Abstract
Malformations of cortical development (MCD) result from abnormal neuronal positioning during corticogenesis. MCD are believed to be the morphological and perhaps physiological bases of several neurological diseases, spanning from mental retardation to autism and epilepsy. In view of the fact that during development, an appropriate blood supply is necessary to drive organogenesis in other organs, we hypothesized that vasculogenesis plays an important role in brain development and that E15 exposure in rats to the angiogenesis inhibitor thalidomide would cause postnatal MCD. Our results demonstrate that thalidomide inhibits angiogenesis in vitro at concentrations that result in significant morphological alterations in cortical and hippocampal regions of rats prenatally exposed to this vasculotoxin. Abnormal neuronal development was associated with vascular malformations and a leaky blood–brain barrier. Protein extravasation and uptake of fluorescent albumin by neurons, but not glia, was commonly associated with abnormal cortical development. Neuronal hyperexcitability was also a hallmark of these abnormal cortical regions. Our results suggest that prenatal vasculogenesis is required to support normal neuronal migration and maturation. Altering this process leads to failure of normal cerebrovascular development and may have a profound implication for CNS maturation.
Keywords: cortical dysplasia, angiogenesis, brain development, vasculogenesis, blood–brain barrier, endothelium
Malformations of cortical development (MCD) result from abnormal neuronal positioning during corticogenesis. Genetic/epigenetically-induced MCD represent one of the most common etiologic factors associated with CNS pathologies, i.e. neurological deficits, autism, developmental delay, and epilepsy (Taylor et al., 1971; Robain, 1996). While the exact mechanisms underlying MCD are unknown, it is clear that single genes may be responsible for distinct MCD including lissencephaly, subcortical band heterotopias, periventricular nodular heterotopia, and tuberous sclerosis (Gardiner, 1999; Steinlein, 2004). Although the exact pathogenesis of MCD remains unknown, environmental factors also appear to be involved (Montenegro et al., 2002; Montenegro, 2003; Bassanini and Battaglia, 2006).
Animal models of CNS disorders have provided a crucial step toward both the understanding of mechanisms involved and discovery of therapeutic factors. In the case of MCD, the animal model that most realistically recapitulates the morphology of human cortical dysplasia is based on prenatal exposure to methylazoxymethanol acetate (MAM; Battaglia et al., 2003a). A prenatal exposure to this putative anti-proliferative and cytotoxic agent (Cattaneo et al., 1995) induces cerebral heterotopias that share striking similarities with those observed in humans (Colacitti et al., 1999; Battaglia et al., 2003a). MAM-induced ablation of early cortical neurons may alter migration and differentiation of subsequently generated neurons, leading to the formation of heterotopia.
While the commonly accepted mechanism of action of MAM and related neuro-teratogenic compounds implicates death of vulnerable neuronal precursors (Cattaneo et al., 1995), other reports have emphasized that MAM-treated animals display an array of pathological features encompassing vascular (Bardosi et al., 1985a,b, 1987) and glial (Gierdalski and Juliano, 2003; Hasling et al., 2003) changes. For example, vascularization of the malformed cortex showed severe damage to the layered distribution of vascular trunks. A pathological course and marked variability in radial vessel density were seen in cortical areas where neuroblast migration was most severely affected. A decisive role of neuroblast migration and maturation in cortical angioarchitecture development was hypothesized (Bardosi et al., 1985a,b, 1987; Hallene et al., 2005). Thus, in addition to neuroblast toxicity, a parallel process targeting the vasculature may be responsible for MCD. Supporting the notion that prenatal vasculogenesis and postnatal (PN) angiogenesis may parallel neurogenesis is the recent finding that neurogenesis is accompanied by the formation of new capillary networks (Palmer et al., 2000; Haigh et al., 2003). Furthermore, the angiogenesis inhibitor thalidomide (THAL) (Franks et al., 2004) causes brain malformations in humans and animal models (Narita et al., 2002; Teitelbaum, 2003; Miyazaki et al., 2005). Finally, prenatal exposure to THAL has been linked to adult or neonatal onset of seizures (Stephenson, 1976; Kanno, 1987).
This prompted us to test the hypothesis that vascular development is an essential step in corticogenesis and that agents which interfere with this process may cause malformations of brain development. To assess this, we monitored cortical development of E15 Sprague–Dawley rats exposed to the angiogenesis inhibitor THAL.
EXPERIMENTAL PROCEDURES
In vitro experiments
Bovine aortic endothelial cells (BAEC; provided by Dr. Helen Sage, University of Washington, Seattle, WA, USA), human endothelial cells (Dr. Bingaman, Cleveland Clinic, Cleveland, OH, USA), and rat microvascular endothelial cells (Dr. Ljiljiana Bengez, Cleveland Clinic) were cultured as previously described (Stanness et al., 1997; Marroni et al., 2003b). Cells were maintained in a humidified 5% CO2 incubator at 37 °C in DMEM (BioWhittaker, Walkersville, MD, USA) with 10% FBS (Sigma, St. Louis, MO, USA) supplemented with 100 U/ml penicillin G, 100 U/ml streptomycin (Gibco BRL, Gaithersburg, MD, USA). THAL (Sigma) was added in increasing concentrations (0–200 µM). Cultures were grown for 2–3 days following drug administration and were then examined for signs of toxicity. Cell counts were performed as described (Cucullo et al., 2005) and data from at least six wells per concentration were pooled together. Data are presented as mean±S.E.M. and statistical analysis was performed by ANOVA (Origin V6, Microcal Software, Inco, Northampton, MA, USA).
For experiments with Matrigel (0.20 mg/ml; BD Bioscience, Bedford, MA, USA), cells were first pre-treated for 2 days by culturing on tissue culture dishes. When at 40–60% confluence, cells were released with trypsin/EDTA, washed, spun down and re-suspended in medium. Matrigel preparation was added at 0.2 ml/cm2 to 48-well plates (Fisher, Pittsburgh, PA, USA); plates were incubated for 30 min at 37 °C in a 5% CO2 humidified chamber; cells were transferred to Matrigel-coated wells and plated at a density of 30,000 cells/cm2. Following 8 h of cell adherence, THAL was added directly to culture media. Cultures were grown for 2 days and the anti-angiogenic effects of the drug were examined. Estimation of tube formation was performed by analyzing images with QCapture Pro Software (Media Cybernetics, San Diego, CA, USA).
Animal handling
E12 pregnant Sprague–Dawley rats were acquired from the vendor (Harlan, Indianapolis, IN, USA). Animals were housed in the Cleveland Clinic Foundation (CCF) animal facility where food, water, and enrichment materials were provided in accordance with CCF and the National Institutes of Health guidelines for the Care and Use of Laboratory Animals. Procedures involving animals and their care were conducted in conformitiy with institutional guidelines that are in compliance with national (D.L.n.116, G.U., suppl.40, Feb. 18, 1992; UK legislation under the 1986 Animals [Scientific Procedures] Act) and international laws and policies (EEC Council Directive 86/609, OJL 358, 1, Dec. 12, 1987; Guide for the Care and Use of Laboratory Animals, U.S. National Research Council, 1996).
Prenatal exposure to THAL
Pregnant Sprague–Dawley rats received two doses of THAL (30 mg/kg maternal body weight, i.p. in sterile saline; 12 h apart), or vehicle alone (sterile saline) on day E15.
At various PN stages, control or THAL-treated rats were deeply anesthetized with ice (P1–P7) or by an i.p. injection of 40 mg/kg sodium pentobarbital (P15–adulthood; Abbott Laboratories, Abbott Park, IL, USA). Animals were moved to a designated surgery area and positioned supine on a surgical towel to expose the ventral surface, and the thoracic skin was removed. After surgical exposure of the ribcage, the midline thoracic cutis was incised and the sternum opened. The apex of the left ventricle was cannulated with a 24-gauge polyethylene catheter (BDInsyte, BDVialon, Becton Dickinson, Mountain View, CA, USA), and perfusion (1 ml/min) was performed with a fluorescent dye solution (fluorescein isothiocyanate, FITC; see (Cavaglia et al., 2001) and “solution composition” below). The right atrium was incised to prevent elevation in systemic blood pressure. Immediately following perfusion, animals were decapitated and the brains removed. The lag time between decapitation and immersion fixation never exceeded 2 min.
