Abstract
Asbestos causes asbestosis and malignancies by molecular mechanisms that are not fully understood. The modes of action underlying asbestosis, lung cancer, and mesothelioma appear to differ depending on the fiber type, lung clearance, and genetics. After reviewing the key pathologic changes following asbestos exposure, we examine recently identified pathogenic pathways, with a focus on oxidative stress. Alveolar epithelial cell apoptosis, which is an important early event in asbestosis, is mediated by mitochondria- and p53-regulated death pathways and may be modulated by the endoplasmic reticulum. We review mitochondrial DNA (mtDNA)-damage and -repair mechanisms, focusing on 8-oxoguanine DNA glycosylase, as well as cross talk between reactive oxygen species production, mtDNA damage, p53, OGG1, and mitochondrial aconitase. These new insights into the molecular basis of asbestos-induced lung diseases may foster the development of novel therapeutic targets for managing degenerative diseases (e.g., asbestosis and idiopathic pulmonary fibrosis), tumors, and aging, for which effective management is lacking.
Keywords: asbestosis, epithelium, mitochondria, OGG1, aconitase, p53
INTRODUCTION
Asbestos-related lung diseases are a common clinical problem and a major health concern worldwide. Epidemiologic studies have established that exposure to asbestos fibers causes pulmonary fibrosis (asbestosis), pleural abnormalities (effusion and plaques), and malignancies (bronchogenic carcinoma and mesothelioma) (see References 1–5 for a review). Extensive investigations over the past several decades have identified many important pathogenic mechanisms, yet the precise molecular mechanisms involved and the cross talk between implicated pathways are not fully understood. A search of the term asbestos yielded 11,324 citations in PubMed and more than 46 million listings in Google, but there is still no consensus about the best definition of asbestos, and as a result, there is considerable uncertainty regarding exposure-response relationships in mediating pulmonary toxicity (see Reference 1 for a review). The term asbestos as used herein refers to a select category of naturally occurring mineral silicate fibers, including chrysotile (the only serpentine fiber) and amphiboles (e.g., crocidolite, amosite, tremolite, anthophyllite, and actinolite). In 1987, the International Agency for Research on Cancer (6) classified asbestos as a group 1 (definite) human carcinogen. Although the use of asbestos has been nearly eliminated in the United States since the 1970s, its considerable presence in buildings, combined with the long latency period of 15 to 40 years between exposure and pulmonary toxicity, indicates that asbestos-related lung diseases will continue to pose a challenge (5). The long latency period also affords an opportunity to prevent the progression from exposure to disease. Importantly, the asbestos paradigm should provide key pathophysiologic insights into other, more common lung diseases [e.g., idiopathic pulmonary fibrosis (IPF) and lung cancer], which require effective pharmacologic management.
We focus on asbestosis because it is present in nearly all patients with asbestos-related lung cancers and because asbestosis has similarities to IPF, a much more common lung disease with a worse prognosis, for which effective treatment is urgently required. After briefly reviewing the term asbestos and the key pathologic changes following asbestos exposure, we examine recent investigations highlighting important pathogenic pathways involving oxidative stress, apoptosis, and inflammation. Alveolar epithelial cell (AEC) apoptosis is a crucial early event observed in asbestosis as well as in patients with IPF. We explore the evidence implicating the mitochondria- and p53-regulated death pathways, as well as a possible emerging role for the endoplasmic reticulum (ER) in modulating AEC apoptosis. We review mitochondrial DNA (mtDNA)-damage and -repair mechanisms focusing on 8-oxoguanine DNA glycosylase 1 (OGG1) and mitochondrial aconitase (ACO2), which is a redox-sensor molecule involved in mtDNA maintenance. Mitochondrial OGG1 and Aco2 have recently been implicated in attenuating oxidant (asbestos and H2O2)-induced AEC apoptosis. Collectively, these studies are providing insight into the molecular basis of asbestos-induced lung diseases.
PATHOLOGIC CHANGES
Asbestos Fiber Types
Asbestos is the name given to numerous naturally occurring fibrous silicate minerals that have high tensile strength, the ability to be woven, and resistance to heat and most chemicals. Figure 1 depicts four common types of asbestos fibers. The World Health Organization, the National Institute for Occupational Safety and Health, and the Occupational Safety and Health Administration define the regulated form of asbestos as fibers whose length is greater than 5 μm and whose length:width ratio is 3:1 (1). The serpentine chrysotile, or white asbestos, accounts for more than 95% of the asbestos previously used for industrial purposes in the United States. Chrysotile consists of a 1:1 silicate layer with a chemical formula represented as Mg3[Si2O5](OH)4, where layers of a silicate sheet are connected to octahedral sheets of MgO2(OH)4. Because of misalignment between the two layers, the fibers are curled, wavy, flexible, easily breakable, and soluble in tissues. Some chrysotile fibrils exist in cylindrical layers and others in spiral configurations, so the surface groups exposed to the environment are unique in each configuration (1).
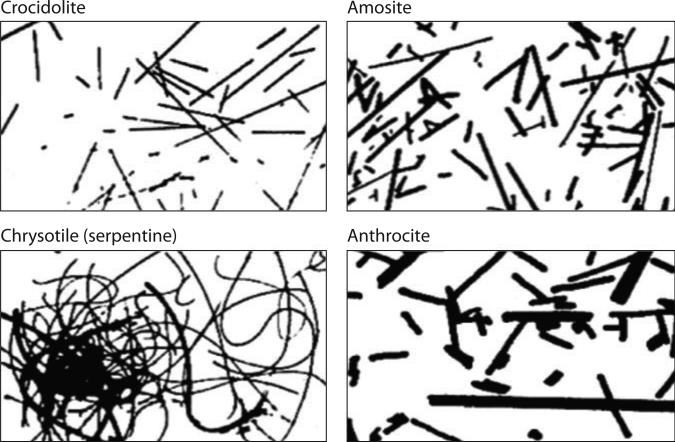
Figure 1.
Asbestos fiber types. Amphiboles (e.g., crocidolite, amosite, anthrocite, and others not shown) are straight, rod-like fibers, whereas serpentines (e.g., chrysotile) are curvilinear fibers.
Amphibole asbestos fibers are rigid, sharp, and highly resistant to chemical and biological solution, and they have a longer biological persistence compared with that of chrysotile (1, 7). Amphibole asbestos consists of double-chain silicates that are also characterized by units similar to chrysotile. Crocidolite, or Cape Blue asbestos, is an amphibole asbestos whose chemical unit is Na2(Fe3+)2(Fe2+)3 Si8O22(OH)2. Crocidolite consists of a double-chained, tetrahedrally coordinated SiO4 that is separated by ribbons of octahedrally coordinated cations (7). Amosite (or brown amphibole asbestos) is named for the company Asbestos Mines of South Africa. The unit structure of amosite is (Mg,Fe2+)7Si8O22(OH)2, where iron exists in the ferrous ionic state (7). Other asbestos fiber types include tremolite Ca2Mg5Si8O22(OH), anthophyllite (Mg,Fe2+)7Si8O22(OH)2, and actinolite Ca2(Mg,Fe)5Si8O22(OH)2. Although the core structures of crocidolite and amosite contain considerable iron (~27–30%) that can be redox activated, the surface of chrysotile can also contain redox-active iron (1–6%) (7, 8).
The determinants of fiber toxicity depend on multiple factors, including (a) dose, (b) dimension, (c) biodurability, (d ) surface reactivity, and (e) the genetic background of the host exposed. A detailed analysis of these areas is beyond the scope of this review, but they have recently been extensively assessed elsewhere (1, 2, 7, 9). In this section, we highlight a few important principles; the roles of surface reactivity and genetics are discussed further below.
Asbestos dose is a crucial determinant for triggering inflammation; high doses over short periods promote an acute neutrophil-predominant inflammation, whereas low doses over prolonged exposure periods promote alveolar macrophage (AM)-predominant chronic inflammation. Fiber dimensions are important because only very thin fibers (diameter <0.4 μm and length <10 μm) are respirable to the distal alveolar space; long fibers cannot be fully engulfed by AMs because they are biodurable. Phagocytosis of fibers is also limited by the size of the AMs (generally 14–21 μm). The clearance of short chrysotile asbestos fibers is rapid, whereas clearance of the long chrysotile fibers is slow or insignificant (10). In general, fibers greater than 20 μm in length are associated with asbestosis, and fibers greater than 10 μm in length are the most carcinogenic; however, the carcinogenicity of amphiboles is two orders of magnitude greater than that of chrysotile (1). There is some evidence that fibers less than 5 μm in length can also promote pulmonary fibrosis and malignancy, especially when administered as a lung overload condition, as can occur in dust clouds (2). The biopersistence of chrysotile fibers is greater than that of amphibole fibers (months versus years, respectively), but chrysotile has less surface area (27 versus ~8 m2 g–1) (7). Tobacco smoking, a common confounder in human studies involving asbestos workers, impairs asbestos clearance, which probably accounts in part for the observation that tobacco smoke augments asbestos pulmonary toxicity (11, 12). Despite these advances in our understanding, future work is necessary to more precisely define the term asbestos, especially in the context of epidemiologic and pathophysiologic studies (1, 2). Better knowledge of the complex interplay among fiber type, dimensions, chemistry, and factors controlling biopersistence will help investigators and regulators working in the field and, ideally, eliminate human diseases resulting from asbestos exposure.
Role of Inflammation
AEC injury and recruitment of inflammatory cells are important early events in the pathogenesis of chronic interstitial lung fibrosis, including asbestosis (13–16). The alveolar epithelium is composed of (a) alveolar epithelial type I cells that cover ~90% of the alveolar surface area and are susceptible to an oxidant stress and (b) alveolar epithelial type II (AT2) cells that produce surfactant and can proliferate and differentiate into type I cells. The early stage of asbestosis is characterized by discrete foci of fibrosis within the respiratory bronchiole walls and alveolar duct bifurcations associated with the accumulation of asbestos bodies (2, 13–16). Asbestos triggers the accumulation of AMs and an inflammatory reaction, followed by more diffuse pulmonary involvement characterized by (a) loss of alveolar epithelial type I and AT2 cells, (b) fibroblast proliferation, and (c) collagen deposition. The pulmonary fibrosis of asbestosis is associated with fibrosis of the walls of the respiratory bronchioles and alveolar ducts (Figure 2). The site of asbestos-induced inflammation occurs at the area of fiber deposition along airways and in alveolar spaces. Patients with asbestosis typically have radiographic and histologic evidence of distal lung fibrosis, and there is controversy about whether bronchiolar wall fibrosis without distal parenchymal fibrosis should also be subsumed under the term asbestosis (13, 16). Macrophage ingestion of asbestos fibers triggers a fibrogenic response from fibroblasts via the release of growth factors, such as transforming growth factor β (TGF-β) and platelet-derived growth factor, as well as cytokines, such as tumor necrosis factor α (TNF-α) and interleukin-1β (IL-1β), that collectively promote collagen deposition (see References 2 and 17 for a review). As discussed below, accumulating evidence also implicates an important role for macrophage-derived reactive oxygen species (ROS) in mediating asbestosis.
