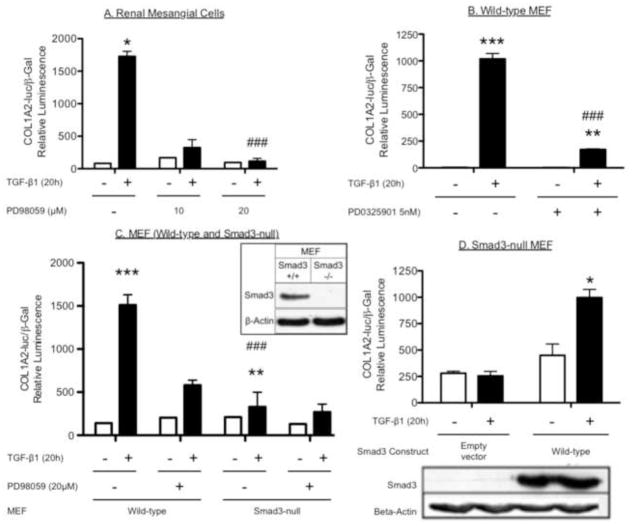
Because TGF-β-stimulated Smad activity contributes to the collagen-I response in human renal mesangial cells [6, 7], we examined whether ERK supports Smad3-mediated collagen gene activation in embryonic fibroblasts (MEF) (Fig. 2). TGF-β stimulation (20 h) significantly increased COL1A2 promoter activity in human renal mesangial cells (HMC) (Fig. 2A) or wild-type MEF (Fig. 2B and Fig. 2C), compared to vehicle-treated cells. These responses were attenuated by the MEK/ERK inhibitors: PD98059 (20 μM) in HMC (Fig. 2A) and wild-type MEF (Fig. 2C) and PD0325901 (5 nM) in wild-type MEF (Fig. 2B). As expected, Smad3-null MEF did not show a COL1A2 response to TGF-β compared with a normal response in wild-type MEF (Fig. 2C), and transfection of Smad3-null MEF with wild-type Smad3 reconstituted the COL1A2 response (Fig. 2D). A lactate dehydrogenase (LDH), assay was performed to assess MEF viability. In comparison to vehicle-treated MEFs (wild-type or Smad3-null) there was no change in LDH release (cytotoxicity) following treatment with PD98059 (20 μM) or PD0325901 (5 nM) for 20h (Fig. S2, Supplemental data). This confirmed that the lack of a collagen response in either ERK-inhibitor treated- or Smad3-null MEF was not attributed to cytotoxicity. The absence of Smad3 from the null-MEF and its reconstitution by transfection of the Smad3 expression construct were verified (Fig. 2D). Together, these results demonstrate that optimal TGF-β-induced COL1A2 promoter activity in MEF requires both ERK and Smad3.
Figure 2. ERK MAP kinases are required for optimal Smad3-mediated TGF-β-induced COL1A2 promoter activity in fibroblasts and renal mesangial cells.
A, Blocking ERK in renal mesangial cells inhibits TGF-β-stimulated COL1A2 promoter activity. Renal mesangial cells were transfected with COL1A2-luciferase and β-galactosidase constructs for 3 hours. Transfected cells were then pre-treated with PD98059 (10 and 20 μM) or vehicle (DMSO) for 1 h, before vehicle- or TGF-β- (2 ng/ml) stimulation for a further 20 h. B, Blocking ERK in wild-type murine embryonic fibroblasts (MEF) inhibits TGF-β-stimulated COL1A2 promoter activity. MEF were exposed to the same experimental conditions as in (A); however a different MEK/ERK inhibitor, PD0325901 (5 nM), was used. C, TGF-β-stimulated COL1A2 promoter activity is both ERK- and Smad3-dependent. Wild-type and Smad3-null MEF were exposed to the same experimental conditions as in (A). D, Reconstitution of Smad3-mediated COL1A2 promoter activity. Smad3-null MEFs were transfected with empty vector or pCS2-Smad3-wild-type, including the COL1A2-luciferase and β-galactosidase constructs for 3 hours. Transfected cells were stimulated with TGF-β (2 ng/ml) for a further 20 h. Relative luciferase activity (mean ± S.E.M., n=3) was corrected for β-galactosidase activity. Western blotting shows the absence of Smad3 in Smad3-null MEF versus wild-type MEF. Shown here is a representative experiment of three independent experiments. *P<0.05, **P<0.01 vs. vehicle-treated cells. #P<0.05, ##P<0.01 vs. vehicle-treated cells.