Brains were immersion fixed for 24–48 h in 10% formalin solution, then cryoprotected in 30% sucrose solution. Brains were mounted on a cryostat and cut in the coronal plane at 10 µm. Sections were collected and floated in 0.1 M phosphate buffer solution and mounted on positive-charged, glass slides. Sections were dried, processed and coverslipped using Vectashield mounting medium with 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA, USA). For some experiments, including those implicating morphological processing of slices used for electrophysiology, tissue was fixed and cut on a Vibratome at 200–400 µm.
Bovine desiccate albumin–fluorescein isothiocyanate (FITC–albumin) solution composition
The fluorescent albumin solution was prepared by reconstituting 500 mg of FITC–albumin (MW 69 kDa; 12 mol/mol albumin; Sigma) in 50 ml of phosphate-buffered saline (0.1 M PBS) lacking magnesium and calcium ions. The FITC solution was stirred at room temperature in the dark for 5 min prior to injection.
Microscopy and immunohistochemistry
To investigate the expression of anti-angiogenic properties induced by THAL in cell cultures, bright-field microscopy and fluorescent microscopy were used in conjunction; Vectashield mounting medium with DAPI was used to visualize cell nuclei. Vessel length was estimated by QCapture Pro Software (Media Cybernetics). At least 12 representative tubes per well were measured. Only the initial part of the tube was measured in tubes characterized by visible gaps.
For morphological analysis, brains from control and THAL-treated animals were removed, fixed in 10% formalin and cryoprotected in 30% sucrose until use. Sections were processed with Cresyl Violet and/or fluorescence to visualize blood vessels and cells. Immunostaining was performed as previously described (Marroni et al., 2003a). Primary antibodies used were: mouse anti-neuronal nuclei (NeuN) monoclonal antibody (1:500; Chemicon International, Temecula, CA, USA), rabbit anti-neurofilament 200 kDa polyclonal antibody (1:200; Chemicon International), monoclonal anti-glial fibrillary acidic protein (GFAP) clone G-A-5 mouse ascites fluid (1:200; Sigma), and guinea-pig anti-doublecortin (DCX; 1:3000; Chemicon International). Secondary antibodies used were as follows: Texas-Red dye conjugated Affinipure donkey anti-mouse IgG (1:100; Jackson Immunoresearch, West Grove, PA, USA) and FITC-conjugated Affinipure donkey anti-rabbit IgG (1:200; Jackson Immunoresearch).
Rat brain sections were also stained with lectin to analyze densities and profiles of cerebral blood vessels. Sections were blocked in PBS (1% BSA and 0.5% Triton X-100) for 1 h at room temperature. They were rinsed in PBS, washed twice with PBLec (phosphate-buffered saline, pH 6.8, containing in mM: 0.1 CaCl2, 0.1 MgCl2, 0.1 MnCl2, and 1% Triton X-100), and incubated in biotinylated isolectin B4 (20 µg/ml; Bandeiraea simplicifolia, Sigma L-2140), in PBLec at room temperature for 2 h. After five washes with PBS, sections were incubated with streptavidin conjugates (CY3; Sigma), diluted 1:100 in PBS containing 0.5% BSA and 0.25% Triton X-100 at room temperature for 2 h. Sections were then rinsed with PBS and coverslipped with Vectashield mounting medium containing DAPI.
To examine if there was a presence of degenerating neuron populations, Fluoro-Jade antibodies (Histo-Chem, Inc.; Fernandes et al., 2004) were used. Mounted sections were dried and placed in 100% ethanol for 3 min followed by 70% ethanol for 1 min. Sections were then placed in water for 1 min and subsequently placed in 0.06% KMnO4 for 15 min on a shaker. After this, sections were rinsed in water for 1 min and incubated in 0.001% Fluoro-Jade for 30 min on a shaker. Slides were washed in water (3× for 3 min each), dried, and mounted with DPX mounting medium (Electron Microscopy Services, Ft. Washington, PA, USA).
Western blot
Brains from control and THAL-treated E16 prenatal animals were removed, snap frozen in liquid nitrogen, and stored at −80 °C until use. Identification of vascular endothelial growth factor (VEGF) protein in E16 prenatal rats was performed by Western blotting techniques. Protein extracts from THAL-treated prenatal rat brains were dissolved in radial immunoprecipitation assay (RIPA) buffer (0.5% deoxycholic acid, 1% NP-40, 0.1% SDS) containing protease inhibitors (0.17 mg/ml PMSF, 2 µg/ml leupeptin, and 0.7 µg/ml aprotinin; Sigma). Prior to electrophoresis, protein extracts were denatured by heating at 100 °C for 5 min in a running buffer solution containing RIPA, β-mercaptoethanol, and Bromophenol Blue tracking dye. The acrylamide gel (12%, pre-cast gels; Biorad Laboratories, Hercules, CA, USA) was run for 2.5–3 h at constant voltage (80 V) until the Bromophenol Blue tracking dye migrated to the bottom edge of the gel. Proteins from each gel were transferred onto a blot of PVDF membrane using a 192 mM glycine, 25 mM Tris-base, 20% methanol buffer system at constant current (40 mA) overnight at 4 °C. VEGF primary antibody was probed overnight at 4 °C (VEGF A-20; 1:500; Santa Cruz Biotechnologies, Santa Cruz, CA, USA). Blots were washed and treated with goat anti-rabbit IgG HRP-conjugated secondary antibody (1:2000; Dako Corporation, Carpentaria, CA, USA). Protein concentration was estimated according to the Bradford assay method. Relative expressions of protein were determined by densitometric analysis using Phoretix 1D Advanced Software (Newcastle upon Tyne, UK).
Matrigel in vivo angiogenesis assay and hemoglobin content
Matrigel (0.20 mg/ml; BD Bioscience) matrix and supplies were kept on ice; a wide s.c. pocket was formed by swaying the needle right and left after a routine s.c. injection. Following this, 500 µl of Matrigel alone or Matrigel+THAL (100 µM) was mixed on ice and subsequently injected into mice s.c. with a 21G1 needle. After 3 days of incubation, the mice were anesthetized and ~5 mm square tissue segments containing s.c. tissue, peritoneum, and skin were excised with scissors to ensure complete removal of the Matrigel plug. The levels of hemoglobin were analyzed using the Drabkin’s Reagent method following the manufacturer’s protocol (Sigma).
Slice electrophysiology
PN rats were deeply anesthetized with isoflurane and then decapitated. The brain was quickly removed from the skull and immediately placed into ice-cold oxygenated artificial cerebrospinal fluid (aCSF). The brainstem and cerebellum were dissected out. The central section of the cerebral hemisphere containing somatosensory cortical regions was subsequently cut in the sagittal plane into slices of 400–500 µm thickness using a vibratome. Slices were kept in a pre-incubation chamber containing oxygenated (95% O2; 5% CO2) Mg2+-free aCSF at room temperature and pH 7.4 prior to recording. For electrophysiological recordings, a slice was placed in a submerged recording chamber and constantly superfused with oxygenated Mg2+-free aCSF at a flow rate of 3–4 ml/min at 32 °C. Extracellular field potentials (FP) were recorded using borosilicate glass micropipettes (0.5–2 MΩ) filled with aCSF.
Tissue morphology and cellular composition were observed using a Hamamatsu infrared camera. Images were acquired with QCapture Pro Software (Media Cybernetics). Electrophysiological recordings were performed from areas where visible vascular malformations could be detected. These consisted of unusually large, penetrating pial vessels and large vessels running parallel to the pial surface.
RESULTS
For the experiments described here, we used a total of 186 rat pups born from eight control and eight THAL-treated dams. Examination of gross brain anatomy unveiled several major differences between treated and untreated animals (Fig. 1A). THAL animals were microcephalic compared with controls; this persisted at all ages tested and was not gender specific. In addition, the number of live births per dam was also significantly affected by prenatal THAL exposure. THAL treatment led to fetal resorption, resulting in a lower number of pups per mother (THAL: 10.5±0.87; control: 13±0.73, P<0.05; Fig. 1B). Table 1 summarizes gross brain anatomy findings and histological properties found in this study, while Table 2 quantitatively summarizes specific defects found in both pre- and PN THAL-treated animals.