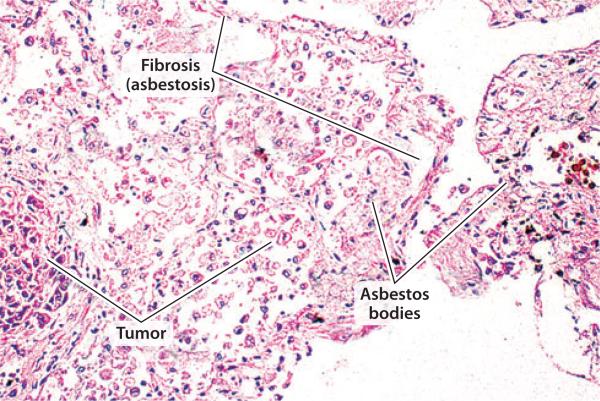
Figure 2.
Pulmonary damage due to asbestosis. Hematoxylin and eosin stain of human lungs showing areas of fibrosis, tumor, and accumulation of asbestos bodies (lines).
An asbestos body (Figure 3) is a fibrous structure (20–200 μm in length and 2–6 μm in width) with asbestos in its core encased by mucopolysaccharides and iron-rich proteins (e.g., ferritin and hemosiderin) that can be redox activated (9, 18, 19). Asbestos bodies, which represent nearly one-third of the lung fiber burden of coated to uncoated asbestos fibers, are diagnostic of asbestosis if detected in patients with pulmonary fibrosis either in a histological specimen or in the bronchoalveolar lavage fluid (BALF) at a level greater than one asbestos body per milliliter (16, 20). A diagnosis of asbestosis does not necessarily require a tissue biopsy if the following are present: (a) reliable asbestos exposure; (b) an appropriate latency period, typically >20 years; (c) abnormal chest imaging showing subpleural, basal-predominant reticular abnormalities typically with pleural plaques (80–90%); (d ) restrictive pulmonary physiology with reduced gas exchange; and (e) end-inspiratory crackles on physical examination (16).
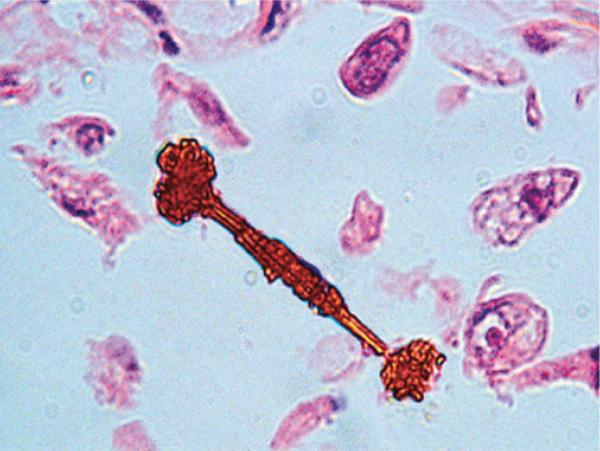
Figure 3.
An asbestos body. The typical dimensions of an asbestos body are a length of 20 to 200 μm and a width of 2 to 6 μm.
Chronic inflammation, including that following asbestos exposure, is prominently implicated in the formation of numerous malignancies (see References 21–23 for a review). Approximately 20% of cancer deaths worldwide, especially those related to gastrointestinal and lung cancer (21, 22), are caused by chronic infection and/or inflammation. In general, neoplasm prevention is a key function of normal adaptive immune responses, but tumors can also arise from dysregulated innate and/or adaptive immune responses. Similar to the well-established increased risk of lung cancer in patients with IPF, there is a direct relationship between excess asbestosis cases and lung cancer mortality (24). Asbestos-related lung cancer accounts for 5% to 7% of all lung cancers worldwide (23). Although asbestosis is a well-recognized risk factor for lung cancer following asbestos exposure, controversy persists about whether asbestosis is required for the causal attribution of lung cancer to asbestos exposure (24–26). There is a direct relationship between the number of asbestos bodies in the lung tissue and the risk of lung cancer (26). As reviewed in detail elsewhere (23), there is substantial and convincing evidence showing that chronic inflammation, as occurs following exposure to asbestos, tobacco, and many other agents, can promote all stages of tumorigenesis, including DNA damage, continuous replication, apoptosis evasion, prolonged angiogenesis, self-sufficient growth signaling, resistance to antigrowth signaling, and tissue invasion/metastasis. Extensive studies by Mossman and colleagues (2, 27, 28) have established some of the important cell signaling pathways, especially the epidermal growth factor receptor (EGFR)-related extracellular signal–regulated kinase (ERK) signaling that promote lung epithelial cell and fibroblast proliferation and early response protooncogene expression that occur in the setting of pulmonary inflammation following chronic inhalation of asbestos (e.g., chrysotile and crocidolite). These signaling pathways also appear important in human epithelioid malignant mesothelioma (28). Although these studies have detected numerous potentially important pathogenic pathways that are amenable to therapeutic manipulation, further work is required to assess the in vivo relevance of fibrosis and malignancy in animal models and in humans with asbestos pulmonary toxicity.
Role of Alveolar Epithelial Cell Apoptosis
Considerable in vitro and in vivo data show that asbestos can induce both lytic cell death and apoptosis (see References 2, 4, and 29 for a review). Apoptosis is a regulated, ATP-dependent process characterized by membrane blebbing, cell shrinkage, nuclear chromatin condensation, and DNA fragmentation. Unlike the inflammatory signaling arising from lytic cell death, apoptosis enables cells with extensive DNA damage to be eliminated without inciting an inflammatory response.
Substantial evidence convincingly confirms that AEC apoptosis is important in the patho-physiology of pulmonary fibrosis (29, 30). First, patients with IPF and animal models of asbestosis demonstrate significant injury to the alveolar epithelium (15, 31–33). Second, the AECs of patients with IPF have DNA strand-break formation and apoptosis, as assessed by TUNEL (terminal deoxynucleotidyl transferase–mediated dUTP-biotin nick end labeling) (31–33). As discussed below, asbestos induces AEC DNA damage and apoptosis. Third, various novel transgenic murine models show that AEC apoptosis is sufficient for inducing pulmonary fibrosis, an observation that is supported in part by the finding that the blocking of AEC-targeted apoptosis is protective (34–37). Fourth, prevention of αvβ6 integrin release from lung epithelial cells, a key activator of latent TGF-β, prevents TGF-β activation and pulmonary fibrosis (38). Although these data firmly implicate AEC apoptosis in the pathophysiology of pulmonary fibrosis following exposure to various agents, including asbestos, future studies are necessary to define the precise molecular mechanisms involved in apoptosis, to determine how apoptosis-resistant cells promote mutagenesis, and to characterize the translational significance of apoptosis in animals and humans exposed to asbestos.
ASBESTOS-INDUCED PRODUCTION OF REACTIVE OXYGEN SPECIES
Asbestos-induced ROS generation is implicated in the development of asbestos-induced pulmonary toxicity (see References 4, 8, and 29 for a review). Although asbestos stimulates the production of reactive nitrogen species that probably contribute to DNA damage and subsequent pulmonary toxicity (see References 4 and 39 for a review), we focus on the various mechanisms by which asbestos causes oxidative stress. There are at least three sources of ROS production following asbestos exposure, including (a) fiber surface reactivity; (b) release from inflammatory cells, especially AMs; and (c) mitochondria-derived ROS released from inflammatory and other target cells, such as lung epithelial cells and mesothelial cells.
Asbestos Fiber Surface Reactivity
The hydroxyl radical (HO·) is a potent oxidizing agent that can initiate lipid peroxidation, kill bacteria, damage cellular DNA, and react with most organic molecules (8). Electron spin resonance spectroscopy studies have established that ROS are produced by asbestos (e.g., chrysotile, crocidolite, and amosite) in cell-free systems from a normal by-product of metabolism (H2O2) and that iron chelators are protective (Figure 4) (8, 40). Even small amounts of iron (<2% total weight) complexed with asbestos can augment the generation of the highly reactive HO· from H2O2 via the Fenton-catalyzed Haber–Weiss reaction shown in Equation 1 (8, 40, 41). Alkoxyl radicals are also formed by asbestos-associated iron catalysis of organic hydroperoxides, as shown in Equation 2:
| 1 |
| 2 |
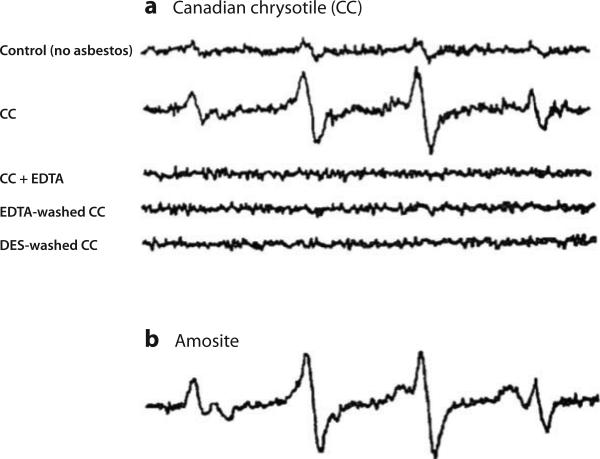
Figure 4.
Electron paramagnetic resonance spectroscopy studies. (a) Chrysotile and (b) amosite asbestos induce a hydoxyl radical signal in the presence of hydrogen peroxide and a reducing agent. Abbreviations: DES, desferoxamine; EDTA, ethylene diamene tetraacetic acid. Reproduced with permission from Reference 40.
The surface of asbestos fibers deposited in the lungs acquires iron that is redox active; this iron cycles between the reduced and oxidized forms and can cause oxidative DNA damage in nearby cells (4, 8, 42). Iron homeostasis in the lungs is abnormal in asbestos-exposed individuals, as evidenced by increased levels of BALF iron, transferrin, transferrin receptors, lactoferrin, and ferritin (19). As recently reviewed in detail elsewhere (2, 4, 29), numerous groups have documented that oxidative stress generated by asbestos fibers causes both alterations in antioxidant enzymes and DNA damage in all the relevant target cells (e.g., lung epithelium, AMs, and mesothelial cells) in vitro and, to a lesser extent, in vivo. These effects generally occur in an asbestos dose–dependent manner (the dose ranges from 0.05 to 250 μg cm–2); they preferentially impact AECs and mesothelial cells, rather than bronchial epithelial cells, and are attenuated by iron chelators (e.g., deferoxamine and phytic acid). Asbestos-induced ROS produce various DNA lesions soon after exposure, which, if not efficiently repaired, can promote apoptosis, gene mutations, chromosomal aberrations, and ultimately cell transformation (see Reference 4 for a review). Additional work is necessary to determine how asbestos-induced ROS production regulates DNA-damage signaling, p53 activation, and inflammation in relevant target cells in vitro and in vivo.
Inflammatory Cells
Inflammatory cells are another important source of ROS production, given that all forms of asbestos activate rodent and human neutrophil and AM ROS generation during so-called frustrated phagocytosis, a process that is accompanied by the release of cytokines, chemokines, proteases, and growth factors that collectively contribute to fiber pathogenicity (see References 2, 4, and 8 for a review). As described in detail below, asbestos-induced ROS generation arises from the mitochondria of target cells (e.g., inflammatory cells, AECs, and mesothelial cells) as well as by the augmentation of cytosolic NADPH oxidases that generate ROS in activated neutrophils and macrophages during the respiratory burst. Iron-derived free radicals from various asbestos fibers are a major determinant of AM transcriptional activation and gene expression (8). Oxidative stress following asbestos exposure can activate several signaling pathways involving mitogen-activated protein kinases, nuclear factor κB (NF-κB), and activator protein 1, all of which have been linked to increases in early response genes (e.g., jun and fos) that govern cell proliferation, apoptosis, and inflammatory signaling (2, 27). As stated above, asbestos fibers can also directly interact with cell-surface receptors (e.g., EGFR), thereby activating these pathways via metals that induce protein aggregation and phosphorylation (2, 27, 28).