Role of the Smad3LR Phosphoacceptor sites in TGF-β-induced COL1A2 Promoter Activity in Fibroblasts
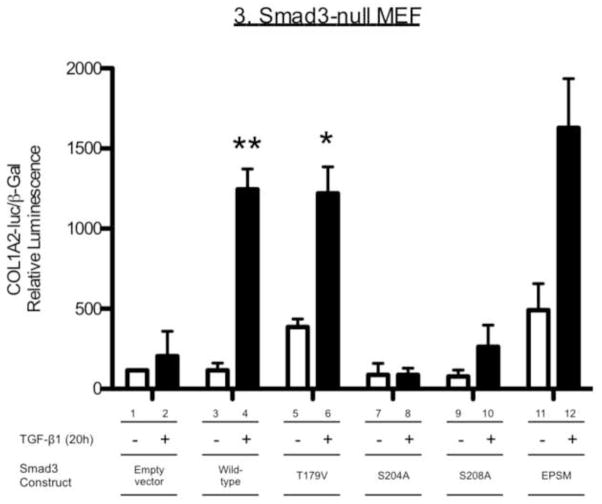
We then tested the role of the specific phosphorylation sites in the Smad3LR on TGF-β-induced COL1A2 promoter activity. Smad3-null MEF were transfected with wild-type Smad3, the single site-specific Smad3LR mutants or Smad3-EPSM (all four LR sites mutated: T179V, S204A, S208A and S213A) (Fig. 3). As was shown in Figure 2D, empty vector-transfected Smad3-null MEF did not show a COL1A2 promoter response to TGF-β (Fig. 3, bars 1–2). In contrast a 9-fold increase in COL1A2 promoter activity was observed in TGF-β-treated Smad3-null MEF transfected with wild-type Smad3 compared with those treated with vehicle (P<0.01, bars 3–4). T179V-expressing Smad3-null MEF exhibited a strong COL1A2 promoter activity response to TGF-β (P<0.01, bars 5–6). In contrast, S204A- and S208A-expressing cells failed to reconstitute significant COL1A2 promoter responses to TGF-β (bars 7–10). Smad3-EPSM-expressing cells showed enhanced basal and a strong COL1A2 promoter activity by TGF-β (P<0.05, bars 11–12). Interestingly, blocking ERK with PD98059, did not inhibit the COL1A2 promoter response to TGF-β in Smad3-EPSM-expressing cells (data not shown). These results suggest that S204 and S208 in the Smad3LR are necessary for an optimal collagen-I response when the LR phosphoacceptor sites are intact.
Figure 3. Effect of site-specific Smad3LR mutants (T179V, S204A, S208A or EPSM) on TGF-β-induced COL1A2 promoter activity in Smad3-null murine embryonic fibroblasts.
Smad3-null MEFs were transfected with empty vector or pCS2-Smad3 constructs (wild-type, T179V, S204A, S208A, S213A or EPSM) including the COL1A2-luciferase and β-galactosidase constructs for 3 hours. Transfected cells were then stimulated with TGF-β (2 ng/ml) for a further 20 h. Relative luciferase activity (mean ± S.E.M., n=3) was corrected for β-galactosidase activity. Shown here is a representative experiment of five independent experiments. *P<0.05, **P<0.01 vs. vehicle-treated cells expressing the same construct. #P<0.05,
Role of ERK and the S204 Smad3LR Phosphoacceptor Site in Smad3-mediated COL1A2 Promoter Activity in Renal Epithelial Cells
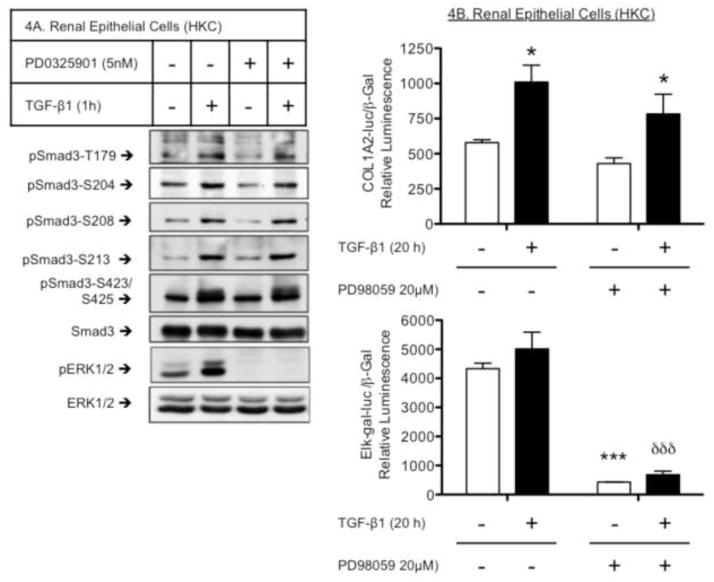
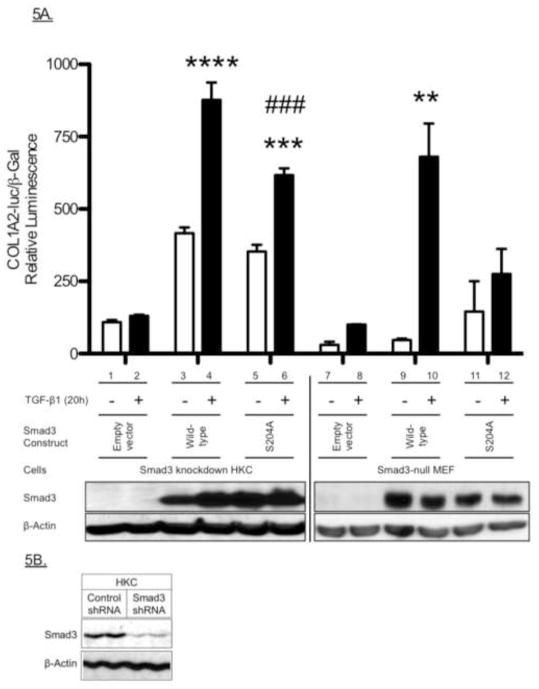
We next examined a role for ERK in both Smad3LR phosphorylation and Smad3-mediated COL1A2 promoter activity in renal epithelial cells (HKC). TGF-β treatment (1 h) increased site-specific Smad3LR phosphorylations at T179, S204, S208 and S213 in HKC, as was seen in the fibroblasts and mesangial cells. However, these phosphorylations were unaffected by the MEK/ERK inhibitor, PD98059 (Fig. 4A). TGF-β also significantly stimulated COL1A2 promoter activity in HKC. Unlike our observations in fibroblasts or mesangial cells, PD98059 did not block the collagen response (Fig. 4B), despite blocking Elk-gal transactivation activity (Fig. 4B, lower panel), a downstream readout of ERK-mediated transcriptional activity. A cytotoxicity assay (LDH assay) was performed and confirmed that there was no significant increase in