Fig. 1.
Anatomical and developmental effects of THAL. (A) Assessment of gross brain anatomy. Upon examination of brain anatomy from both treated and untreated animals, several major differences were unveiled. When compared with control animals, THAL-treated animals were found to be microcephalic. These findings were evident at all ages and were independent of gender. (B) Live births. Treatment with THAL in E15 pregnant rats led to fetal resorption; the numbers of live births per treated dams were significantly decreased when compared with control animals.
Table 1.
Summary of gross anatomical findings and physical characteristics found in THAL-treated rats
| Characteristic | MAM | THAL |
|---|---|---|
| Dysplasia–heterotopia | Mainly CA1 dispersion; heterotopia; rostro-caudal gradient; defects increase with age; thickness of superficial layers | Heterotopic nodules; rostro-caudal gradient; defects increase with age; thickness of superficial layers (i.e. Fig. 9A) |
| Vascularity | Large vessels; minimal vessel leak | Large vessels; leaky vessels |
| Gross findings | Abnormal cellular distribution; microcephalic | Abnormal cellular distribution; microcephalic; exaggerated thickness of sub-pial cortical layers |
| Electrophysiology | Increased excitability | Increased excitability/synchronization |
These findings are compared with changes reported by us and others after exposure to MAM acetate (Bardosi et al., 1985a, 1987; Sancini et al., 1998; Chevassus-Au-Louis et al., 1999; Baraban et al., 2000; Battaglia et al., 2003a,b).
Table 2.
Quantitative analysis of pre- and postnatal defects present in THAL-treated animals
| Defect | Prenatal | Postnatal |
|---|---|---|
| Brain size | Decreased; 100% (n=6) | Decreased; 100% (n=28) |
| Cortical thickness | Increased; 80% (n=3) | No change (n=28) |
| Heterotopic cells | Not obvious (n=6) | |
| Hippocampus | Hippo: 80% (n=28) | |
| Cortex | Cortex: 50% (n=28) | |
| Abnormal cortical layering | 90% (n=6) | 90% (n=28) |
| Abnormal blood vessels | 100% (n=6) | 100% (n=28) |
| BBB leakage | NA | 100% (n=12) |
| Electrophysiological change | NA | 100% (n=7) |
Morphological changes in prenatal development after exposure to THAL
In animals killed at E16, E19 or E21 after THAL treatment (E15), we observed little or no change in gross head histology (Fig. 2A). However, brain cortical thickness was significantly increased in exposed pups, possibly due to an enlarged extracellular space reflecting interstitial edema, as shown in Fig. 2B–C. A similar increase in cortical thickness was seen after acute exposure to MAM (not shown). Staining for apoptotic markers revealed that no toxicity was exerted by the treatment. In general, the most striking acute effect of THAL was seen when analyzing the profiles and densities of cerebral blood vessels stained with lectin (Fig. 3A). Note that following THAL treatment, the fine branching of penetrating pial vessels was dramatically decreased. This was paralleled by an increased diameter of the principal arteriolar elements (white arrows in Fig. 3). The quantitative evaluation of these qualitative findings is shown in Fig. 3B. Note that treated cortex exhibited longer high-caliber vessels while displaying a significantly lower capillary density.
Fig. 2.
Histological changes induced by THAL at pre-natal day 16. (A) THAL did not induce any significant, gross changes in the histology of the head as revealed by Cresyl Violet staining of coronal sections. (B) THAL caused acute, significant changes in cortical organization. Note the disorganization of neuroepithelial cells, as well as the thickening of the cortical plate. No evidence of cell death was seen at this stage. Overall, the cortical thickness of THAL-treated embryos was slightly, but significantly increased (C).
Fig. 3.
THAL suppresses angiogenesis both in vitro and in vivo and decreases fetal VEGF expression. (A) The most prominent effect of THAL was on the development of the capillary network of the cortex. Note that in control, a thick network of vessels was observed, while in THAL-treated animals, penetrating pial vessels did not branch to form capillaries. (B) Quantitative evaluation of the findings found in A. Although THAL-treated cortex had significantly decreased capillary densities, it did exhibit larger vessels. (C–E) Note that all concentrations of THAL dramatically reduced Matrigel-induced tube formation in BAECs. All THAL data points were statistically significant from control (* P<0.05; ** P<0.001 by ANOVA; n=6 wells per concentration). Prenatal exposure to THAL also drastically reduced VEGF levels in fetal brain. The differences were statistically significant (P<0.05). Note that additional in vivo experiments utilizing the Matrigel plug assay revealed a remarkable potency of THAL to inhibit angiogenesis (right panel in C, P<0.05).
Anti-angiogenic effects of THAL in vitro and in vivo
In the embryo, vascular networks are developed through both vasculogenesis, the assembly of vessels from endothelial progenitor cells or hemangioblasts, and angiogenesis, the sprouting of vessels from pre-existing capillaries. Since a major effect of early exposure to THAL was composed of changes consistent with altered vasculogenesis, and because THAL is a specific inhibitor of angiogenesis, we explored concentrations of THAL necessary to inhibit BAEC tube formation in the in vitro Matrigel assay. The results are shown in Fig. 3C. Parallel cultures were established on fibronectin-coated Petri dishes to determine the toxicity of THAL on BAEC (not shown). At all concentrations tested (range 1–200 µM), THAL caused a dramatic and statistically significant reduction of the mean length of vessel-like protrusions induced by Matrigel. Identical results were obtained with cultures of rat brain microvascular endothelial cells (data not shown). At the concentrations used for these experiments, THAL failed to exert any toxicity. Additional in vivo experiments measuring hemoglobin content confirmed the unusual potency of THAL (100 µM) in inhibiting angiogenesis in a mouse Matrigel model (right panel of Fig. 3C; P<0.05).
The anti-angiogenic effects of THAL appear to involve VEGF (D’Amato et al., 1994). Therefore, we measured VEGF levels in fetal brain after exposure to THAL to demonstrate a sharp decline in VEGF levels 48 h after exposure. These experiments were performed by Western blot analysis (Fig. 3E) and confirmed in other animals by ELISA (Fig. 3D). The other target of THAL action, tumor necrosis factor-α, was unaffected (not shown).
Prenatal neuroarchitectural changes
While the most remarkable effect of intrauterine exposure to THAL was seen when sections were processed for vascular markers; Cresyl Violet sections revealed slightly altered cytoarchitecture of the developing cortex (E16; Fig. 4C). Sections stained with the immature neuronal marker DCX (Clark, 2001; Fernandes et al., 2004), demonstrated mild disorganization of normal layering that paralleled the obvious cortical swelling. Since the cortex of THAL-treated fetuses was thicker, this cytoarchitectonic change was best appreciated when the two cortices were scaled to the same final size (as shown in Fig. 4B, C). Note that the increased DCX positive band was fully accounted for by increased cortical thickness. To confirm that cortical swelling may be due to brain edema, we measured the levels of aquaporins 1 and 4, both associated with brain edema (Amiry-Moghaddam and Ottersen, 2003; Manley et al., 2004). Fluoro-Jade was also used to assess populations of degenerating or otherwise damaged neurons. Note that there were no changes seen between control and THAL-treated animals (Fig. 4D), suggesting that no direct cytotoxicity was exerted by THAL.
Fig. 4.
Prenatal neuroarchitectural changes. (A–C) Sections stained with Cresyl Violet and DCX revealed disorganization in normal cellular layering and paralleled noticeable cortical swelling. The thickened cortex of THAL-treated animals is best observed when compared side by side with control animals (B and C). Antibodies against Aquaporins 1 and 4 were used to confirm the presence of cortical swelling due to brain edema (left panel in C). (D) Staining with Fluoro-Jade was performed to examine degenerating neuron populations in THAL-treated animals. Note that there were no significant differences observed between control and THAL treatments.