Several groups investigating the link between oxidative stress and inflammatory signaling following asbestos exposure have implicated multiple mechanisms, including: (a) prevention of asbestos toxicity by extracellular superoxide dismutase, in part by reducing the oxidative shedding of syndecan-1 expression (43); (b) protein kinase Cδ (PKCδ)-dependent modulation of ERK1/2 and c-Jun N-terminal kinase ( JNK) 1/2 phosphorylation and Bim-associated intrinsic apoptosis in lung epithelial cells (44); (c) H2O2-induced release of high-mobility group box 1 from mesothelial and inflammatory cells around asbestos deposits when colocalized with TNF-α (45); and (d ) ROS-mediated Nalp3 inflammasome sensing (46, 47). Inflammasomes, which are contained in macrophages and neutrophils, consists of a complex of proteins with a unique role in IL-1β-driven innate host defense for cellular danger sensing (48). Nalp3, which is 1 of more than 20 members of the NLR family of proteins, contains an N-terminal protein-protein interaction domain that is crucial for caspase activation, a caspase recruitment domain (CARD), a central nucleotide-binding domain, and a C-terminal leucine-rich repeat domain (48). Activation of the Nalp3 inflammasome configuration is triggered when Nalp3 recruits ASC, an adaptor molecule, and caspase-1 by CARD-CARD interactions. Notably, asbestos- and silica-induced lung inflammatory cell recruitment and cytokine produc tion (e.g., IL-1β and IL-18) are decreased in mice deficient in Nalp3, ASC, or caspase-1 (46, 47). Moreover, through the use of specific pharmacologic ROS inhibitors, iron chelator–treated asbestos, and targeted murine knockouts, Nalp3 inflammasome activation appears to require fiber uptake into phagocytic cells as well as ROS generated by NADPH oxidase, mitochondrial oxidative phosphorylation, or both. Accumulating evidence in other inflammatory models implicates mitochondrial ROS as the crucial upstream regulator of Nalp3 inflammasome activation as well as inflammasome-independent cytokines (e.g., TNF-α and IL-6) production (49, 50, 51). However, Nalp–/– mice do not block all asbestos-induced cytokine expression, which implicates alternative signaling pathways activated by mitochondrial ROS, such as those noted above or others suggested by gene-profiling and computational proteomic experiments (2, 50, 52–54). TNF-α as well as Nalp3 inflammasome signaling and downstream IL-1β expression have been detected in human malignant mesotheliomas, which are implicated in the promotion of cell survival and tumor growth (54). Libby amphibole asbestos induces Nalp3 inflammasome signaling in rat lungs; these effects are blocked by iron-loaded fibers (55). Thus, asbestos-induced oxidative stress from the mitochondria of inflammatory cells induces multiple downstream signaling pathways, which has suggested novel therapeutic targets. Future studies are necessary to determine whether any of these molecular targets are relevant for the mediation of asbestosis or mutagenesis in animals and humans.
Mitochondrial Production of Reactive Oxygen Species
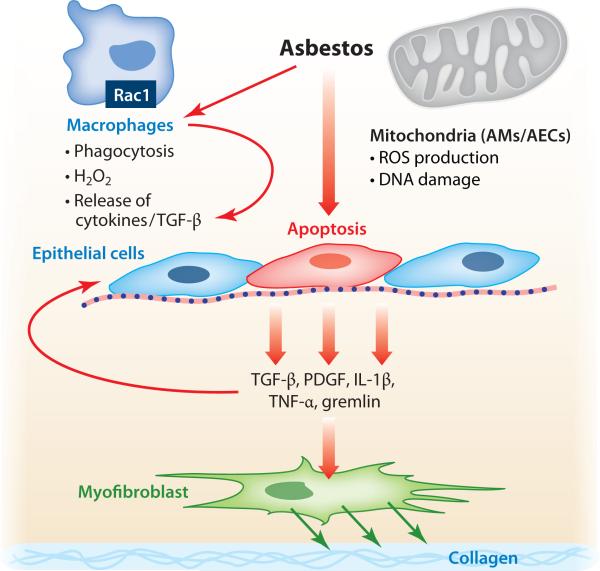
Accumulating evidence strongly indicates that ROS generated from the mitochondria of key target cells mediate asbestos pulmonary toxicity. In the case of inflammatory cells, recent elegant studies by Carter and colleagues (56–59) using novel murine models have established a prominent role for AM mitochondrial H2O2 production in mediating asbestosis. This finding is based on the following experimental observations: (a) AMs exposed to asbestos produce H2O2 that is blocked by catalase or by mitigation of AM mitochondrial oxidative stress; (b) Ras-related C3 botulinum toxin substrate 1 (Rac1), one of the Rho family of GTP-binding proteins known to regulate NADPH oxidase, augments AM mitochondrial H2O2 production; (c) knockdown of the iron-sulfur protein of complex III in the mitochondrial electron transport chain, a major site of mitochondrial ROS production, reduces asbestos-induced AM H2O2 production; (d ) Rac1 is localized in the mitochondria of AMs from patients with asbestosis; and (e) asbestos-exposed mice whose AMs have conditional deletion of Rac1 show reduced oxidative stress as well as pulmonary fibrosis. Collectively, these studies show that asbestos triggers AM H2O2 production by transferring electrons from complex III to Rac1 and that this transfer can culminate in asbestosis in mice. Carter and colleagues reason that AM Rac1 may be a biomarker for pulmonary fibrosis. As discussed in detail below, asbestos also activates mitochondrial ROS production in other important target cells, such as lung epithelial and mesothelial cells. Low levels of mitochondrial ROS production generally cause cell proliferation and activation of antioxidant defenses, but higher levels of mitochondrial oxidative stress trigger DNA damage, p53 activation, cell-cycle blockade, and cell death via both necrotic and apoptotic pathways. However, the site of mitochondrial ROS generation following asbestos exposure in these cells is not as well understood as in AMs. Figure 5 depicts a hypothetical model highlighting some of the important early events that occur following asbestos exposure; these events emphasize the pathways discussed above and below.
Figure 5.
Hypothetical model of asbestos-induced pulmonary fibrosis. Asbestos elicits a macrophage response to phagocytize and clear the fibers, but this response results in reactive oxygen species (ROS) production by a Rac1-dependent mechanism as well as the release of cytokines and growth factors. Asbestos-induced alveolar macrophage (AM) and alveolar epithelial cell (AEC) mitochondrial ROS production promotes AEC apoptosis that may be important for myofibroblast differentiation, collagen deposition by myofibroblasts, and ultimately pulmonary fibrosis. Abbreviations: PDGF, platelet-derived growth factor; IL, interleukin; Rac1, Ras-related C3 botulinum toxin substrate 1; TGF, transforming growth factor; TNF, tumor necrosis factor.
ASBESTOS-INDUCED MITOCHONDRIA-REGULATED APOPTOSIS
Mitochondria-Regulated Apoptosis
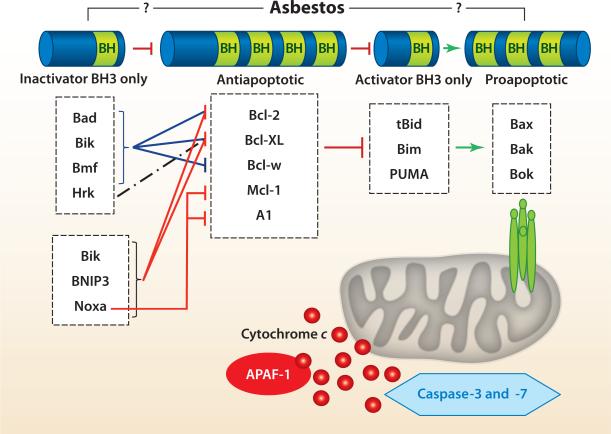
The two mechanisms by which cells undergo apoptosis include the extrinsic (death receptor–related) and intrinsic (mitochondria-regulated) death pathways. We focus on the latter because diverse stimuli, including ROS, DNA damage, and asbestos, activate the intrinsic death pathway by increasing the permeability of the outer mitochondrial membrane; reducing the mitochondrial membrane potential (Δψm); and releasing apoptotic proteins, including cytochrome c (see References 29 and 60–63 for a review). The B cell lymphoma 2 (Bcl-2) family of proteins is grouped into a hierarchy of four classes according to structure and function (pro- or antiapoptotic) (Figure 6). The first class consists of a common BH3-only domain that functions as an inactivator BH3-only protein [e.g., Bad, Bik (Bcl-2-interacting killer), and Noxa] by inactivating a second class of Bcl-2 antiapoptotic family members that contain all four BH3 domains [e.g., Bcl-2, Bcl-XL (B cell lymphoma extra large), and Mcl-1 (myeloid cell leukemia sequence 1)]. The antiapoptotic family members act on a third class of proapoptotic, activator BH3-only proteins [e.g., tBid, Bim (Bcl-2-like 11), and PUMA (p53-upregulated modulator of apoptosis)], which activate a fourth set of proapoptotic proteins [i.e., Bax (Bcl-2-associated X protein), Bak (Bcl-2 homologous antagonist killer), and Box]. Bax- and Bak-induced outer-mitochondrial membrane permeabilization is implicated as the point of no return in the intrinsic apoptotic pathway; it causes the release of apoptotic molecules and caspase activation (29, 60–63). The Bcl-2 family members function at both the mitochondria and the ER. Although the detailed molecular mechanisms regulating cross talk between these two organelles and the Bcl-2 family members is not fully understood, and is beyond the scope of this review, some pertinent studies exploring asbestos pulmonary toxicity are discussed below.
Figure 6.
The B cell lymphoma 2 (Bcl-2) protein family and its role in mitochondria-regulated apoptosis. Abbreviations: A1, Bcl-2-related protein A1; Bak, Bcl-2 homologous antagonist killer; Bax, Bcl-2-associated X protein; APAF-1, apoptotic protease–activating factor 1; Bcl-XL, B cell lymphoma extra large; Bik, Bcl-2-interacting killer; Bim, Bcl-2-like 11; Bmf, Bcl-2-modifying factor; BNIP3, Bcl-2/adenovirus E1B 19-kDa protein–interacting protein 3; Bok, Bcl-2-related ovarian killer; Hrk, Harakiri Bcl-2-interacting protein; Mcl-1, myeloid cell leukemia sequence 1; PUMA, p53-upregulated modulator of apoptosis. Adapted from Reference 64.