toxicity in HKC treated with PD98059 (20 βM, 20h) compared to vehicle-treated cells (Fig. S3, Supplemental data). Finally, while Smad3 knock down eliminated the COL1A2 response to TGF-β in HKC, re-expression of either wild-type Smad3 (P<0.0001) or the S204A mutant (P<0.001) reconstituted the response (Fig. 5A). However, the level of the response supported by the S204A mutant was significantly less than that observed in TGF-β treated HKC that had been transfected with wild-type Smad3 (Fig. 5A, bar 4 vs. 6). As a comparison, re-expression of the S204A mutant in Smad3-null MEF failed to reconstitute the response (Fig. 5A, bar 11 vs. 12), as previously shown in Figure 3. Knockdown of Smad3 in HKC was confirmed by western blotting (Fig. 5B). These results suggest that ERK-mediated S204 phosphorylation is less important for the collagen response to TGF-β in epithelial cells than it is in mesenchymal cells.
Figure 4. Effects of MEK/ERK inhibition on Smad3LR phosphorylation and Smad3-mediated COL1A2 promoter activity in renal epithelial cells.
A, S204 phosphoacceptor is not an ERK target in renal epithelial cells. Renal epithelial cells (HKC) were pre-treated (1 h) with vehicle (DMSO) or PD0325901 (5 nM) prior to vehicle- or TGF-β1 (2 ng/ml) stimulation (1 h). Phosphorylations at T179, S204, S208, S213 and S423/S425 were analyzed by western blotting with specific phospho-peptide Smad3 antibodies. S204 phosphorylation is not prevented by the MEK/ERK inhibitor. B, Smad3-mediated COL1A2 promoter activity in renal epithelial cells does not require ERK. HKC were transfected with β-galactosidase, COL1A2-luciferase (upper panel) or Elk-gal (lower panel) and constructs for 3 hours. The pFA-Elk plasmid expresses an activation domain of Elk linked to the DNA-binding region of yeast Gal4, along with a Gal4-luciferase reporter construct. Transfected cells were then pre-treated with vehicle (DMSO) or PD98059 (20 μM) for 1 h, before vehicle- or TGF-β (2 ng/ml) stimulation for a further 20 h. Relative luciferase activity (mean ± S.E.M., n=3) corrected for β-galactosidase activity. Shown here is a representative experiment of three independent experiments. *P<0.05, **P<0.01 vs. vehicle-treated cells. δδδP<0.001 vs. TGF-β-treated cells.
Figure 5. Role of the Serine-204 (S204) Smad3LR Phosphorylation Site in Smad3-mediated renal epithelial cell COL1A2 promoter activity.
A, Effect of the S204A-Smad3 mutant on Smad3-mediated COL1A2 promoter activity in renal epithelial cells. Smad3-knockdown HKC (bars 1–6) and Smad3-null MEFs (bars 7–12) were transfected with empty vector or pCS2-Smad3 constructs (wild-type or S204A), including the COL1A2-luciferase and β-galactosidase constructs for 3 hours. The transfected cells were then stimulated with TGF-β (2 ng/ml) for a further 20 h. Relative luciferase activity (mean ± S.E.M., n=3) was corrected for β-galactosidase activity. Shown here is a representative experiment of three independent experiments. *P<0.05, **P<0.01 vs. vehicle-treated cells. B, Smad3-knockdown in renal epithelial cells. Western blotting analysis of HKC stably expressing control- or Smad3-pGIPZ lentiviral shRNA.