Neuroarchitectural changes and vascular profiles induced by THAL: early PN development
Brain morphology and vascularity were studied at various PN stages (days 4–60). The results are shown in Figs. 5, 6 and 7. Note that abnormal cellular distribution and vascular abnormalities were seen as early as 4–8 days PN (Fig. 5A–G). In these animals, clusters of misplaced cells were observed in white matter bundles running along both cortical and hippocampal structures. These abnormal cellular clusters were invariably accompanied by either unusually large vascular formations or leaky vessels. In the cortex, large bundles of cells were often observed in THAL-treated but not control animals (Fig. 5D). Strikingly, these agglomerates of cells in the former were seen as migrating cells parallel to the pial surface (see also Fig. 7E), while in control animals, neuronal migration occurred as expected, perpendicular to the pial surface and was completed after birth.
Fig. 5.
THAL induced neuro-architectural changes and vascular profiles in early postnatal development: Pregnant Sprague–Dawley rats were injected at E15. Brains were studied at various postnatal stages as indicated. (A, B) Hippocampal changes in early (PN 4) development: Note the areas of leakage in the vasculature indicated by the arrows and the overall normal laminar structure of the hippocampus. Neuro-architectonic and vascular defects in PN 8 animals. Note the abnormal ectopic neuronal cluster in C, as well as the pear-shaped cluster in D. An additional heterotopic nodule is shown in E. The original micrographs were stained with DAPI to visualize cell nuclei and FITC–albumin to show blood vessels and BBB leakage. Notice that the figures were also split into individual colors (black and white) to emphasize the relationship between cellular and vascular changes. (F, G) Lack of pyramidal cell banding in the entorhinal cortex and distorted white matter bands were observed in THAL-treated rats as early as PN 8. (H–K) Vascular abnormalities and leakage were present throughout the brain, as shown in these pictures taken from a PN day 12 animal. (L1–M) In PN 20 animals, the continuity of CA1 and CA3 subfields in hippocampal pyramidal layers was interrupted by large gaps (L1). The FITC–albumin-labeled section (L2) revealed that these gaps corresponded to large, leaky vascular formations. Leakage and intrapyramidal cell layer large vessels were rarely observed in untreated animals (M).
Fig. 6.
Neuro-architectural disorganization and vascular changes in mature rats. THAL-treated animals (ranging from 26 to 60 days PN) demonstrated an exaggerated thickening in the sub-pial cortical layers. The layers were infiltrated with large penetrating pial vessels (asterisks in A, B). Unusually large, penetrating pial vessels running deep into the cortical layers, sometimes reaching white matter, characterized these layers. Vessels were traced in red with a camera lucida program to emphasize the cerebral vasculature. The dashed line represents the pial surface (C). In control animals, these vessels run parallel to the axonal fibers. THAL-treated animals also experienced extensive disorganization in hippocampal architecture (D–G2). Sub-cortical white matter was also found to have scattered large vascular elements present. Red arrows in D–G point to abnormal vasculature characterized by exaggerated size and erratic topography. Dispersion of DG and CA-1 cells was almost invariably seen in these hippocampi. Additional ectopic nuclei associated with leaky vessels are shown in G (dotted lines). At more rostral levels, the hippocampus and cortex of treated rats were not immediately distinguishable from naïve controls (H1). However, more ventral/caudal areas were commonly characterized by massively altered DG morphology (H2). For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.
Fig. 7.
Cellular and BBB abnormalities in THAL brain. (A–E) NeuN staining confirmed the neuronal nature of dysplastic cell clusters (dotted lines in A). Bands of migrating cells forming columnar structures perpendicular to the ventricular surface and grossly abnormal borders in CA1 and CA3 regions of the hippocampus (dotted lines in B) were common features in THAL-treated but not in control animals. (C) Exaggerated cortical layer found in the entorhinal cortex (dotted line) of THAL-treated animals. Note the “normal” appearing, but leaky, vessels. Small vascular networks occasionally “housed” heterotopic areas positive for NeuN (D). Numerous cortical abnormalities demonstrated by NeuN staining were present throughout postnatal development in treated animals. Although the vast majority of cells in cortical areas were NeuN-negative, NeuN-positive cells were still evident in white matter layers of cortices of PN day 26 animals (E). The inset shows the relationship of the actual micrograph to the overall morphology of the actual brain slice.
The layering of the entorhinal cortex was less pronounced in THAL versus control rats (Fig. 5F, G). In particular, the most superficial layers of the cortex were not as clearly defined as in control. While abnormal, leaky vessels were seen most commonly in cortical structures, all brain regions analyzed were affected (Fig. 5H–K).
Neuroarchitectural changes and vascular profiles induced by THAL: late PN development
In older animals (e.g. PN20, Fig. 5L–M), abnormal neuronal clusters persisted in both cortical and hippocampal structures. An additional abnormality was observed in hippocampal pyramidal layers, where large gaps were seen interrupting the continuity of CA1 and CA3 pyramidal cells (Fig. 5L1, L2). In FITC-labeled sections, these gaps were shown to correspond to large vascular formations characterized by leakiness. In untreated animals, leakage and transverse vessels were not observed (Fig. 5M).
A common feature of cortical structures in THAL-treated animals was an exaggerated thickness of the sub-pial cortical layers (Fig. 6A–B). These superficial layers were crossed by large penetrating pial vessels (asterisks in Fig. 6A–B). In THAL-treated animals, cortical and subcortical regions were invariably associated with abnormal, large penetrating pial vessels that run deep into the cortical layers sometimes reaching into the white matter, whereas these vessels run parallel to the axonal fibers in control animals (Cavaglia et al., 2001). Fig. 6C shows a side-by-side comparison of penetrating pial vessels in control and THAL-treated animals.
In addition to cortical abnormalities, adult THAL-treated rats were also characterized by extensive disorganization of the hippocampal cytoarchitecture (Fig. 6D–G). Large vascular elements were observed in the sub-cortical white matter (red arrows in Fig. 6D–E); these were associated with clusters of heterotopic neurons in all hippocampal layers (blue arrow in E and inset). Fig. 6F1–G2 shows additional evidence for granular cell dispersion and abnormal pyramidal cell layering. In more rostral regions, these departures from normal cytoarchitecture become less pronounced, and there were in fact no obvious differences between treated and untreated animals (Fig. 6H1). However, areas more ventral/caudal were characterized by massively altered dentate gyrus (DG) morphology in THAL rats (Fig. 6H2).
The neuronal nature of heterotopic clusters was confirmed by NeuN staining (Fig. 7A). A grossly abnormal CA3–CA1 border was also a common feature, with bands of seemingly migrating cells forming columnar structures perpendicular to the ventricular surface (Fig. 7B). In control animals of comparable age, all pyramidal cell layers in the hippocampus as well as the granule cell layer, were characterized by sharp boundaries as expected.
Band heterotopia or double cortex syndrome is a common feature of MCD. In one THAL-treated animal, an exuberant cortical layer was found in the entorhinal cortex (dotted line in Fig. 7C). This layer appeared to originate above the normal pial surface and was situated on top of “normal” appearing (but leaky) pial vessels. Heterotopic structures were occasionally found to be enveloped in small vascular networks (Fig. 7D). Neocortical abnormalities were presented throughout PN development, as shown in Fig. 7E. In contrast to control animals where clear-cut cortical layering was observed after birth, NeuN positive cells could still be found in white matter layers in PN 26 cortices.
Leakage of serum protein
In addition to shielding the brain from potentially harmful agents, the blood–brain barrier serves as a shield to protein extravasation (Grant and Janigro, 2004). Recently, Seiffert et al. (2004) have established a model for focal cortical disruption of the blood–brain barrier resulting in extravasation of serum albumin to the brain extracellular space. This was associated with epileptiform activity that could also be induced by direct cortical application of serum or albumin. The authors concluded that direct uptake of albumin by neurons may be one of the mechanisms leading to seizures. We tested this by analyzing the sections used to determine vascularity and not used for light microscopy or immunofluorescence (Fig. 8A, B). Note that brief exposure to intravascular FITC–albumin (less than 5 min following intra-cardial injection) resulted in a significant accumulation of albumin in neuronal (but, surprisingly, not glial (Fig. 8C, D, and E)) cells. It should also be noted that in areas of abnormal pyramidal layers or in DG, defective cellular layering was often accompanied by vascular hypertrophy. Thus, it appears that the leakage of the blood–brain barrier observed in these animals was sufficient to cause extravasation of serum albumin to levels that allowed significant intra-neuronal accumulation.