The mitochondrial death pathway is important in mediating asbestos-induced apoptosis in all the relevant lung target cells (see References 2, 4, and 29 for a review). Asbestos increases Δψm, which causes the release of cytochrome c from the mitochondria into the cytosol, where caspase-9 and -3 are then activated (2, 4, 29). Notably, in AECs these effects were blocked by phytic acid (an iron chelator), benzoic acid (a free-radical scavenger), and the overexpression of Bcl-XL (65). Asbestos-induced AEC intrinsic apoptosis was blocked in cells lacking mtDNA and the ability to generate ROS, suggesting that that mitochondrial ROS mediates asbestos-induced apoptosis (66). Evidence for a crucial role for AEC mitochondrial ROS production following asbestos exposure was obtained by use of a highly sensitive Rho-GFP (green fluorescent protein) probe targeted to the mitochondria to detect ROS production (67). Mesothelial cells exposed to asbestos also undergo intrinsic apoptosis, in part because mtDNA is more sensitive than nuclear DNA to damage following crocidolite asbestos exposure (68). Studies by Mossman and colleagues (44, 69, 70) demonstrated a key role of PKCδ in the mediation of asbestos-induced AEC intrinsic apoptosis. PKCδ is activated and migrates to the mitochondria of bronchiolar cells and AECs following asbestos exposure both in vitro and in vivo. Administration of rottlerin, a specific inhibitor of PKCδ, or overexpression of a dominant-negative form of PKCδ abolishes the effects of asbestos in a murine pulmonary epithelial cell line. PKCδ phosphorylates ERK and JNK, which leads to Bim activation and subsequent intrinsic apoptosis. Collectively, these studies link altered mitochondrial function by PKCδ and mitochondrial ROS production to asbestos-induced intrinsic apoptosis. Although there is some evidence that ROS production and apoptosis require internalization of asbestos fibers via integrins or other receptors (see References 2 and 3 for a review), the precise molecular mechanisms underlying asbestos entry into noninflammatory cells, causing mitochondrial ROS production and apoptosis, are largely unknown. There are also some in vivo data demonstrating that asbestos (crocidolite, amosite, and chrysotile) triggers apoptosis in cells at the bronchoalveolar duct junction, distal alveolar epithelium, and mesothelial cells, as assessed by various techniques (see References 2, 4, and 29 for a review). However, the role of the mitochondria and the pathophysiologic significance of apoptosis in the context of asbestosis, as well as malignant transformation following asbestos exposure, require further investigation.
Mitochondria–Endoplasmic Reticulum Cross Talk
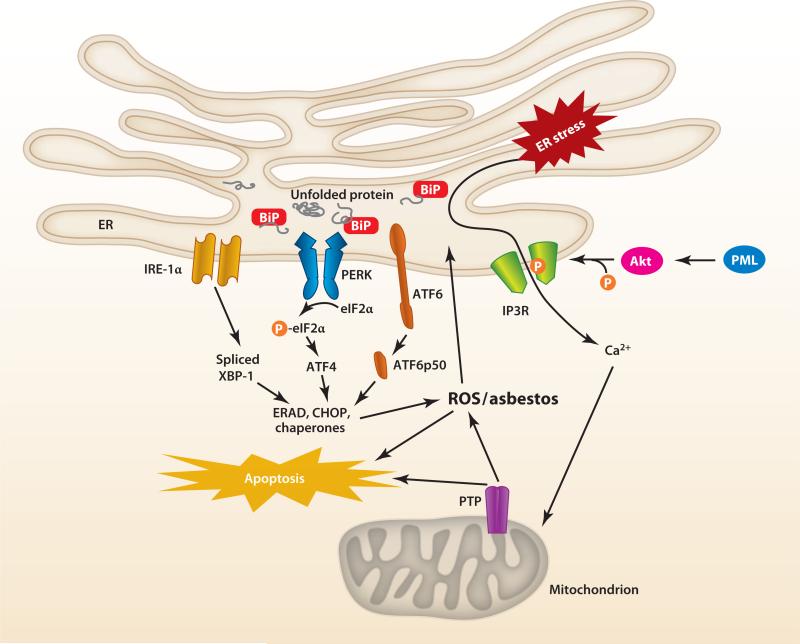
The ER is an important regulator of intrinsic apoptosis, but the detailed molecular mechanisms underlying cross talk between the ER and mitochondria and how this cross talk regulates mitochondrial metabolism, mitochondrial Ca2+ levels, and cell survival/death signaling are not fully understood (see References 29, 61, and 71–73 for a review). The ER is dedicated to protein synthesis, folding, and transport, as well as maintenance of Ca2+ homeostasis. Ca2+ is important for triggering proapoptotic membrane permeabilization and intrinsic apoptosis (61, 71, 74, 75). Close tethering of the ER to the mitochondria appears crucial in mediating these various functions (73, 76). For example, Mitofusin 2, one of several recently described proteins localized at mitochondrion-associated membranes (MAMs), physically links the mitochondrion to the ER and is necessary for mitochondrial Ca2+ uptake following ER release (73, 76). Chemical and structural stressors cause unfolded proteins to accumulate in the ER, leading to ER stress and activation of three unfolded protein response (UPR) signaling cascades, namely (a) inositol-requiring kinase 1α (IRE-1α)/X box–binding protein 1 (XBP1), (b) activated transcription factor 6, and (c) protein kinase RNA–like ER kinase/eukaryotic initiation factor 2a (see References 29, 61, and 71–73 for a review). The UPR coordinates adaptive cellular responses that promote survival but, in the setting of sustained ER stress, can trigger apoptosis.
ER-mitochondrial cross talk is tightly regulated by the expression of various Bcl-2 family members. Prosurvival signaling is generated by transient Ca2+ release, whereas intrinsic apoptotic agents require sustained Ca2+ release accompanied by mitochondrial Bax/Bak binding (61, 74, 75). Agents that induce ER stress also cause intrinsic apoptosis via a Bax/Bak-dependent mechanism involving the modulation of ER to mitochondria home-ostatic Ca2+ levels (61, 74, 75). Sarcoplasmic ER Ca2+ ATPase (SERCA) overexpression in Bax/Bak double-knockout mouse embryonic fibroblasts, cells that are resistant to intrinsic apoptosis, restores ER Ca2+ levels and the intrinsic apoptotic response following oxidative stress. Bcl-2 regulates Ca2+ mobilization from the ER to the mitochondrion by blocking the homodimerization of proapoptotic Bax at the mitochondrion that displaces SERCA from the MAM (74, 75, 77, 78). Sigma-1 receptors, which are located at the MAM, regulate Bcl-2 expression by ROS-dependent NF-κB transcriptional control (79). ER Ca2+ release to the mitochondria is necessary, but not sufficient, for the induction of intrinsic apoptosis by the coordination of ER stress survival signals through IRE-1α/TNF receptor–associated factor 2 that can result in sustained activation of apoptosis signal–regulating kinase 1 and JNK (80). Bcl-2 regulates phosphorylation of the inositol-1,4,5-triphosphate receptors, which are the channels that mediate ER Ca2+ release (81). Protein kinase B (Akt) mitigates apoptosis by phosphorylating these receptors, thereby attenuating ER Ca2+ release and the prevention of mitochondrial Ca2+ overload and subsequent intrinsic apoptosis (82–84). Thus, Bax and/or Bak can act at both the mitochondria and the ER to modulate ER Ca2+ levels and subsequent intrinsic apoptosis following exposure to an apoptotic stimulus. Figure 7 shows some of the key components of ER-mitochondria cross talk, discussed above, that are involved with intrinsic apoptosis.
Figure 7.
Hypothetical model of mitochondria–endoplasmic reticulum (ER) cross talk in asbestos-induced apoptosis. ER stress due to asbestos causes Ca2+ release and activation of an unfolded protein response (UPR), especially inositol-requiring kinase 1α (IRE-1α), which lead to activation of mitochondria-regulated apoptosis. Abbreviations: Akt, protein kinase B; ATF, activating transcription factor; BiP, binding immunoglobulin protein; CHOP, CCAAT/enhancer-binding protein homologous protein; eIF2α, eukaryotic initiation factor 2α; ERAD, endoplasmic reticulum–associated protein degradation; IP3R, inositol-1,4,5-triphosphate receptor; PERK, protein kinase RNA–like endoplasmic reticulum kinase; PML, promyocytic leukemia oncogenic protein; PTP, permeability transition pore; ROS, reactive oxygen species; XBP1, X box–binding protein 1. Modified from Reference 61.
Several lines of evidence indicate that ER stress responses play a potentially important role in patients with IPF that may be relevant to asbestos pulmonary toxicity. First, several studies have established that AECs in patients with IPF undergo intrinsic apoptosis that colocalizes with markers of ER stress (85, 86). Second, ER stress promotes epithelial-mesenchymal transition (EMT) that can generate myofibroblasts, one of the key effector cell types in IPF (87–89). Third, a mutant, misfolded form of surfactant protein C (SP-Cδ exon4) that is associated with familial pulmonary fibrosis causes AEC ER stress and EMT (89–92). Another SP-C mutant, SP-CL188Q, is associated with familial interstitial fibrosis (92). Transgenic mice that conditionally express SP-CL188Q only in their AT2 cells do not develop pulmonary fibrosis when exposed to ER stress (e.g., intratracheal tunicamycin) unless a second profibrotic stimulus (e.g., bleomycin) is added (93). These data suggest that ER stress that occurs following exposure to various toxins, perhaps including asbestos, produces abnormal lung epithelium that promotes fibrosis. Abnormal AEC ER has been found in a rat model of asbestosis (15). Our group has observed that asbestos-exposed human A549 and rat AT2 cells release ER Ca2+ within 5 min and activate the UPR signaling proteins, IRE-1α and XBP1, within 1 h (94). Preliminary studies show that simultaneous treatment with the ER chemical chaperone 4-phenyl butyric acid abolishes asbestos-induced increases in IRE-1α and XBP1 but does not prevent mobilization of ER Ca2+ or apoptosis. Additional work is required to more fully understand how asbestos-induced ER stress alters intrinsic AEC apoptosis and whether this process will afford a novel therapeutic target for the remediation of pulmonary fibrosis.
ROLE OF p53 IN ASBESTOS PULMONARY TOXICITY
p53 is considered the gatekeeper of the genome because it integrates various signals and initiates the appropriate cellular responses, including cell-cycle arrest, differentiation, apoptosis, senescence, and antiangiogenesis (see References 4, 29, and 95 for a review). The functions of p53 are mediated by transcriptional activation that regulates the expression of downstream target genes, including distinct p53 transcriptional programs involved in acute DNA-damage responses and tumor suppression (96). A normal-functioning p53 response after exposure to DNA-damaging agents prevents mutations from accumulating. The importance of the p53 tumor-suppressor function is underscored by the facts that over half of all human cancers have p53 mutations and that p53 null mice have a marked increase in cancer predisposition (97). p53 is also redox sensitive, and its transcriptional function is integrally linked to oxidative stress, which allows it to orchestrate downstream cellular effects including the induction of apoptotic cell death (4, 29, 95). ROS activate p53 expression, but p53 stabilization can also promote further ROS generation, often via effects on the mitochondria (4, 29, 95). By regulating thousands of genes, either directly or indirectly, p53 modulates numerous vital cellular roles, including mtDNA maintenance (discussed below) (98, 99).
Altered p53 expression is implicated in the pathophysiology of pulmonary fibrosis, including that caused by asbestos exposure, and asbestos-associated malignancies, especially bronchogenic lung cancer (100–103). Asbestos activates p53 and p21 expression in lung epithelial and mesothelial cells that result in cell-cycle arrest (104–108). Furthermore, increased p53 levels are detected in lung cancers of patients with asbestosis (109), and p53 point mutations are present in the lung epithelium of smokers and asbestos-exposed individuals (110). Crocidolite asbestos promotes p53 gene mutations predominantly in axons 9 through 11 in BALB/c-3T3 cells (111). In lung-specific dominant-negative p53 mice, chrysotile asbestos induces TGF-β and other proinflamma-tory/fibrotic signaling and increases adenocarcinoma formation (112). Finally, studies in lung epithelial and mesothelial cells, performed with gene-expression microarray techniques, hierarchical clustering analyses, and a systems biology approach to examine asbestos-induced whole-genome expression profiling (54,000 genes), confirm that p53 activation plays a crucial role, along with the activation of nearly 2,500 other genes, in the regulation of tumor suppression, cell-cycle arrest, apoptosis/antiproliferation, and cell survival/antiapoptosis (113, 114). Thus, p53 plays an important role in the regulation of lung cellular DNA-damage response following exposure to oxidative stress, as occurs with asbestos and tobacco smoke.