Discussion
TGF-β/Smad3 signaling plays a central role in renal fibrogenesis. Our laboratory has characterized a number of additional, non-canonical signals that contribute to TGF-β-mediated, type-I collagen expression. Critical among these is ERK-mediated signaling. We previously showed in human glomerular mesangial cells that ERK and Smad3 linker-region (LR) phosphorylation plays a role in TGF-β induction of collagen expression [6, 7]. Because the role of the LR in Smad signaling is variably reported [12–25] the present series of experiments was designed to determine (1) whether and how specific LR phosphorylations occur in human mesangial cells, and (2) the role such phosphorylations plays in the collagen response. Our initial studies confirmed the specificity of the phosphorylation site-specific Smad3LR antibodies. We then determined that inhibiting different known mediators caused various patterns of phosphorylation, but only ERK inhibition consistently blocked the phosphorylation of the serine at amino acid position 204, suggesting that S204 phosphorylation is ERK dependent. Next, we tested whether S204 phosphorylation was important in the collagen-I response. Because the Smad3-EPSM construct (lacking all four LR phosphoacceptor sites) does not function as a dominant negative mutant, we utilized Smad3-null fibroblasts reconstituted with various Smad3 mutants. By doing this, we were able to test whether each specific phosphoacceptor site of the Smad3LR is required for reestablishing the collagen promoter response. The T179V-Smad3 mutant supported normal or increased responses and in some experiments showed increased basal activity. In contrast, Smad3 mutants -S204A and -S208A did not support the collagen response. Importantly, the Smad3-EPSM construct, in which all four LR phosphoacceptor sites are mutated, did support a response in Smad3-deficient MEFs. Moreover, ERK inhibitor treatment did not prevent TGF-β-stimulated collagen promoter activity in the Smad3-reconstituted cells. Taken together, these data are consistent with a complex role for Smad LR phosphorylation (Fig. 6). In the absence of LR phosphorylation, Smad3 can activate the COL1A2 promoter, likely through the TβR-regulated C-terminal phosphorylation as previously shown [31]. In the presence of intact C-terminal serines and their phosphorylation, phosphorylation of T179 or S213 is likely to be inhibitory, whereas phosphorylation at S204 and S208 enhances responses, likely by releasing the inhibition caused by phosphorylation at the T179 and S213 sites. Since ERK inhibition prevents S204 phosphorylation, we propose that S204 phosphorylation is an important, ERK-mediated step in TGF-β–stimulated collagen expression in fibroblasts and renal mesangial cells. This complex model could explain both our results and the often-contradictory findings reported in the literature.
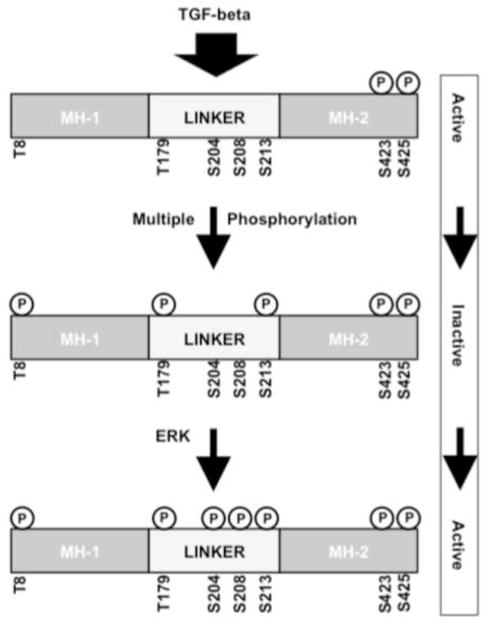
Figure 6. Proposed model for regulation of Smad3 activity in TGF-β-stimulated collagen expression.
TGF-β stimulates the TβR to phosphorylate essential serines at the carboxyl terminus of Smad3. In the absence of linker region phosphorylation, Smad3 can activate the COL1A2 promoter. Phosphorylation of T179 and S213 is likely to be inhibitory, whereas ERK-mediated phosphorylation at S204 and possibly S208 enhances responses, likely by releasing the inhibition caused by phosphorylation at the T179 and S213 sites. Since ERK inhibition targets S204 phosphorylation, we propose that S204 phosphorylation is an important, ERK-mediated step in TGF-β–stimulated collagen expression. This complex model could explain both our results and the often-contradictory findings reported in the literature.
In contrast, when we performed similar studies in Smad3-knockdown HKC, a renal tubular epithelial line, we did not find a similar set of results. In HKC cells, re-expression of either wild-type Smad3 or the Smad3-S204A mutant reconstituted a strong Smad3-mediated COL1A2 promoter response to TGF-β. This observation is consistent with our finding that ERK inhibition did not block TGF-β-stimulated collagen expression in epithelial cells, despite blocking the transactivational activity of a downstream ERK substrate, Elk. Together, our findings suggest the presence of a mesenchymal cell-specific mechanism regulating Smad3-mediated fibrogenesis.