Fig. 8.
Cellular and structural abnormalities in older animals. The blood–brain barrier serves to protect the brain from harmful toxins. In addition to this, the barrier functions to block protein extravasation. Both properties appeared compromised in THAL-treated animals. (A, B) Abnormalities in hippocampal (HI) structures were accompanied by large regions of vascular leakage. Observe that brief exposure to intravascular FITC–albumin (less than 5 min) resulted in a considerable accumulation of albumin in neuronal cells (B) but, surprisingly, not glial cells (C). Figure D and E shows that GFAP-positive glia do not accumulate fluorescent albumin, while CA-1 pyramidal cells do.
Electrophysiological properties
Disturbances of neuronal migration result in abnormal electrical activity, and heterotopic neurons can contact areas outside of their normal synaptic range (Colacitti et al., 1998; Calcagnotto et al., 2002). Furthermore, extravasation of serum protein in the CNS is epileptogenic (Seiffert et al., 2004). We performed electrophysiological experiments on slices prepared from brains dissected from THAL-treated animals (Figs. 9–10). Recordings were also performed from age-matched control animals. Fig. 9A and B shows field potentials obtained from different but neighboring cortical regions in a slice from a THAL-treated rat (PN 17). The recordings highlighted in red refer to fields obtained in a region characterized by exaggerated thickening of the pial layers, and an exorbitant number of penetrating pial vessels. Histological and immunocytochemical analysis performed on comparable sections suggested that these regions are often characterized by abnormal neuronal cytoarchitecture. Field potential recordings of zero-Mg-induced bursts revealed a dramatic difference in firing pattern in regions characterized by vascular dysplasia compared with cortex that appeared normal both during the experiment and after staining with Cresyl Violet. Recordings from the trans-cortical axis parallel to the abnormal vasculature were characterized by intermittent bursts separated by longer inter-burst intervals. Each burst was immediately followed by after-discharges which were not present in comparably normal cortex. Burst duration and frequency were also significantly increased in recordings from dysplastic cortex (Fig. 9B, D).
Fig. 9.
Electrophysiological properties of THAL-induced abnormalities in rat brain. (A) Electrophysiological properties of heterotopias. Recordings were obtained from different cortical and hippocampal regions in both THAL-treated and control animals (not shown). Histological examination with Cresyl Violet revealed that these regions appeared endowed with seemingly normal cytoarchitecture characterized by obvious vascular malformations in slices obtained from treated animals. These vascular changes were typical of THAL-treated animals and consisted of unusually large and numerous penetrating pial vessels and layer 1 thickening. In these slices, zero-Mg2+ induced field recordings (red) consisted of intermittent bursts, followed by after-discharges; these discharges were not present in comparable normal cortex (blue). (B) Plots of burst duration and frequency show two distinct populations in dysplastic cortex, while normal cortex has a single population. This is consistent with the presence of regular spiking and after-discharges recorded in the dysplastic but not in the “normal” region. Data were analyzed with Clampfit and fitted with Gaussian functions. (C) Other heterotopic areas yielded similar results. A neuronal cluster in the olfactory cortex (dotted line in Cresyl Violet; scale bar=200 µm) of a PN day 17 THAL-treated animal exhibited an abnormal firing-pattern characterized by several hundred clustered bursts at regularly paced intervals. A comparable control slice from an identical location demonstrated short bursts occurring at variable intervals. This was the most commonly observed difference between dysplastic and normal cortex. (D) Recordings from dysplastic cortex demonstrated a significant increase in bursts. For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.
Fig. 10.
Further electrophysiological properties of THAL-induced abnormalities in rat brain. (A–D) Electrophysiological properties- synaptic recruitment. (A) Simultaneous recordings from two cortical regions of a PN day 25 rat exposed to THAL prenatally. The Cortex II region was chosen for its abnormal vasculature. Panel B shows the actual micrograph of the region as seen in phase contrast with the large vascular structure highlighted in red for clarity. Cortex I was comparably normal. Panel D highlights three samples of the recordings obtained in this region shown as full-scale traces in A. Note the increasing level of coupling between the two signals, the abnormal region (Cortex II) initially leading Cortex 1. The level of synaptic coupling is quantified in C, demonstrating the delay between the burst in Cortex I and Cortex II at different time points during the recording. For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.
Similar results were obtained in other heterotopic regions, as the one shown in Figure 9D (dotted line in Cresyl Violet). This cluster of neurons was found in the olfactory cortex of a THAL-treated animal; a recording from a comparable control slice from identical location is shown as comparison. Note that the predominant activity in normal olfactory cortex was composed of short, zero-Mg-induced bursts occurring at variable intervals, while the firing pattern in the dysplastic region consisted of several hundred action potentials clustered at regularly paced intervals.
The data presented so far demonstrate the alternately normal and abnormal regions of cortex, at least morphologically and by determining Mg-free bursting patterns. We wished to test, during prolonged recordings, if abnormal activity can eventually spread to “normal cortex.” The data are shown in Fig. 10A–D. Similar to the recordings shown before, a field electrode was placed in proximity of a dysplastic pial vessel (Fig. 10B; highlighted area in the phase contrast image). Another recording electrode was placed distally at approximately 4 mm in a seemingly normal region (Cortex I). Note that Cortex II behaved in a fashion that resembled the abnormal firing pattern shown in A. Cortex I was firing in a non-synchronous pattern at the beginning of the recording. A few action potentials were initiated in II and appeared in I with a mean delay of 187 ms (see Fig. 10C), while most action potentials did not spread either way. After approximately 15 min, the temporal firing convergence was clearly increased and propagation took approximately 109 ms. Finally, the firing occurred synchronously in both areas, with an inter-burst interval of 60 ms.
DISCUSSION
Two novel findings resulted from the present work: 1) we discovered a novel prenatal effect of the angiogenesis inhibitor and teratogen THAL, and 2) prenatal exposure to THAL resulted in gross PN CNS abnormalities that spanned from microcephaly to MCD. We have also provided evidence supporting the fact that drugs or events that affect vasculogenesis may promote the development of neurological defects by a parallel action on neurogenesis and neuronal migration.
Role of vasculogenesis in neuronal migration and cortical development
Despite numerous reports describing the events that promote CNS maturation, surprisingly little is known on how the development of the cerebral vasculature promotes, guides and/or sustains corticogenesis. By drawing on analogies with other organs, we hypothesized that affecting vasculogenesis may severely impact brain development. This was demonstrated in this study by showing that the inhibitor of vascular formation THAL, causes MCD similar to those observed with the putative neurotoxin MAM (Bassanini and Battaglia, 2006) and, more relevantly, also comparable to the abnormal morphology of the malformed human brain. In support of a significant link between angiogenesis and neuronal/brain development is a report that demonstrated that altered expression of VEGF receptor A causes developmental abnormalities in rodent brain (Haigh et al., 2003). Thus, increasing evidence, including data presented herein, supports a crucial role for vasculogenesis in the etiology of cortical malformations.
Two mechanisms account for the formation of blood vessels: vasculogenesis and angiogenesis. These two terms have the same meaning (genesis of blood vessels), and therefore, we have used in this manuscript the two terms interchangeably. Despite the nomenclature, the two processes are clearly distinct. Vasculogenesis is driven by the recruitment of undifferentiated mesodermal cells to the endothelial lineage, while angiogenesis is the generation of new blood vessels from endothelial cells of existing blood vessels or from circulating endothelial cell precursors (Asahara et al., 1997; Risau, 1997). Vasculogenesis in the embryo is a composite of two processes: the direct in situ formation of blood vessels from mesodermally derived angioblasts and the incorporation and differentiation of circulating endothelial cell progenitors into forming embryonic blood vessels (LaRue et al., 2003). Our experimental design did not mean to directly address how THAL affects these processes, but the data obtained by us and others (Denekamp, 1993; D’Amato et al., 1994; Kerbel and Folkman, 2002; Akhtar et al., 2002) suggest that endothelium-dependent vascular formation is a key target, possibly involving VEGF and bFGF. In support of this are the marked early effect of THAL on fetal VEGF expression and the dramatic effects on tube formation in the Matrigel assay. Interestingly, Palmer et al. (2000) have recently implicated VEGF in adult neurogenesis, opening the possibility that interference with adult angiogenesis may also cause formation of impaired neuronal networks. This is anecdotally supported by the fact that therapeutic THAL may severely, but transiently, impair cognitive function (Morgan et al., 2003).