The precise mechanisms by which p53 regulates apoptosis are complex and not fully established, but they include both p53 transcription-dependent and transcription-independent mechanisms (4, 29, 95). p53 can induce intrinsic apoptosis by increasing the gene expression of proapoptotic stimuli (e.g., BAX, NOXA, and PUMA) while inhibiting the expression of antiapoptotic Bcl-2 family members. One of the p53 transcription-independent mechanisms that promote apoptosis involves wild-type p53 protein interacting with the antiapoptotic Bcl-XL protein in the cytoplasm to augment Bax/Bak-induced outer mitochondrial membrane permeabilization. Tumor-derived p53 mutants that block the interaction between p53 and Bcl-XL cause a double hit on the mitochondria-regulated apoptotic pathway by preventing both transcription-dependent and direct mitochondrial effects of p53 (29, 95). Considerable evidence has established that p53 is a crucial regulator of mitochondrial functions, including ROS generation and mtDNA repair following oxidative damage, as well as mitochondrial biogenesis and mtDNA replication (see Reference 29 for a review). For example, p53 mediates asbestos-induced, mitochondria-regulated apoptosis in lung epithelial cells; this effect is blocked in cells that are incapable of producing mitochondrial ROS (115). Further, amosite asbestos triggers mitochondrial translocation of Bax and p53 in cultured lung epithelial cells and induces p53 expression in rat lungs two weeks following intratracheal instillation; these effects are blocked by phytic acid, an iron chelator (Figure 8). Notably, the loss of p53 results in mtDNA depletion, altered mitochondrial function, and increased H2O2 production (98, 99). Further studies are necessary to more fully understand how asbestos-induced p53-dependent signaling alters mitochondria-regulated AEC apoptosis and whether this signaling represents a novel therapeutic target for the remediation of asbestos-induced pulmonary fibrosis and malignant transformation. Figure 9 shows a hypothetical model highlighting some of the key p53-dependent pathways that are activated following asbestos exposure in mediating intrinsic apoptosis.
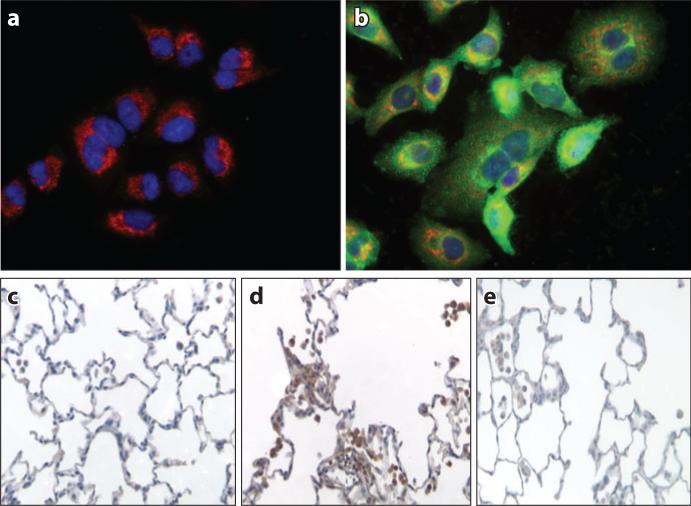
Figure 8.
Asbestos-induced changes in p53 localization and expression in alveolar epithelial cells and rat lung tissue. Compared with untreated controls (a), amosite asbestos induces human A549 cell nuclear (DAPI-stained; blue) and mitochondrial (GRP78-stained; red) p53 green fluorescent protein colocalization (b). Compared with saline-treated rat lungs (c), amosite asbestos induces p53 expression in lung cells at the bronchoalveolar junction (d ) that is inhibited by phytic acid, an iron chelator (e). Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; GRP78, 78-kDa glucose-regulated protein/binding immunoglobulin protein. Modified with permission from Reference 115.
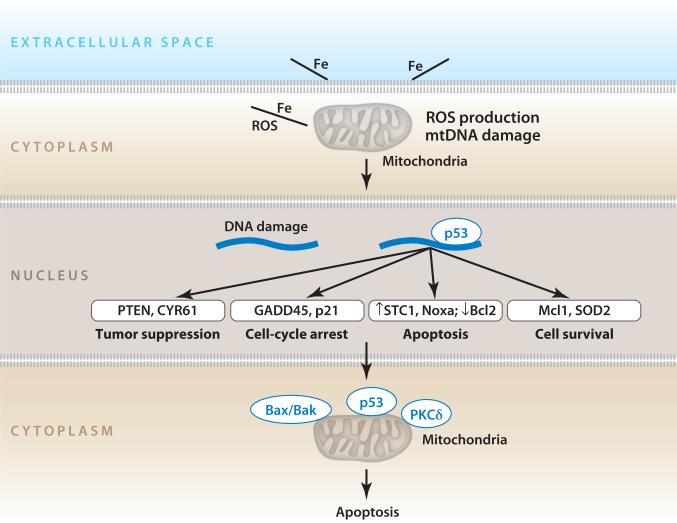
Figure 9.
Asbestos induces more than 2,500 genes, including a prominent p53 response. Interaction between asbestos and cells causes reactive oxygen species (ROS) production, DNA damage, p53 activation, and apoptosis. Abbreviations: Bax/Bak, Bcl-2 homologous antagonist killer/Bcl-2-associated X protein; Bcl-2, B cell lymphoma 2; CYR61, cysteine-rich, angiogenic inducer, 61; GADD45, growth arrest and DNA damage; Mcl-1, myeloid cell leukemia sequence 1; Noxa, nicotinamide adenine dinucleotide phosphate oxidase activator 1; PKC, protein kinase C; PTEN, phosphatase and tensin homolog; p21, cyclin-dependent kinase inhibitor 1/WAF-1; SOD2, superoxide dismutase 2; STC1, stanniocalcin 1. Modified with permission from Reference 5.
ROLE OF MITOCHONDRIAL OGG1/ACO2
Mitochondrial DNA Repair
Because approximately 1–5% of the total molecular oxygen utilized by mammalian mitochondria is converted into ROS, the mitochondria are one of the main cellular targets of oxidative damage (see References 60, 116, and 117 for a review). Mitochondrial ROS production following asbestos exposure causes DNA damage that must be efficiently repaired; otherwise, it could trigger apoptosis or other mutagenic abnormalities that would contribute to its malignant potential (see References 4, 8, and 29 for a review). Oxidative stress causes multiple types of DNA-base damage, but one of the most abundant lesions is 8-hydroxyguanine (4, 117). The 8-hydroxyguanine residue base pairs with adenine, rather than cytosine, which causes transversion mutations in replicating cells. These mutations have been implicated in aging and the development of cancer (4, 117, 118). There is evidence that polymorphisms of DNA-repair genes could identify asbestos-exposed workers at risk of developing a malignancy (119–122). Inefficient repair of oxidative mtDNA damage augments the accumulation of mtDNA damage and mutations that can lead to mitochondrial dys-function and apoptosis. Lung mesothelial cell mtDNA damage occurs following exposure to a fourfold-lower dose of crocidolite asbestos than is necessary for the induction of nuclear DNA damage (68). As recently reviewed elsewhere (23), several lines of evidence implicate mtDNA oxidative injury as a key trigger of apoptosis that can promote inflammation-associated cancer. These findings include the following: (a) Cell death is more closely associated with mtDNA oxidative lesions than with nuclear DNA damage, (b) mtDNA damage causes ATP depletion and mitochondrial dysfunction, (c) enhancement of mtDNA repair can prevent cell death, and (d ) defective mtDNA repair speeds up cell death. Herein, we focus on the repair of 8-hydroxyguanine by mitochondrial human 8-oxoguanine DNA glycosylase 1 (mt-hOGG1) because it is the best-characterized mitochondrial base excision–repair protein.
The base excision–repair pathway is responsible for most of the mtDNA repairs that are important for genome stability and long-term cell survival (see References 29 and 117 for a review). All mtDNA-repair enzymes, including those involved in base excision repair, are encoded in the nucleus and imported into the mitochondria. Base excision repair occurs over four sequential enzymatic steps involving DNA glycosylase, apurinic/apyrimidinic (AP) endonuclease, DNA polymerase, and DNA ligase. The initial base excision–repair step involves recognition and removal of the aberrant 8-hydroxyguanine base pair by a DNA glycosylase. OGG1 is a bifunctional protein located on chromosome 3p26.2 that contains a DNA glycosylase that recognizes and removes 8-hydroxyguanine, but it also has AP-lyase activity that cleaves DNA at abasic sites through a β-elimination mechanism (123, 124). Mice that are deficient in OGG1 show substantial accumulation of 8-hydroxyguanine in mtDNA and nuclear DNA, which suggests that this enzyme is important in most of the base excision–repair activity following oxidative stress, as occurs with asbestos (125). Interestingly, the mitochondria, rather than the nucleus, are the primary site of OGG1 DNA-repair activity, as determined with assessments that used fluorometric techniques to identify the site of OGG1 DNA-repair activity following exposure to oxidative stress (126). Not surprisingly given the above findings, OGG1 gene mutations or polymorphisms increase the risk of various malignancies, including lung, kidney, gastric, head and neck, and colorectal cancers, as well as leukemia (see References 23, 117, and 125 for a review).
Role of OGG1 and ACO2 in Preventing Asbestos-Induced Apoptosis
Several groups have shown that mt-hOGG1 overexpression attenuates mtDNA damage and intrinsic apoptosis caused by ROS-exposed vascular endothelial and asbestos-exposed cells (67, 127–131). This evidence indicates that mt-hOGG1 plays a key role in the regulation of intrinsic apoptosis in diverse types of oxidative stress, including that induced by asbestos. Alternative splicing of the OGG1 transcript creates two isoforms: α-OGG1 and β-OGG1 (117, 125). β-OGG1 levels in the mitochondria are 20-fold higher than α-OGG1 levels are, but surprisingly, β-OGG1 lacks OGG1 activity (132). This finding led our group to suggest that OGG1 plays a cellular role that is independent of DNA repair (67). We reported that overexpression of mitochondrial α-OGG1 mutants lacking 8-hydroxyguanine DNA-repair activity were as effective as wild-type mt-OGG1 in preventing oxidant (asbestos and H2O2)-induced caspase-9 activation and intrinsic apoptosis. Mitochondria-targeted OGG1 did not alter the levels of mitochondrial ROS produced but, interestingly, preserved ACO2; this finding suggests a novel role for OGG1, as discussed further below.