Several other signaling molecules also affect LR phosphorylation. The ability of CDK inhibitors to block T179, S204 or S208 phosphorylation in TGF-β-stimulated mink lung epithelial (Mv1Lu) cells [23] suggests that CDK family members may target Smad3LR phosphorylation sites in addition to the S204 ERK target. Others have reported TGF-β-stimulated S204 phosphorylation is mediated by GSK3 in epithelial-like cells [23, 24] and in MEF [23]. We also confirmed that GSK3 inhibition (SB216763) reduced TGF-β-induced S204 phosphorylation without changing the phosphorylation of T179, S208 nor S423/S425 in MEF (Fig. S1E, Supplemental data). SB216763 also attenuated TGF-β-stimulated COL1A2 promoter activity in MEFs (data not shown) consistent with the role we have proposed for S204 and suggesting a role for GSK3 as a modulator of Smad3 activity, either in concert with or distinct form the effect of ERK. The ability of TGF-β to phosphorylate S208 in JNK+/+ but not in JNK−/− MTE cells implicates JNK in S208 phosphorylation [28]. ROCK or p38 inhibitors block basal- and TGF-β-stimulated S204- and S208- phosphorylation in human mammary carcinoma cells [21], suggesting that ROCK and p38 and may also target these sites.
The mechanism by which S204 phosphorylation enhances Smad3 activity is uncertain. Kretschmar and colleagues reported that LR phosphorylation inhibits Smad3 responses by blocking the nuclear translocation of Smad3 [13]. However, others have described strong nuclear staining for S204 phosphorylation in TGF-β-treated AML12 mouse hepatocytes [24]. Another proposed mechanism of modulated Smad3 signaling is regulation of protein decay. Indeed, CDK8/9-mediated (but not MAPK-mediated) T179 phosphorylation has been shown to be required for the linker region to interact with NEDD4L, an E3 ubiquitin ligase that targets proteins for degradation [35]. On the other hand, mutations at either S204 or S208 were reported not to alter basal Smad3 degradation [22]. Finally, differential LR phosphorylation could affect Smad3 transactivational activity. The ability of the LR to regulate Smad3 interactions with other transcriptional regulators is supported by work that shows a Smad3 phospho-mimetic (T179E/S204D/S208D) to enhance--and a Smad3-EPSM (all LR sites ablated) to inhibit--the Smad3-Smad4 interaction in alveolar type II epithelial (C10) cells [28]. The authors of that paper also reported that the Smad3-EPSM reduced basal- and TGF-β-stimulated Smad3-binding element (SBE) promoter activities. LR phosphorylation could affect the interaction of Smad3 with the transcriptional coactivator, p300/CBP [29, 30]. Therefore, the S204 and S208 sites could enhance the binding of Smad3 to coactivators involved in the transcriptional regulation of the COL1A2 promoter. However, in the presence of a constitutively active TβRI, the S204A and the S204A/S208A mutants were highly associated with overexpressed CBP compared to wild-type Smad3 [24]. This suggests that S204 and/or S208 inhibit the Smad3-CBP interaction, which fits in their model that shows S204 and S208 to inhibit downstream Smad3 activity [24]. Conversely, S204 and S208 may inhibit the binding of Smad3 with co-repressors of the COL1A2 promoter. These discrepant results further highlight the cell context-dependent role of the Smad3LR.
In summary, ERK-induced S204 phosphorylation in the Smad3LR may be a critical event in mesenchymal cells, to allow ERK to enhance Smad3-mediated COL1A2 promoter activity. This finding may provide new insights into the potential mechanism whereby ERK promotes fibrosis. Further investigation into the mechanism through which S204 and S208 in the Smad3LR enhance TGF-β-stimulated collagen-I accumulation may provide a therapeutic strategy for the treatment of fibrotic disorders.
Supplementary Material
A, Wild-type and Smad3-null MEFs were stimulated with vehicle or TGF-β (2 ng/ml, 1 h). HEK (B) and wild-type-MEF (C) were transfected with pCS2-Smad3 constructs (wild-type, T179V, S204A, S208A or S213A) for 18h. The transfected wild-type-MEF (but not the transfected HEK) were then treated with vehicle- or TGF-β- (2 ng/ml) for 1 h. Effect of GSK3 inhibition on Smad3 Linker Region Phosphorylation (D). Wild-type murine embryonic fibroblasts (MEF) were pre-treated (1 h) with vehicle (DMSO) or SB216763 (5μM) prior to vehicle- or TGF-β1 (2 ng/ml) stimulation for 1h. Smad3 phosphorylation at T179, S204, S208, S213 and S423/S425 were analyzed by western blotting using site-specific phospho-Smad3 antibodies. The upper of the two bands detected by the anti-phospho-Smad3-T179 antibody represents cross-reaction with the analogous site (T220) in the linker region of Smad2. Shown here are representative experiments of three independent experiments.
Wild-type and Smad3-null MEFs were exposed to experimental conditions as described in Figure 2B and C (± COL1A2-luciferase and β-galactosidase constructs, ± 20 μM PD98059 or 5 nM PD0325901, ± TGF-β1). Media was assayed for relative Lactate Dehydrogenase (LDH) activity (mean ± S.E.M., n=3). A positive control of the “total” possible LDH released from cells at equivalent confluence is shown. ****P<0.0001 vs. vehicle-treated cells.