Why is angiogenesis a factor in brain development?
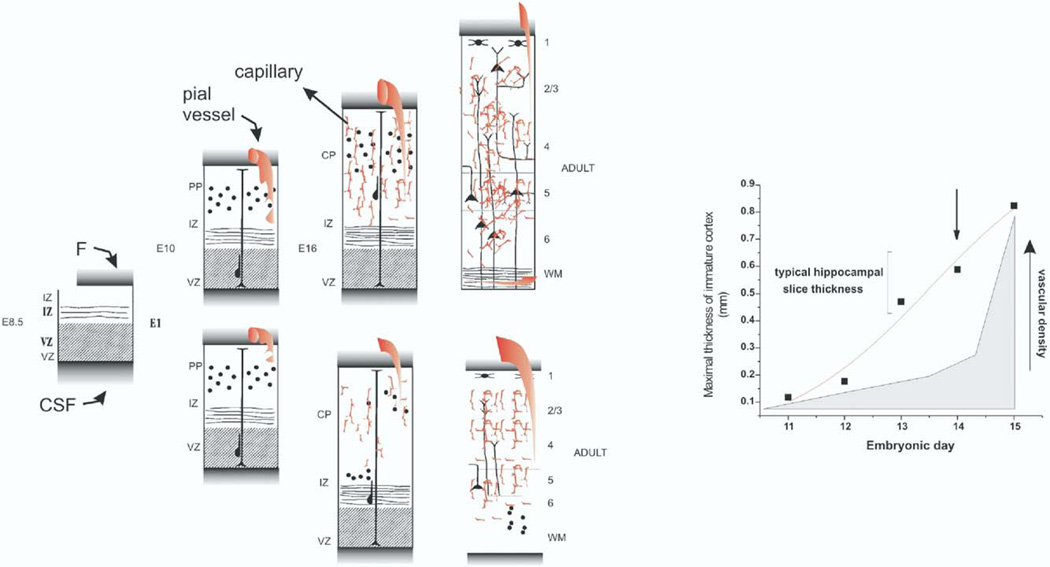
How relevant is angiogenesis for correct neuronal positioning and differentiation? From CNS slice experiments, it is known that brain tissue from newborn or adult animals can survive for several hours in an in vitro environment without intraparenchymal blood flow, and only by percolatory perfusion. A crucial thickness not to exceed 1 mm is commonly used (Bianchi et al., 1986; Wu et al., 2005). It is also known that metastatic growth into the brain is associated with a pronounced angiogenic response characterized by leaky, MRI-enhancing vessels (Leenders et al., 2003). Under both conditions, a critical thickness of less than 1 mm in diameter triggers release of angiogenesis promoters, such as hypoxia-inducible factor or VEGF. A predictor of these findings is that exposure to an anti-angiogenetic factor during brain development should reach its maximal effects during formation of a structure that is beginning to exceed this value.Our treatment with THAL produced significant cortical malformations when the drug was administered at E15. Given the rapid metabolism of the drug (Franks et al., 2004), it is likely that the peak effects on either neurons or vessels were achieved in a 24 h time frame. During this time, cortical development is greatly accelerated, the cortical structures achieve thickness well above 1 mm, and vascular infiltration in the cortex is pronounced (Altman and Bayer, 1994). Taken together, these findings suggest that angiogenesis is crucially involved in neuronal development only when the superfusion with cerebrospinal fluid becomes insufficient (see Fig. 11).
Fig. 11.
Cartoon representation of the hypothesis and results of the present work: These data were modified from data presented in the atlas by Altman and Bayer (1995). In particular, the thickness of cortical layers and vascular density were derived from Fig. 5 in the aforementioned book. The left panel represents the various stages of embryonic development. The acronyms have the usual meanings (CP, cortical plate; IZ, intermediate zone; PP, pre-plate; WM, white matter; VZ, ventricular zone). The normal development of the brain is shown in the upper panels. Note that in between E10 and E16 a large network of blood vessels is formed in parallel to the thickening of the cortical layers. In the adult animal, the capillary network is fully developed and originates from thin penetrating vessels. The capillary network in the adult originates from thin penetrating pial vessels as shown in the rightmost part of the figure. The bottom panel summarizes the events that may happen following an inhibition of angiogenesis. Vascular formation is aborted at a crucial time point (indicated by the arrow in the right panel) and as a result, neuronal migration and cortical development are reduced. In the adult animal, embryonic-type vessels remain in the cortical layers. These vessels are of unusually large proportions, and common in regions of abnormal brain development. The right panel shows data summarizing the concept expressed on the left. Note that the maximal thickness of the hippocampal and cortical layer develops rapidly throughout the embryonic gestation phase. Also note that the vascular density increases sharply following crossing of a crucial point of 0.5 mm threshold. See Results and Discussion for full details.
THAL, epilepsy and brain development
The annual incidence of epilepsy in the first seven years of life is five times that of the general population, and the prevalence of active epilepsy is significantly increased in the teenage THAL population. That this increased incidence and prevalence of epilepsy is not a chance observation is supported by published clinical and experimental evidence of CNS abnormalities in THAL embryopathy, in addition to the known neurological effects of the drug in the adult (Stephenson, 1976; Kanno, 1987).
While this is the first report of cortical and hippocampal disorganization following prenatal exposure to THAL, a recent report demonstrated that embryonic exposure to THAL or valproic acid (VPA) before neural tube closure causes abnormalities of the serotoninergic system similar to what is often observed in human autism (Narita et al., 2002; Miyazaki et al., 2005). When pregnant rats were exposed to THAL or VPA on embryonic day 9, a dramatic shift of the distribution of serotoninergic neurons in the dorsal raphe nucleus was observed postnatally. The relevance of this study is underscored by the fact that THAL exposure during human embryogenesis may lead to autism and epilepsy (Kanno, 1987; Stromland et al., 1994). How these data relate to what is shown in the present study remains to be fully elucidated. In particular, it is not clear why, regardless of the phenotype studied (raphe nuclei or hippocampus/cortex) or the drugs used (VPA or THAL), the neuronal or behavioral deficits require time to develop, even when the exposure is as early as E9; how different species respond to these early insults, and how this relates to what is seen in humans.
Functional consequences of THAL exposure
Ample electrophysiological evidence suggests that the MAM brain is characterized by abnormal electrical activity, even though frank behavioral or electrographic spontaneous seizures have not been reported. THAL-treated animals exhibited normal gross behavior, but further studies need to be performed to fully evaluate the impact of THAL on learning, memory, etc. However, in vitro electrophysiological recordings disclosed subtle, but reproducible changes in excitability in cortical regions associated with large, abnormal blood vessels. This was evaluated in a fairly crude paradigm based on zero-Mg-induced bursting, which revealed that neurons located in proximity to detectable vascular malformations were capable of exhibiting hyperexcitable and hypersynchronous firing that sometimes spread over time to neighboring, yet normal-appearing cortex. These preliminary experiments necessitate further investigations to understand the delicate balance of altered inhibition/excitation in these animals.
The results by Seiffert et al. (2004) suggested that neurons tend to fire abnormally when exposed to molecules that extravasate through a leaky blood–brain barrier. Our results clearly demonstrate that this was also the case in dysplastic or otherwise abnormal cortex. It is therefore possible that the altered neuronal properties of THAL and MAM rats are due to a combined “circuitry effect” (e.g. abnormal wiring of neurons) and a concomitant effect of molecules that are normally segregated into peripheral blood.