ACO2, an enzyme that is crucial for carbohydrate and energy metabolism, is responsible for the interconversion of citrate and isocitrate in the tricarboxylic acid (TCA) cycle and is a key enzyme regulating bioenergy transformation, given that loss of its activity decreases cell survival (133). ACO2 is an iron-sulfur protein that is susceptible to oxidative inactivation causing the release of redox-active iron from the (4Fe–4S)2+ center; there is evidence that ACO2 is a mitochondrial redox sensor (134). Aconitase inactivation augments the deficiency of mitochondrial manganese superoxide dismutase (135); is associated with decreased life span in Drosophila (136); and is implicated in numerous neurodegenerative diseases, including progressive supranuclear palsy (137), Friedreich's ataxia (138), and Huntington's disease (139). A study in yeast found that ACO2 preserves mtDNA independently of aconitase's catalytic activity, which suggests that ACO2 plays a novel dual role as a mitochondrial TCA enzyme and in mtDNA maintenance (140). ACO2 coprecipitates with frataxin, an iron chaperone protein that prevents ACO2 oxidative inactivation and/or augments ACO2 reactivation and is implicated in the pathogenesis of Friedreich's ataxia (141). Our group reported that overexpression of both mt-hOGG1 wild type and mutants completely blocks (a) oxidant (asbestos and H2O2)-induced decreases in AEC mitochondrial ACO2 activity and (b) protein expression in lung epithelial cells (67). Moreover, with immunoprecipitation techniques we showed that ACO2 colocalizes with both wild-type and mutant mt-hOGG1. Notably, overexpression of ACO2 also blocks oxidant-induced AEC apoptosis, whereas OGG1 underexpression obtained with short-hairpin RNA techniques decreases basal ACO2 levels and augments oxidant-induced AEC apoptosis. The latter findings are in accord with those from several recent studies, which showed that OGG1 deficiency increases oxidant-induced apoptosis (142–144). Collectively, these findings implicate a novel interaction between a mtDNA-repair enzyme (mt-OGG1) and ACO2 in preventing intrinsic AEC apoptosis following exposure to oxidative stress (e.g., stress from asbestos or H2O2).
The mechanisms underlying the interactive protective effect of mt-hOGG1 and ACO2 against asbestos-induced AEC apoptosis require further study, but there are at least two possibilities, which are not mutually exclusive. First, mt-hOGG1 may prevent oxidative modification of ACO2 that is responsible for triggering ACO2 degradation by mitochondrial Lon protease (145, 146). Because Lon protease selectively degrades oxidatively modified ACO2 at a much higher rate than unexposed aconitase does, ACO2 oxidative modification may be important in mitochondrial dysfunction, and it may ensure that such cells undergo intrinsic apoptosis (145, 146). Support for this hypothesis is our finding that MG132, a protease inhibitor that blocks mitochondrial Lon protease (147), attenuates asbestos-induced reductions in ACO2 activity (67). Second, overexpression of mt-hOGG1 or ACO2 may preserve the mtDNA levels necessary to prevent activation of intrinsic apoptosis. Future studies are required to clarify these possibilities and to determine precisely how mt-hOGG1 interacts with aconitase and whether other mtDNA repair proteins act similarly.
Several lines of evidence implicate a potentially important link among p53, OGG1, and ACO2. First, OGG1 is transcriptionally regulated by p53 in colon and renal epithelial cells (143). The expression and activity of OGG1 are reduced in HCT116p53–/– cells, which is explained by the facts that p53 binds to the putative cis elements within the OGG1 promoter and that overexpression of p53 in HCT116p53–/– cells restores OGG1 transcription. Second, p53 may regulate ACO2 levels because thymoquinone, a p53-dependent antineoplastic drug, reduces ACO2 enzyme activity in isolated rat liver mitochondria (148). Third, ACO2 is a p53-downregulated gene in some cells; ACO2 gene expression in prostate carcinoma cells is inhibited by both endogenous p53 induction by camptothecin treatment and exogenous p53 induction by transient overexpression of p53 (149). Camptothecin does not affect ACO2 reporter activity in p53-null PC-3 cells, which suggests that the decrease in ACO2 gene expression by camptothecin occurs via p53 activation. The relevance of these findings to asbestos exposure and to other cell types, as well as their significance in vivo, requires further study.
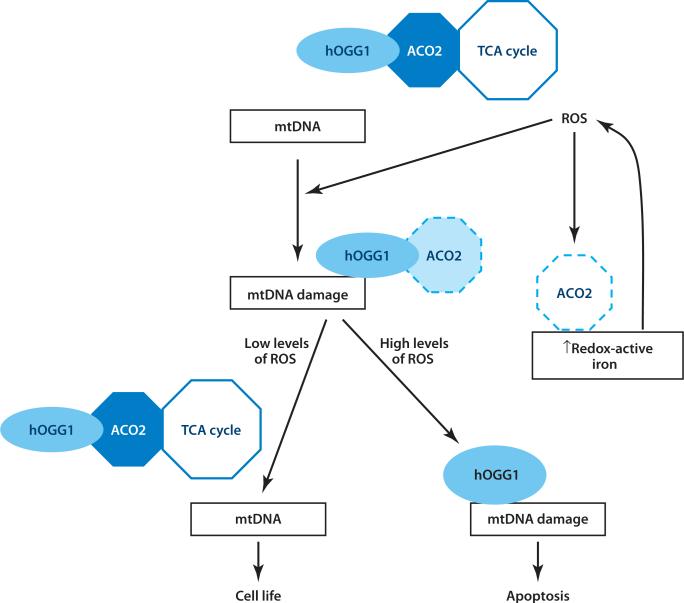
Figure 10 shows a hypothetical model that highlights some of the key OGG1/ACO2-dependent pathways described above in the mediation of apoptosis. Under normal physiological conditions, OGG1 is associated with ACO2's promotion of normal mitochondrial function and TCA cycling by reducing ACO2 oxidative degradation that may occur following exposure to low levels of ROS. However, in the setting of additional oxidative stress, such as that which follows exposure to asbestos or H2O2, mtDNA damage occurs, causing the engagement of α-OGG1 binding to mtDNA. This binding pulls ACO2 out of the TCA cycle, thereby reserving energy production for DNA repair. Although the β-OGG1 isomer cannot bind DNA or repair the 8-oxoguanine byproduct of DNA, it may play a role in sheltering ACO2 from oxidative damage. However, high levels of oxidative stress that exceed the antioxidant defenses and DNA-repair capacity of the cell result in oxidative modification of ACO2 and degradation by Lon proteases, which promotes the release of additional redox-active iron, irreversible mitochondrial dysfunction, and apoptosis. Additional studies are warranted to assess whether OGG1 functions as an ACO2 chaperone to protect mtDNA from asbestos and other forms of oxidative stress. If so, investigators should determine whether the ACO2 protection of mtDNA is an important regulated target for novel pharmacological therapy to prevent oxidant-induced mitochondrial dys-function and apoptosis that are important in respiratory disorders, including asbestosis and pulmonary fibrosis, aging, neurodegenerative disease, and cardiovascular disease.
Figure 10.
Hypothetical model showing mitochondrial human oxoguanine glycosylase 1 (hOGG1) and aconitase interactions that promote cell life or death. Abbreviations: ACO2, mitochondrial aconitase; mtDNA, mitochondrial DNA; ROS, reactive oxygen species; TCA, tricarboxylic acid. Reprinted with permission from Reference 67.
CONCLUSIONS
Table 1 summarizes some of the important molecular mechanisms underlying asbestos-induced pulmonary toxicity that are discussed herein. Emerging evidence demonstrates that mitochondria- and p53-regulated death pathways mediate AEC apoptosis, which is an important early event in patients with asbestosis and IPF, both of which may be modulated by the ER. There may also be an important interactive effect among mtOGG1, ACO2, and p53 in mtDNA repair and oxidant-induced intrinsic apoptosis. Although we focus on the role of oxidative stress caused by exposure to asbestos fibers, many of the interactive effects among mtOGG1, ACO2, p53, and intrinsic apoptosis described herein will probably have important broader implications regarding pulmonary fibrosis and mutagenesis. However, future studies exploring the role of these various interactive effects in transgenic animal models and in humans will be required. Additional studies will be necessary to further characterize the role of mt-OGG1 and ACO2 in the prevention of mtDNA damage (including following asbestos exposure), p53 activation, and intrinsic apoptosis. It will also be interesting to better understand the molecular mechanisms by which mt-OGG1 binds ACO2. Table 1 highlights some of the crucial areas that require further investigation. Finally, and perhaps most importantly, the asbestos paradigm will shed light onto the molecular basis underlying pulmonary toxicity, which will provide a better understanding of the pathogenesis of other, more common diseases, such as lung cancer and IPF, for which effective treatment regimens are urgently required. Strategies focused on promoting mtDNA integrity in key target cells by limiting oxidative stress or p53 signaling and by augmenting mitochondrial OGG1 and/or ACO2 levels may prove useful in developing novel therapeutic treatments for tumors and degenerative diseases, as well as in modulating the effects of aging.
Table 1.
Summary of some of the important molecular mechanisms underlying asbestos-induced pulmonary toxicity and areas requiring further investigation
| What we know | What we need to know |
|---|---|
| Determinants of fiber toxicity (dose, dimension, biodurability, surface reactivity) have been identified. | How should the term asbestos best be defined? |
| The number of asbestos bodies in lung tissue is directly associated with the risk of lung cancer. | What is the most reproducible technique to quantitate asbestos bodies in tissue and BALF? |
| Asbestos induces iron-derived ROS from AMs as well as target cells, such as AECs and mesothelial cells. | 1. What is the primary source of iron mediating ROS production (e.g., fiber, inflammatory cells, AECs, or mesothelial cells) after asbestos exposure? |
| 2. Are iron-derived ROS necessary for mediating asbestosis and malignant transformation? | |
| Asbestosis increases the risk of lung cancer. | Is inflammation associated with asbestosis sufficient for inducing lung cancer, and if so, which cell and/or product is responsible? |
| Apoptosis of AECs occurs in patients with asbestosis and IPF. | Is AEC apoptosis essential for mediating asbestosis or IPF? |
| Asbestos activates Nalp3 inflammasomes. | What role do Nalp3 inflammasomes play in asbestos-related fibrosis and malignancies? |
| Asbestos induces AM Racl-dependent mitochondrial ROS production that is important in murine asbestosis. | What role does AM Racl play in humans with asbestosis, and is it a useful biomarker of pulmonary fibrosis? |
| p53-dependent transcription mediates asbestos-induced intrinsic AEC apoptosis in vitro and is evident in gene-profiling studies of normal and malignant cells. | Is p53-dependent transcription necessary for pulmonary fibrosis and/or malignant transformation following asbestos exposure in animal models or humans? |
| Mitochondria-targeted hOGGl preserves AEC mitochondrial function and ACO2 and thereby prevents intrinsic apoptosis in vitro. | What is the in vivo relevance of these findings in the context of asbestosis and malignancies? |
| Asbestos induces AEC ER stress in vitro. | Is asbestos-induced ER stress important in mediating pulmonary toxicity? |
Abbreviations: ACO2, mitochondrial aconitase; AEC, alveolar epithelial cell; AM, alveolar macrophage; BALF, bronchoalveolar lavage fluid; ER, endoplasmic reticulum; hOGGl, human 8-oxoguanine-DNA glycosylase 1; IPF, idiopathic pulmonary fibrosis; ROS, reactive oxygen species.