HKC were exposed to experimental conditions as described in Figure 4B (± COL1A2-luciferase and β-galactosidase constructs, ± 20 μM PD98059, ± TGF-β1). Media was assayed for relative Lactate Dehydrogenase (LDH) activity (mean ± S.E.M., n=3). A positive control of the “total” possible LDH released from cells at equivalent confluence is shown. ****P<0.0001 vs. vehicle-treated cells.
Highlights.
Regulation of TGF-β1/Smad3 signaling in fibrogenesis is complex
Ser204 in the Smad3 Linker Region is a primary ERK target in mesenchymal cells
ERK is required for Smad3-mediated TGF-β-induced COL1A2 promoter activity
Ser204 phosphorylation by ERK is critical for ERK to enhance this collagen response
Acknowledgments
We thank E. Böttinger (Mount Sinai Medical Center, NY) and L. Racusen (John Hopkins Medical School, Baltimore, MD) for the providing the mouse embryonic fibroblasts (MEF) and human renal tubular epithelial cells (HKC) respectively. CS2-Smad3 expression constructs (wild-type, T179V, S204A, S208A and EPSM) were generous gifts from F. Liu (Rutgers, Piscataway, NJ).
Grants
This work was in part supported by the grant R01-DK049362 from the National Institute of Diabetes, Digestive and Kidney Diseases to H.W.S.
Footnotes
Disclosure
No conflicts of interest, financial or otherwise, are declared by the authors(s).
Author contributions
Author contributions: J.A.B., X.L., performed experiments; J.A.B prepared figures; J.A.B., T.H., and H.W.S., drafted manuscript; J.A.B, T.H., X.L. and H.W.S., approved final version of manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 2.Runyan CE, Schnaper HW, Poncelet AC. Smad3 and PKCdelta mediate TGF-beta1-induced collagen I expression in human mesangial cells. American journal of physiology Renal physiology. 2003;285:F413–422. doi: 10.1152/ajprenal.00082.2003. [DOI] [PubMed] [Google Scholar]
- 3.Runyan CE, Schnaper HW, Poncelet AC. The phosphatidylinositol 3-kinase/Akt pathway enhances Smad3-stimulated mesangial cell collagen I expression in response to transforming growth factor-beta1. The Journal of biological chemistry. 2004;279:2632–2639. doi: 10.1074/jbc.M310412200. [DOI] [PubMed] [Google Scholar]
- 4.Hubchak SC, Runyan CE, Kreisberg JI, Schnaper HW. Cytoskeletal rearrangement and signal transduction in TGF-beta1-stimulated mesangial cell collagen accumulation. Journal of the American Society of Nephrology : JASN. 2003;14:1969–1980. doi: 10.1097/01.asn.0000076079.02452.92. [DOI] [PubMed] [Google Scholar]
- 5.Basu RK, Hubchak S, Hayashida T, Runyan CE, Schumacker PT, Schnaper HW. Interdependence of HIF-1alpha and TGF-beta/Smad3 signaling in normoxic and hypoxic renal epithelial cell collagen expression. American journal of physiology Renal physiology. 2011;300:F898–905. doi: 10.1152/ajprenal.00335.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayashida T, Poncelet AC, Hubchak SC, Schnaper HW. TGF-beta1 activates MAP kinase in human mesangial cells: a possible role in collagen expression. Kidney Int. 1999;56:1710–1720. doi: 10.1046/j.1523-1755.1999.00733.x. [DOI] [PubMed] [Google Scholar]
- 7.Hayashida T, Decaestecker M, Schnaper HW. Cross-talk between ERK MAP kinase and Smad signaling pathways enhances TGF-beta-dependent responses in human mesangial cells. FASEB J. 2003;17:1576–1578. doi: 10.1096/fj.03-0037fje. [DOI] [PubMed] [Google Scholar]
- 8.Hayashida T, Wu MH, Pierce A, Poncelet AC, Varga J, Schnaper HW. MAP-kinase activity necessary for TGFbeta1-stimulated mesangial cell type I collagen expression requires adhesion-dependent phosphorylation of FAK tyrosine 397. J Cell Sci. 2007;120:4230–4240. doi: 10.1242/jcs.03492. [DOI] [PubMed] [Google Scholar]
- 9.Hayashida T, Jones JC, Lee CK, Schnaper HW. Loss of beta1-integrin enhances TGF-beta1-induced collagen expression in epithelial cells via increased alphavbeta3-integrin and Rac1 activity. J Biol Chem. 2010;285:30741–30751. doi: 10.1074/jbc.M110.105700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujimoto M, Maezawa Y, Yokote K, Joh K, Kobayashi K, Kawamura H, Nishimura M, Roberts AB, Saito Y, Mori S. Mice lacking Smad3 are protected against streptozotocin-induced diabetic glomerulopathy. Biochemical and biophysical research communications. 2003;305:1002–1007. doi: 10.1016/s0006-291x(03)00885-4. [DOI] [PubMed] [Google Scholar]