CONCLUSIONS
THAL as an etiologic factor for neurological diseases
The most prominent teratogenic effect of THAL is phocomelia, but increasing evidence suggests that when taken at later stages, THAL causes a spectrum of neurological effects including autism and seizure disorders (Stephenson, 1976; Archer, 1978; Kanno, 1987; Stromland et al., 1994; Rodier et al., 1996). Since the only known mechanism of action of THAL implicated angiogenesis (D’Amato et al., 1994; Franks et al., 2004), the results presented here confirm that when given to pregnant rodents, THAL causes significant disturbances in brain cytoarchitecture and that this is due to altered development of CNS vasculature.
Acknowledgments
This work was supported by NIH 2RO1 HL51614, NIH RO1 NS43284, NIH RO1 NS38195, and NIH NS149514.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- BAEC
bovine aortic endothelial cells
- CCF
Cleveland Clinic Foundation
- DAPI
4′,6-diamidino-2-phenylindole
- DCX
doublecortin
- DG
dentate gyrus
- FITC
fluorescein isothiocyanate
- FITC–albumin
bovine desiccate albumin–fluorescein isothiocyanate
- GFAP
glial fibrillary acidic protein
- MAM
methylazoxymethanol
- MCD
malformations of cortical development
- NeuN
neuronal nuclei
- PBLec
phosphate-buffered saline containing in mM: 0.1 CaCl2, 0.1 MgCl2, 0.1 MnCl2, and 1% Triton X-100
- PBS
phosphate-buffered saline
- PN
postnatal
- RIPA
radial immunoprecipitation assay
- THAL
thalidomide
- VEGF
vascular endothelial growth factor
- VPA
valproic acid
REFERENCES
- Akhtar N, Dickerson EB, Auerbach R. The sponge/Matrigel angiogenesis assay. Angiogenesis. 2002;5:75–80. doi: 10.1023/a:1021507031486. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Atlas of the prenatal rat brain development. Boca Raton, FL: CRC Press; 1995. [Google Scholar]
- Altman R, Bayer SA. Atlas of Prenatal Rat Brain Development. CRC Press; 1994. The E14 rat brain; p. 149. [Google Scholar]
- Amiry-Moghaddam M, Ottersen OP. The molecular basis of water transport in the brain. Nat Rev Neurosci. 2003;4:991–1001. doi: 10.1038/nrn1252. [DOI] [PubMed] [Google Scholar]
- Archer J. Thalidomide and neurological damage revisited. JAMA. 1978;239:1608–1609. [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der ZR, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Baraban SC, Wenzel HJ, Hochman DW, Schwartzkroin PA. Characterization of heterotopic cell clusters in the hippocampus of rats exposed to methylazoxymethanol in utero. Epilepsy Res. 2000;39:87–102. doi: 10.1016/s0920-1211(99)00104-7. [DOI] [PubMed] [Google Scholar]
- Bardosi A, Ambach G, Friede RL. The angiogenesis of micrencephalic rat brains caused by methylazoxymethanol acetate. I Superficial venous system. A quantitative analysis. Acta Neuropathol (Berl) 1985a;66:253–263. doi: 10.1007/BF00688591. [DOI] [PubMed] [Google Scholar]
- Bardosi A, Ambach G, Friede RL. The angiogenesis of microencephalic rat brains caused by methylazoxymethanol acetate. II. Superficial and basal arterial system. Acta Neuropathol (Berl) 1985b;68:59–64. doi: 10.1007/BF00688957. [DOI] [PubMed] [Google Scholar]
- Bardosi A, Ambach G, Hann P. The angiogenesis of the micrencephalic rat brains caused by methylazoxymethanol acetate. III. Internal angioarchitecture of cortex. Acta Neuropathol (Berl) 1987;75:85–91. doi: 10.1007/BF00686797. [DOI] [PubMed] [Google Scholar]
- Bassanini S, Battaglia G. Neuronal migration and malformations of cortical development (MCDs) In: Janigro D, editor. Cell cycle in the nervous system. Humana Press; 2006. [Google Scholar]
- Battaglia G, Bassanini S, Granata T, Setola V, Giavazzi A, Pagliardini S. The genesis of epileptogenic cerebral heterotopia: clues from experimental models. Epileptic Disord. 2003a;5(Suppl 2):S51–S58. [PubMed] [Google Scholar]
- Battaglia G, Pagliardini S, Saglietti L, Cattabeni F, Di Luca M, Bassanini S, Setola V. Neurogenesis in cerebral heterotopia induced in rats by prenatal methylazoxymethanol treatment. Cereb Cortex. 2003b;13:736–748. doi: 10.1093/cercor/13.7.736. [DOI] [PubMed] [Google Scholar]
- Bianchi R, Janigro D, Milan F, Giudici G, Gorio A. In vivo treatment with GM1 prevents the rapid decay of ATPase activities and mitochondrial damage in hippocampal slices. Brain Res. 1986;364:400–404. doi: 10.1016/0006-8993(86)90856-5. [DOI] [PubMed] [Google Scholar]
- Calcagnotto ME, Paredes MF, Baraban SC. Heterotopic neurons with altered inhibitory synaptic function in an animal model of malformation-associated epilepsy. J Neurosci. 2002;22:7596–7605. doi: 10.1523/JNEUROSCI.22-17-07596.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo E, Reinach B, Caputi A, Cattabeni F, Di Luca M. Selective in vitro blockade of neuroepithelial cells proliferation by methylazoxymethanol, a molecule capable of inducing long lasting functional impairments. J Neurosci Res. 1995;41:640–647. doi: 10.1002/jnr.490410510. [DOI] [PubMed] [Google Scholar]
- Cavaglia M, Dombrowski SM, Drazba J, Vasanji A, Bokesch PM, Janigro D. Regional variation in brain capillary density and vascular response to ischemia. Brain Res. 2001;910:81–93. doi: 10.1016/s0006-8993(01)02637-3. [DOI] [PubMed] [Google Scholar]
- Chevassus-Au-Louis N, Baraban SC, Gaiarsa JL, Ben Ari Y. Cortical malformations and epilepsy: new insights from animal models. Epilepsia. 1999;40:811–821. doi: 10.1111/j.1528-1157.1999.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Clark GD. Cerebral gyral dysplasias: molecular genetics and cell biology. Curr Opin Neurol. 2001;14:157–162. doi: 10.1097/00019052-200104000-00004. [DOI] [PubMed] [Google Scholar]
- Colacitti C, Sancini G, DeBiasi S, Franceschetti S, Caputi A, Frassoni C, Cattabeni F, Avanzini G, Spreafico R, Di Luca M, Battaglia G. Prenatal methylazoxymethanol treatment in rats produces brain abnormalities with morphological similarities to human developmental brain dysgeneses. J Neuropathol Exp Neurol. 1999;58:92–106. doi: 10.1097/00005072-199901000-00010. [DOI] [PubMed] [Google Scholar]
- Colacitti C, Sancini G, Franceschetti S, Cattabeni F, Avanzini G, Spreafico R, Di Luca M, Battaglia G. Altered connections between neocortical and heterotopic areas in methylazoxymethanol-treated rat. Epilepsy Res. 1998;32:49–62. doi: 10.1016/s0920-1211(98)00039-4. [DOI] [PubMed] [Google Scholar]
- Cucullo L, Dini G, Hallene KL, Fazio V, Ilkanich EV, Igboechi C, Kight KM, Agarwal MK, Garrity-Moses M, Janigro D. Very low intensity alternating current decreases cell proliferation. Glia. 2005;51:65–72. doi: 10.1002/glia.20188. [DOI] [PubMed] [Google Scholar]
- D’Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci U S A. 1994;91:4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denekamp J. Review article: angiogenesis, neovascular proliferation and vascular pathophysiology as targets for cancer therapy. Br J Radiol. 1993;66:181. doi: 10.1259/0007-1285-66-783-181. [DOI] [PubMed] [Google Scholar]
- Fernandes AM, Maurer-Morelli CV, Campos CB, Mello ML, Castilho RF, Langone F. Fluoro-Jade, but not Fluoro-Jade B stains non-degenerating cells in brain and retina of embryonic and neonatal rats. Brain Res. 2004;1029:24–33. doi: 10.1016/j.brainres.2004.09.036. [DOI] [PubMed] [Google Scholar]