IPF: idiopathic pulmonary fibrosis
AEC: alveolar epithelial cell
ER: endoplasmic reticulum
mtDNA: mitochondrial DNA
ACO2: mitochondrial aconitase
AM: alveolar macrophage
AT2: alveolar epithelial type II
TGF-β: transforming growth factor β
TNF-α: tumor necrosis factor α
IL-1β: interleukin-1β
ROS: reactive oxygen species
BALF: bronchoalveolar lavage fluid
EGFR: epidermal growth factor receptor
ERK: extracellular signal–regulated kinase
PKCδ: protein kinase Cδ
JNK: c-Jun N-terminal kinase
δψm: mitochondrial membrane potential
MAM: mitochondrion-associated membrane
UPR: unfolded protein response
IRE-1α: inositol-requiring kinase 1α
XBP1: X box–binding protein 1
SERCA: sarcoplasmic ER Ca2+ ATPase
Akt: protein kinase B
EMT: epithelial-mesenchymal transition
SP-C: surfactant protein C
mt-hOGG1: mitochondrial human 8-oxoguanine DNA glycosylase 1
ACKNOWLEDGMENTS
The authors acknowledge the important work of many investigators in the field, only some of whom are mentioned here because of space constraints. We cite multiple recent reviews that include a more thorough listing of all the relevant older literature. The authors are grateful to Dr. David Cugell for allowing us to use his material for Figures 1–3. Our work was supported by a Merit Review grant from the US Department of Veterans Affairs (to D.W.K.) and by National Institutes of Health grant RO1ES020357 (to D.W.K.).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Case BW, Abraham JL, Meeker G, Pooley FD, Pinkerton KE. Applying definitions of “asbestos” to environmental and “low-dose” exposure levels and health effects, particularly malignant mesothelioma. J. Toxicol. Environ. Health. 2011;14:3–39. doi: 10.1080/10937404.2011.556045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mossman BT, Lippmann M, Hesterberg TW, Kelsey KT, Barchowsky A, Bonner JC. Pulmonary endpoints (lung carcinoma and asbestosis) following inhalation exposure to asbestos. J. Toxicol. Environ. Health. 2011;14:76–121. doi: 10.1080/10937404.2011.556047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broaddus VC, Everitt JI, Black B, Kane AB. Non-neoplastic and neoplastic pleural endpoints following fiber exposure. J. Toxicol. Environ. Health. 2011;14:153–78. doi: 10.1080/10937404.2011.556049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang SXL, Jaurand M-C, Kamp DW, Whysner J, Hei TK. Role of mutagenicity in asbestos fiber–induced carcinogenicity and other diseases. J. Toxicol. Environ. Health. 2011;14:179–245. doi: 10.1080/10937404.2011.556051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamp DW. Asbestos-induced lung diseases: an update. Transl. Res. 2009;153:143–52. doi: 10.1016/j.trsl.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Int. Agency Res. Cancer (IARC) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. IARC, World Health Org; Lyon, Fr.: 1987. Overall evaluations of carcinogenicity: an updating of IARC Monographs volumes 1 to 42. [PubMed] [Google Scholar]
- 7.Yao S, Della Ventura G, Petibois C. Analytical characterization of cell–asbestos fiber interactions in lung pathogenesis. Anal. Bioanal. Chem. 2010;379:2079–89. doi: 10.1007/s00216-010-3773-x. [DOI] [PubMed] [Google Scholar]
- 8.Kamp DW, Graceffa P, Pryor WA, Weitzman SA. The role of free radicals in asbestos-induced diseases. Free Radic. Biol. Med. 1992;12:293–315. doi: 10.1016/0891-5849(92)90117-y. [DOI] [PubMed] [Google Scholar]
- 9.Aust AE, Cook PM, Dodson RF. Morphological and chemical mechanisms of elongated mineral particle toxicities. J. Toxicol. Environ. Health. 2011;14:40–75. doi: 10.1080/10937404.2011.556046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warheit DB, Snajdr SI, Hartsky MA, Frame SR. Lung proliferative and clearance responses to inhaled para-aramid RFP in exposed hamsters and rats: comparisons with chrysotile asbestos fibers. Environ. Health Perspect. 1997;105(Suppl. 5):1219–22. doi: 10.1289/ehp.97105s51219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Churg A, Stevens B. Enhanced retention of asbestos fibers in the airways of human smokers. Am. J. Respir. Crit. Care Med. 1995;151:1409–13. doi: 10.1164/ajrccm.151.5.7735593. [DOI] [PubMed] [Google Scholar]
- 12.Nelson HH, Kelsey KT. The molecular epidemiology of asbestos and tobacco in lung cancer. Oncogene. 2002;21:7284–88. doi: 10.1038/sj.onc.1205804. [DOI] [PubMed] [Google Scholar]
- 13.Roggli VL, Gibbs AR, Attanoos R, Churg A, Popper H, et al. Pathology of asbestosis—an update of the diagnostic criteria. Report of the asbestosis committee of the College of American Pathologists and Pulmonary Pathology Society. Arch. Pathol. Lab. Med. 2010;134:462–80. doi: 10.5858/134.3.462. [DOI] [PubMed] [Google Scholar]
- 14.Brody AR, Overby LH. Incorporation of tritiated thymidine by epithelial and interstitial cells in bronchiolar-alveolar regions of asbestos-exposed rats. Am. J. Pathol. 1989;134:133–40. [PMC free article] [PubMed] [Google Scholar]
- 15.Pinkerton KE, Young SL, Fram EK, Crapo JD. Alveolar type II cell responses to chronic inhalation of chrysotile asbestos in rats. Am. J. Respir. Cell Mol. Biol. 1990;3:543–52. doi: 10.1165/ajrcmb/3.6.543. [DOI] [PubMed] [Google Scholar]
- 16.Guidotti TL, Miller A, Christiani D, et al. Diagnosis and initial management of nonmalignant diseases related to asbestos. Am. J. Respir. Crit. Care Med. 2004;170:691–715. doi: 10.1164/rccm.200310-1436ST. [DOI] [PubMed] [Google Scholar]
- 17.Mossman BT, Churg A. Mechanisms in the pathogenesis of asbestosis and silicosis. Am. J. Respir. Crit. Care Med. 1998;157:1666–80. doi: 10.1164/ajrccm.157.5.9707141. [DOI] [PubMed] [Google Scholar]
- 18.Churg AM, Warnock ML. Asbestos and other ferruginous bodies: their formation and clinical significance. Am. J. Pathol. 1981;102:447–56. [PMC free article] [PubMed] [Google Scholar]
- 19.Ghio AJ, Stonehuerner J, Richards J, Devlin RB. Iron homeostasis in the lung following asbestos exposure. Antioxid. Redox Signal. 2008;10:371–77. doi: 10.1089/ars.2007.1909. [DOI] [PubMed] [Google Scholar]
- 20.Warnock ML, Wolery G. Asbestos bodies or fibers and the diagnosis of asbestosis. Environ. Res. 1987;44:29–44. doi: 10.1016/s0013-9351(87)80084-1. [DOI] [PubMed] [Google Scholar]
- 21.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 22.Aggarwal BB, Vijayalesshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin. Cancer Res. 2009;15:425–30. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- 23.Kamp DW, Shacter E, Weitzman SA. Chronic inflammation and cancer: the role of the mitochondria. Oncology. 2011;25:400–13. [PubMed] [Google Scholar]
- 24.Weiss W. Asbestosis: a marker for the increased risk of lung cancer among workers exposed to asbestos. Chest. 1999;115:536–49. doi: 10.1378/chest.115.2.536. [DOI] [PubMed] [Google Scholar]
- 25.Mollo F, Magnani C, Bo P, Burlo P, Cravello M. The attribution of lung cancers to asbestos exposure: a pathologic study of 924 unselected cases. Am. J. Clin. Pathol. 2002;117:90–95. doi: 10.1309/DEDU-V6UC-587A-9CGD. [DOI] [PubMed] [Google Scholar]
- 26.Reid A, de Klerk N, Ambrosini G, Olsen N, Pang SC, Musk AW. The effect of asbestosis on lung cancer risk beyond the dose related effect of asbestos alone. Occup. Environ. Med. 2005;62:885–89. doi: 10.1136/oem.2005.020834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heintz NH, Janssen-Heininger YM, Mossman BT. Asbestos, lung cancers, and mesotheliomas: from molecular approaches to targeting tumor survival pathways. Am. J. Respir. Cell Mol. Biol. 2010;42:133–39. doi: 10.1165/rcmb.2009-0206TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shukla A, Hillegass JM, MacPherson MB, Beuschel SL, Vacek PM, et al. ERK2 is essential for the growth of human epitheliod malignant mesotheliomas. Int. J. Cancer. 2011;129:1075–86. doi: 10.1002/ijc.25763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu G, Beri R, Mueller A, Kamp DW. Molecular mechanisms of asbestos-induced lung epithelial cell apoptosis. Chem. Biol. Interact. 2010;188:309–18. doi: 10.1016/j.cbi.2010.03.047. [DOI] [PubMed] [Google Scholar]
- 30.Noble PW. Epithelial fibroblast triggering and interactions in pulmonary fibrosis. Eur. Respir. Rev. 2008;17:123–29. [Google Scholar]
- 31.Kuwano K, Kunitake R, Kawasaki M, Nomoto Y, Hagimoto N, et al. p21Waf1/Cip1/Sdi1 and p53 expression in association with DNA strand breaks in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 1996;154:477–83. doi: 10.1164/ajrccm.154.2.8756825. [DOI] [PubMed] [Google Scholar]
- 32.Lawson WE, Crossno PF, Polosukhin VV, Roldan J, Cheng DS, et al. Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am. J. Physiol. Lung. Cell Mol. Physiol. 2008;294:1119–26. doi: 10.1152/ajplung.00382.2007. [DOI] [PubMed] [Google Scholar]
- 33.Korfei M, Ruppert C, Mahavadi P, Henneke I, Markart P, et al. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2008;178:838–46. doi: 10.1164/rccm.200802-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X, Rayford H, Uhal BD. Essential roles for angiotensin receptor AT1a in bleomycin-induced apoptosis and lung fibrosis in mice. Am. J. Pathol. 2003;163:2523–30. doi: 10.1016/S0002-9440(10)63607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee PJ, et al. Early growth response gene 1–mediated apoptosis is essential for transforming growth factor β1–induced pulmonary fibrosis. J. Exp. Med. 2004;200:377–89. doi: 10.1084/jem.20040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Budinger GR, Mutlu GM, Eisenbart J, Fuller AC, Bellmeyer AA, et al. Proapoptotic Bid is required for pulmonary fibrosis. Proc. Natl. Acad. Sci. USA. 2006;103:4604–9. doi: 10.1073/pnas.0507604103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sisson TH, Mendez M, Choi K, Subbotina N, Couret A, et al. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2010;181:254–63. doi: 10.1164/rccm.200810-1615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horan GS, Wood S, Ona V, Li DJ, Lukashev ME, et al. Partial inhibition of integrin αvβ6 prevents pulmonary fibrosis without exacerbating inflammation. Am. J. Respir. Crit. Care Med. 2008;177:56–65. doi: 10.1164/rccm.200706-805OC. [DOI] [PubMed] [Google Scholar]
- 39.Hiraku Y, Kawanishi S, Ichinose T, Murata M. The role of iNOS-mediated DNA damage in infection- and asbestos-induced carcinogenesis. Ann. N.Y. Acad. Sci. 2010;1203:15–22. doi: 10.1111/j.1749-6632.2010.05602.x. [DOI] [PubMed] [Google Scholar]
- 40.Weitzman SA, Graceffa P. Asbestos catalyzes hydroxyl and superoxide radical generation from hydrogen peroxide. Arch. Biochem. Biophys. 1984;228:373–76. doi: 10.1016/0003-9861(84)90078-x. [DOI] [PubMed] [Google Scholar]