- 11.Shi Y. Structural insights on Smad function in TGFbeta signaling. Bioessays. 2001;23:223–232. doi: 10.1002/1521-1878(200103)23:3<223::AID-BIES1032>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 12.Engel ME, McDonnell MA, Law BK, Moses HL. Interdependent SMAD and JNK signaling in transforming growth factor-beta-mediated transcription. The Journal of biological chemistry. 1999;274:37413–37420. doi: 10.1074/jbc.274.52.37413. [DOI] [PubMed] [Google Scholar]
- 13.Kretzschmar M, Doody J, Timokhina I, Massague J. A mechanism of repression of TGFbeta/Smad signaling by oncogenic Ras. Genes & development. 1999;13:804–816. doi: 10.1101/gad.13.7.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massague J. Integration of Smad and MAPK pathways: a link and a linker revisited. Genes Dev. 2003;17:2993–2997. doi: 10.1101/gad.1167003. [DOI] [PubMed] [Google Scholar]
- 15.Furukawa F, Matsuzaki K, Mori S, Tahashi Y, Yoshida K, Sugano Y, Yamagata H, Matsushita M, Seki T, Inagaki Y, Nishizawa M, Fujisawa J, Inoue K. p38 MAPK mediates fibrogenic signal through Smad3 phosphorylation in rat myofibroblasts. Hepatology. 2003;38:879–889. doi: 10.1053/jhep.2003.50384. [DOI] [PubMed] [Google Scholar]
- 16.Matsuura I, Denissova NG, Wang G, He D, Long J, Liu F. Cyclin-dependent kinases regulate the antiproliferative function of Smads. Nature. 2004;430:226–231. doi: 10.1038/nature02650. [DOI] [PubMed] [Google Scholar]
- 17.Liu F. Smad3 phosphorylation by cyclin-dependent kinases. Cytokine Growth Factor Rev. 2006;17:9–17. doi: 10.1016/j.cytogfr.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Matsuura I, Wang G, He D, Liu F. Identification and characterization of ERK MAP kinase phosphorylation sites in Smad3. Biochemistry. 2005;44:12546–12553. doi: 10.1021/bi050560g. [DOI] [PubMed] [Google Scholar]
- 19.Mori S, Matsuzaki K, Yoshida K, Furukawa F, Tahashi Y, Yamagata H, Sekimoto G, Seki T, Matsui H, Nishizawa M, Fujisawa J, Okazaki K. TGF-beta and HGF transmit the signals through JNK-dependent Smad2/3 phosphorylation at the linker regions. Oncogene. 2004;23:7416–7429. doi: 10.1038/sj.onc.1207981. [DOI] [PubMed] [Google Scholar]
- 20.Yamagata H, Matsuzaki K, Mori S, Yoshida K, Tahashi Y, Furukawa F, Sekimoto G, Watanabe T, Uemura Y, Sakaida N, Yoshioka K, Kamiyama Y, Seki T, Okazaki K. Acceleration of Smad2 and Smad3 phosphorylation via c-Jun NH(2)-terminal kinase during human colorectal carcinogenesis. Cancer Res. 2005;65:157–165. [PubMed] [Google Scholar]
- 21.Kamaraju AK, Roberts AB. Role of Rho/ROCK and p38 MAP kinase pathways in transforming growth factor-beta-mediated Smad-dependent growth inhibition of human breast carcinoma cells in vivo. The Journal of biological chemistry. 2005;280:1024–1036. doi: 10.1074/jbc.M403960200. [DOI] [PubMed] [Google Scholar]
- 22.Guo X, Ramirez A, Waddell DS, Li Z, Liu X, Wang XF. Axin and GSK3-control Smad3 protein stability and modulate TGF- signaling. Genes & development. 2008;22:106–120. doi: 10.1101/gad.1590908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang G, Matsuura I, He D, Liu F. Transforming growth factor-{beta}-inducible phosphorylation of Smad3. J Biol Chem. 2009;284:9663–9673. doi: 10.1074/jbc.M809281200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Millet C, Yamashita M, Heller M, Yu LR, Veenstra TD, Zhang YE. A negative feedback control of transforming growth factor-beta signaling by glycogen synthase kinase 3-mediated Smad3 linker phosphorylation at Ser-204. The Journal of biological chemistry. 2009;284:19808–19816. doi: 10.1074/jbc.M109.016667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuzaki K, Kitano C, Murata M, Sekimoto G, Yoshida K, Uemura Y, Seki T, Taketani S, Fujisawa J, Okazaki K. Smad2 and Smad3 phosphorylated at both linker and COOH-terminal regions transmit malignant TGF-beta signal in later stages of human colorectal cancer. Cancer research. 2009;69:5321–5330. doi: 10.1158/0008-5472.CAN-08-4203. [DOI] [PubMed] [Google Scholar]
- 26.Rahimi RA, Leof EB. TGF-beta signaling: a tale of two responses. Journal of cellular biochemistry. 2007;102:593–608. doi: 10.1002/jcb.21501. [DOI] [PubMed] [Google Scholar]
- 27.Wilkes MC, Leof EB. Transforming growth factor beta activation of c-Abl is independent of receptor internalization and regulated by phosphatidylinositol 3-kinase and PAK2 in mesenchymal cultures. The Journal of biological chemistry. 2006;281:27846–27854. doi: 10.1074/jbc.M603721200. [DOI] [PubMed] [Google Scholar]