- Franks ME, Macpherson GR, Figg WD. Thalidomide. Lancet. 2004;363:1802–1811. doi: 10.1016/S0140-6736(04)16308-3. [DOI] [PubMed] [Google Scholar]
- Gardiner RM. Genetic basis of the human epilepsies. Epilepsy Res. 1999;36:91–95. doi: 10.1016/s0920-1211(99)00043-1. [DOI] [PubMed] [Google Scholar]
- Gierdalski M, Juliano SL. Factors affecting the morphology of radial glia. Cereb Cortex. 2003;13:572–579. doi: 10.1093/cercor/13.6.572. [DOI] [PubMed] [Google Scholar]
- Grant GA, Janigro D. The blood-brain barrier. In: Winn HR, editor. Youman’s neurological surgery. Philadelphia, PA: Saunders; 2004. pp. 153–174. [Google Scholar]
- Haigh JJ, Morelli PI, Gerhardt H, Haigh K, Tsien J, Damert A, Miquerol L, Muhlner U, Klein R, Ferrara N, Wagner EF, Betsholtz C, Nagy A. Cortical and retinal defects caused by dosage-dependent reductions in VEGF-A paracrine signaling. Dev Biol. 2003;262:225–241. doi: 10.1016/s0012-1606(03)00356-7. [DOI] [PubMed] [Google Scholar]
- Hallene K, Oby E, Marchi N, Janigro D. Impaired vasculogenesis as a mechanism of CNS malformation. Epilepsia. 2005;46:8. [Google Scholar]
- Hasling TA, Gierdalski M, Jablonska B, Juliano SL. A radialization factor in normal cortical plate restores disorganized radial glia and disrupted migration in a model of cortical dysplasia. Eur J Neurosci. 2003;17:467–480. doi: 10.1046/j.1460-9568.2003.02468.x. [DOI] [PubMed] [Google Scholar]
- Kanno O. Electroencephalographic study of 137 patients with thalidomide embryopathy. Jpn J Psychiatry Neurol. 1987;41:197–205. doi: 10.1111/j.1440-1819.1987.tb00403.x. [DOI] [PubMed] [Google Scholar]
- Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002;2:727–739. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- LaRue AC, Lansford R, Drake CJ. Circulating blood island-derived cells contribute to vasculogenesis in the embryo proper. Dev Biol. 2003;262:162–172. doi: 10.1016/s0012-1606(03)00358-0. [DOI] [PubMed] [Google Scholar]
- Leenders W, Kusters B, Pikkemaat J, Wesseling P, Ruiter D, Heerschap A, Barentsz J, de Waal RM. Vascular endothelial growth factor-A determines detectability of experimental melanoma brain metastasis in GD-DTPA-enhanced MRI. Int J Cancer. 2003;105:437–443. doi: 10.1002/ijc.11102. [DOI] [PubMed] [Google Scholar]
- Manley GT, Binder DK, Papadopoulos MC, Verkman AS. New insights into water transport and edema in the central nervous system from phenotype analysis of aquaporin-4 null mice. Neuroscience. 2004;129:983–991. doi: 10.1016/j.neuroscience.2004.06.088. [DOI] [PubMed] [Google Scholar]
- Marroni M, Agarwal M, Kight K, Hallene K, Hossain M, Cucullo L, Signorelli K, Namura S, Janigro D. Relationship between expression of multiple drug resistance proteins and p53 tumor suppressor gene proteins in human brain astrocytes. Neuroscience. 2003a;121:605–617. doi: 10.1016/s0306-4522(03)00515-3. [DOI] [PubMed] [Google Scholar]
- Marroni M, Kight KM, Hossain M, Cucullo L, Desai SY, Janigro D. Dynamic in vitro model of the blood-brain barrier. Gene profiling using cDNA microarray analysis. 2003b;89:419–434. doi: 10.1385/1-59259-419-0:419. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Narita N, Narita M. Maternal administration of thalidomide or valproic acid causes abnormal serotonergic neurons in the offspring: implication for pathogenesis of autism. Int J Dev Neurosci. 2005;23:287–297. doi: 10.1016/j.ijdevneu.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Montenegro MA. Focal cortical dysplasia. Arch Neurol. 2003;60:634–636. doi: 10.1001/archneur.60.4.634. [DOI] [PubMed] [Google Scholar]
- Montenegro MA, Guerreiro MM, Lopes-Cendes I, Guerreiro CA, Cendes F. Interrelationship of genetics and prenatal injury in the genesis of malformations of cortical development. Arch Neurol. 2002;59:1147–1153. doi: 10.1001/archneur.59.7.1147. [DOI] [PubMed] [Google Scholar]
- Morgan AE, Smith WK, Levenson JL. Reversible dementia due to thalidomide therapy for multiple myeloma. N Engl J Med. 2003;348:1821–1822. doi: 10.1056/NEJM200305013481822. [DOI] [PubMed] [Google Scholar]
- Narita N, Kato M, Tazoe M, Miyazaki K, Narita M, Okado N. Increased monoamine concentration in the brain and blood of fetal thalidomide- and valproic acid-exposed rat: putative animal models for autism. Pediatr Res. 2002;52:576–579. doi: 10.1203/00006450-200210000-00018. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Robain O. Introduction to the pathology of cerebral cortical dysplasia. In: Guerrini R, Andermann F, et al., editors. Dysplasias of cerebral cortex and epilepsy. Philadelphia: Lippincott-Raven; 1996. pp. 1–9. [Google Scholar]
- Rodier PM, Ingram JL, Tisdale B, Nelson S, Romano J. Embryological origin for autism: developmental anomalies of the cranial nerve motor nuclei. J Comp Neurol. 1996;370:247–261. doi: 10.1002/(SICI)1096-9861(19960624)370:2<247::AID-CNE8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Sancini G, Franceschetti S, Battaglia G, Colacitti C, Di Luca M, Spreafico R, Avanzini G. Dysplastic neocortex and subcortical heterotopias in methylazoxymethanol-treated rats: an intracellular study of identified pyramidal neurones. Neurosci Lett. 1998;246:181–185. doi: 10.1016/s0304-3940(98)00258-4. [DOI] [PubMed] [Google Scholar]
- Seiffert E, Dreier JP, Ivens S, Bechmann I, Tomkins O, Heinemann U, Friedman A. Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J Neurosci. 2004;24:7829–7836. doi: 10.1523/JNEUROSCI.1751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanness KA, Westrum LE, Fornaciari E, Mascagni P, Nelson JA, Stenglein SG, Myers T, Janigro D. Morphological and functional characterization of an in vitro blood-brain barrier model. Brain Res. 1997;771:329–342. doi: 10.1016/s0006-8993(97)00829-9. [DOI] [PubMed] [Google Scholar]
- Steinlein OK. Genes and mutations in human idiopathic epilepsy. Brain Dev. 2004;26:213–218. doi: 10.1016/S0387-7604(03)00149-9. [DOI] [PubMed] [Google Scholar]
- Stephenson JB. Epilepsy: a neurological complication of thalidomide embryopathy. Dev Med Child Neurol. 1976;18:189–197. doi: 10.1111/j.1469-8749.1976.tb03628.x. [DOI] [PubMed] [Google Scholar]
- Stromland K, Nordin V, Miller M, Akerstrom B, Gillberg C. Autism in thalidomide embryopathy: a population study. Dev Med Child Neurol. 1994;36:351–356. doi: 10.1111/j.1469-8749.1994.tb11856.x. [DOI] [PubMed] [Google Scholar]
- Taylor D, Falconer M, Bruton C, Corsellis J. Focal dysplasia of the cerebral cortex in epilepsy. J Neurol Neurosurg Psychiatry. 1971;34:369–387. doi: 10.1136/jnnp.34.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum P. A proposed primate animal model of autism. Eur Child Adolesc Psychiatry. 2003;12:48–49. doi: 10.1007/s00787-003-0306-6. [DOI] [PubMed] [Google Scholar]
- Wu C, Luk WP, Gillis J, Skinner F, Zhang L. Size does matter: generation of intrinsic network rhythms in thick mouse hippocampal slices. J Neurophysiol. 2005;93:2302–2317. doi: 10.1152/jn.00806.2004. [DOI] [PubMed] [Google Scholar]