- 28.Velden JL, Alcorn JF, Guala AS, Badura EC, Janssen-Heininger YM. c-Jun N-terminal kinase 1 promotes transforming growth factor-beta1-induced epithelial-to-mesenchymal transition via control of linker phosphorylation and transcriptional activity of Smad3. Am J Respir Cell Mol Biol. 2011;44:571–581. doi: 10.1165/rcmb.2009-0282OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prokova V, Mavridou S, Papakosta P, Kardassis D. Characterization of a novel transcriptionally active domain in the transforming growth factor beta-regulated Smad3 protein. Nucleic Acids Res. 2005;33:3708–3721. doi: 10.1093/nar/gki679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang G, Long J, Matsuura I, He D, Liu F. The Smad3 linker region contains a transcriptional activation domain. Biochem J. 2005;386:29–34. doi: 10.1042/BJ20041820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poncelet AC, de Caestecker MP, Schnaper HW. The transforming growth factor-beta/SMAD signaling pathway is present and functional in human mesangial cells. Kidney Int. 1999;56:1354–1365. doi: 10.1046/j.1523-1755.1999.00680.x. [DOI] [PubMed] [Google Scholar]
- 32.Yang YC, Piek E, Zavadil J, Liang D, Xie D, Heyer J, Pavlidis P, Kucherlapati R, Roberts AB, Bottinger EP. Hierarchical model of gene regulation by transforming growth factor beta. Proc Natl Acad Sci U S A. 2003;100:10269–10274. doi: 10.1073/pnas.1834070100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Racusen LC, Monteil C, Sgrignoli A, Lucskay M, Marouillat S, Rhim JG, Morin JP. Cell lines with extended in vitro growth potential from human renal proximal tubule: characterization, response to inducers, and comparison with established cell lines. J Lab Clin Med. 1997;129:318–329. doi: 10.1016/s0022-2143(97)90180-3. [DOI] [PubMed] [Google Scholar]
- 34.Schnaper HW, Kopp JB, Poncelet AC, Hubchak SC, Stetler-Stevenson WG, Klotman PE, Kleinman HK. Increased expression of extracellular matrix proteins and decreased expression of matrix proteases after serial passage of glomerular mesangial cells. Journal of cell science. 1996;109(Pt 10):2521–2528. doi: 10.1242/jcs.109.10.2521. [DOI] [PubMed] [Google Scholar]
- 35.Gao S, Alarcon C, Sapkota G, Rahman S, Chen PY, Goerner N, Macias MJ, Erdjument-Bromage H, Tempst P, Massague J. Ubiquitin Ligase Nedd4L Targets Activated Smad2/3 to Limit TGF-beta Signaling. Mol Cell. 2009;36:457–468. doi: 10.1016/j.molcel.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A, Wild-type and Smad3-null MEFs were stimulated with vehicle or TGF-β (2 ng/ml, 1 h). HEK (B) and wild-type-MEF (C) were transfected with pCS2-Smad3 constructs (wild-type, T179V, S204A, S208A or S213A) for 18h. The transfected wild-type-MEF (but not the transfected HEK) were then treated with vehicle- or TGF-β- (2 ng/ml) for 1 h. Effect of GSK3 inhibition on Smad3 Linker Region Phosphorylation (D). Wild-type murine embryonic fibroblasts (MEF) were pre-treated (1 h) with vehicle (DMSO) or SB216763 (5μM) prior to vehicle- or TGF-β1 (2 ng/ml) stimulation for 1h. Smad3 phosphorylation at T179, S204, S208, S213 and S423/S425 were analyzed by western blotting using site-specific phospho-Smad3 antibodies. The upper of the two bands detected by the anti-phospho-Smad3-T179 antibody represents cross-reaction with the analogous site (T220) in the linker region of Smad2. Shown here are representative experiments of three independent experiments.
Wild-type and Smad3-null MEFs were exposed to experimental conditions as described in Figure 2B and C (± COL1A2-luciferase and β-galactosidase constructs, ± 20 μM PD98059 or 5 nM PD0325901, ± TGF-β1). Media was assayed for relative Lactate Dehydrogenase (LDH) activity (mean ± S.E.M., n=3). A positive control of the “total” possible LDH released from cells at equivalent confluence is shown. ****P<0.0001 vs. vehicle-treated cells.
HKC were exposed to experimental conditions as described in Figure 4B (± COL1A2-luciferase and β-galactosidase constructs, ± 20 μM PD98059, ± TGF-β1). Media was assayed for relative Lactate Dehydrogenase (LDH) activity (mean ± S.E.M., n=3). A positive control of the “total” possible LDH released from cells at equivalent confluence is shown. ****P<0.0001 vs. vehicle-treated cells.