- 41.Turci F, Tomatis M, Lesci IG, Roveri N, Fubini B. The iron-related molecular toxicity mechanism of synthetic asbestos nanofibres: a model study for high-aspect-ratio nanoparticles. Chemistry. 2011;17:350–58. doi: 10.1002/chem.201001893. [DOI] [PubMed] [Google Scholar]
- 42.Shukla A, Gulumian M, Hei T, Kamp D, Rahman Q, Mossman B. Multiple roles of oxidants in the pathogenesis of asbestos-induced diseases. Free Radic. Biol. Med. 2003;34:1117–29. doi: 10.1016/s0891-5849(03)00060-1. [DOI] [PubMed] [Google Scholar]
- 43.Kliment CR, Englert JM, Gochuico BR, Yu G, Kaminski N, et al. Oxidative stress alters syndecan-1 distribution in lungs with pulmonary fibrosis. J. Biol. Chem. 2009;284:3537–45. doi: 10.1074/jbc.M807001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buder-Hoffmann SA, Shukla A, Barrett TF, Macpherson MB, Lounsbury KM, Mossman BT. A protein kinase Cδ–dependent protein kinase D pathway modulates ERK1/2 and JNK1/2 phosphorylation and Bim-associated apoptosis by asbestos. Am. J. Pathol. 2009;174:449–59. doi: 10.2353/ajpath.2009.080180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang H, Rivera Z, Jube S, Nasu M, Bertino P, et al. Programmed necrosis induced by asbestos in human mesothelial cells causes high mobility group box 1 protein release and resultant inflammation. Proc. Natl. Acad. Sci. USA. 2010;107:12611–16. doi: 10.1073/pnas.1006542107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–77. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, et al. The Nalp3 inflammasome is essential for the development of silicosis. Proc. Natl. Acad. Sci. USA. 2008;105:9035–40. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dagenais M, Skeldon A, Saleh M. The inflammasome: in memory of Dr. Jurg Tschopp. Cell Death Differ. 2012;19:5–12. doi: 10.1038/cdd.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naik E, Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J. Exp. Med. 2011;208:417–20. doi: 10.1084/jem.20110367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bulua AC, Simon A, Maddipati R, Pelletier M, Part H, et al. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J. Exp. Med. 2011;208:519–33. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–25. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 52.Sabo-Attwood T, Ramos-Nino M, Bond J, Butnor KJ, Heintz N, et al. Gene expression profiles reveal increased mClca3 (Gob5) expression and mucin production in a murine model of asbestos-induced fibrogenesis. Am. J. Pathol. 2005;167:1243–56. doi: 10.1016/S0002-9440(10)61212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Belitskaya-Levy I, Hajjou M, Su WC, Yie TA, Tchou-Wong KM, et al. Gene profiling of normal human bronchial epithelial cells in response to asbestos and benzo[a]pyrene diol epoxide (BDPE). J. Environ. Pathol. Toxicol. Oncol. 2007;26:281–94. doi: 10.1615/jenvironpatholtoxicoloncol.v26.i4.50. [DOI] [PubMed] [Google Scholar]
- 54.Carbone M, Yang H. Molecular pathways: targeting mechanisms of asbestos and erionite carcinogenesis in mesothelioma. Clin. Cancer Res. 2011;18:598–604. doi: 10.1158/1078-0432.CCR-11-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shannahan JH, Ghio AJ, Schladweiller MC, Richards JH, Andrews D, et al. Transcriptional activation of inflammasome components by Libby amphibole and the role of iron. Inhal. Toxicol. 2012;24:60–69. doi: 10.3109/08958378.2011.633942. [DOI] [PubMed] [Google Scholar]
- 56.Murthy S, Adamcakova-Dodd A, Perry SS, Tephly LA, Keller RM, et al. Modulation of reactive oxygen species by Rac1 or catalase prevents asbestos-induced pulmonary fibrosis. Am. J. Physiol. Lung. Cell Mol. Physiol. 2009;297:846–55. doi: 10.1152/ajplung.90590.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murthy S, Ryan A, He C, Mallampalli RK, Carter AB. Rac1-mediated mitochondrial H2O2 generation regulates MMP-9 gene expression in macrophages via inhibition of SP-1 and AP-1. J. Biol. Chem. 2010;285:25062–73. doi: 10.1074/jbc.M109.099655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.He C, Murthy S, McCormick ML, Spitz DR, Ryan AJ, Carter AB. Mitochondrial Cu,Zn-superoxide dismutase mediates pulmonary fibrosis by augmenting H2O2 generation. J. Biol. Chem. 2011;286:15597–607. doi: 10.1074/jbc.M110.187377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Osborn-Heaford HL, Ryan AJ, Murthy S, Racila AM, He C, et al. Mitochondrial Rac1 import and electron transfer from cytochrome c is required for pulmonary fibrosis. J. Biol. Chem. 2012;287:3301–12. doi: 10.1074/jbc.M111.308387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 61.Malhotra JD, Kaufman RJ. ER stress and its functional link to mitochondria: role in cell survival and death. Cold Spring Harb. Perspect. Biol. 2011;3:a004424. doi: 10.1101/cshperspect.a004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 63.Franco R, Sanchez-Olea R, Reyes-Reyes EM, Panayiotidis MI. Environmental toxicity, oxidative stress and apoptosis: menage a trois. Mutat. Res. 2009;674:3–22. doi: 10.1016/j.mrgentox.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 64.Galonek HL, Hardwick M. Upgrading the BCL-2 network. Nat. Cell Biol. 2006;8:1317–19. doi: 10.1038/ncb1206-1317. [DOI] [PubMed] [Google Scholar]
- 65.Panduri V, Weitzman SA, Chandel N, Kamp DW. The mitochondria-regulated death pathway mediates asbestos-induced alveolar epithelial cell apoptosis. Am. J. Respir. Cell Mol. Biol. 2003;28:241–48. doi: 10.1165/rcmb.4903. [DOI] [PubMed] [Google Scholar]
- 66.Panduri V, Weitzman SA, Chandel NS, Kamp DW. Mitochondrial-derived free radicals mediate asbestos-induced alveolar epithelial cell apoptosis. Am. J. Physiol. Lung. Cell Mol. Physiol. 2004;286:1220–27. doi: 10.1152/ajplung.00371.2003. [DOI] [PubMed] [Google Scholar]
- 67.Panduri V, Liu G, Surapureddi S, Kondapalli J, Soberanes S, et al. Role of mitochondrial hOGG1 and aconitase in oxidant-induced lung epithelial cell apoptosis. Free Radic. Biol. Med. 2009;47:750–59. doi: 10.1016/j.freeradbiomed.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shukla A, Jung M, Stern M, Fukagawa NK, Taatjes DJ, et al. Asbestos induces mitochondrial DNA damage and dysfunction linked to the development of apoptosis. Am. J. Physiol. Lung. Cell Mol. Physiol. 2003;285:1018–25. doi: 10.1152/ajplung.00038.2003. [DOI] [PubMed] [Google Scholar]
- 69.Lounsbury KM, Stern M, Taatjes D, Jaken S, Mossman BT. Increased localization and substrate activation of protein kinase Cδ in lung epithelial cells following exposure to asbestos. Am. J. Pathol. 2002;160:1991–2000. doi: 10.1016/s0002-9440(10)61149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shukla A, Stern M, Lounsbury KM, Flanders T, Mossman BT. Asbestos-induced apoptosis is protein kinase Cδ dependent. Am. J. Respir. Cell Mol. Biol. 2003;29:198–205. doi: 10.1165/rcmb.2002-0248OC. [DOI] [PubMed] [Google Scholar]
- 71.Rong Y, Distelhorst CW. Bcl-2 protein family members: versatile regulators of calcium signaling in cell survival and apoptosis. Annu. Rev. Physiol. 2008;70:73–91. doi: 10.1146/annurev.physiol.70.021507.105852. [DOI] [PubMed] [Google Scholar]
- 72.Heath-Engel HM, Chang NC, Shore GC. The endoplasmic reticulum in apoptosis and autophagy: role of the BCL-2 protein family. Oncogene. 2008;27:6419–33. doi: 10.1038/onc.2008.309. [DOI] [PubMed] [Google Scholar]
- 73.Grimm S. The ER-mitochondria interface: the social network of cell death. Biochim. Biophys. Acta. 2011;1823:327–34. doi: 10.1016/j.bbamcr.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 74.Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–39. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 75.White C, Li C, Yang J, Petrenko NB, Madesh M, et al. The endoplasmic reticulum gateway to apoptosis by Bcl-XL modulation of the InsP3R. Nat. Cell Biol. 2005;7:1021–28. doi: 10.1038/ncb1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–10. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 77.Oakes SA, Scorrano L, Opferman JT, Bassik MC, Nishino M, et al. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA. 2005;102:105–10. doi: 10.1073/pnas.0408352102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ahmad S, Ahmad A, Dremina ES, Sharov VS, Guo X, et al. Bcl-2 suppresses sarcoplasmic/endoplasmic reticulum Ca2+-ATPase expression in cystic fibrosis airways: role in oxidant-mediated cell death. Am. J. Respir. Crit. Care Med. 2009;179:816–26. doi: 10.1164/rccm.200807-1104OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meunier J, Hayashi T. Sigma-1 receptors regulate Bcl-2 expression by reactive oxygen species–dependent transcriptional regulation of nuclear factor κB. J. Pharmacol. Exp. Ther. 2010;332:388–97. doi: 10.1124/jpet.109.160960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Klee M, Pallauf K, Alcala S, Fleischer A, Pimentel-Muinos FX. Mitochondrial apoptosis induced by BH3-only molecules in the exclusive presence of endoplasmic reticular Bak. EMBO J. 2009;28:1757–68. doi: 10.1038/emboj.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pinton P, Ferrari D, Magalhaes P, Schulze-Osthoff K, Di Virgilio F, et al. Reduced loading of intracellular Ca2+ stores and downregulation of capacitative Ca2+ influx in Bcl-2-overexpressing cells. J. Cell Biol. 2000;148:857–62. doi: 10.1083/jcb.148.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vasudevan KM, Garraway LA. AKT signaling in physiology and disease. Curr. Top. Microbiol. Immunol. 2010;347:105–33. doi: 10.1007/82_2010_66. [DOI] [PubMed] [Google Scholar]
- 83.Szado T, Vanderheyden V, Parys JB, De Smedt H, Rietdorf K, et al. Phosphorylation of inositol 1,4,5-trisphosphate receptors by protein kinase B/Akt inhibits Ca2+ release and apoptosis. Proc. Natl. Acad. Sci. USA. 2008;105:2427–32. doi: 10.1073/pnas.0711324105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marchi S, Rimessi A, Giorgi C, Baldini C, Ferroni L, et al. Akt kinase reducing endoplasmic reticulum Ca2+ release protects cells from Ca2+-dependent apoptotic stimuli. Biochem. Biophys. Res. Commun. 2008;375:501–5. doi: 10.1016/j.bbrc.2008.07.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lawson WE, Crossno PF, Polosukhin VV, Roldan J, Cheng DS, et al. Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am. J. Physiol. Lung. Cell Mol. Physiol. 2008;294:1119–26. doi: 10.1152/ajplung.00382.2007. [DOI] [PubMed] [Google Scholar]
- 86.Korfei M, Ruppert C, Mahavadi P, Henneke I, Markart P, et al. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2008;178:838–46. doi: 10.1164/rccm.200802-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am. J. Pathol. 2007;170:1807–16. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J. Clin. Investig. 2002;110:341–50. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhong Q, Zhou B, Ann DK, Minoo P, Liu Y, et al. Role of endoplasmic reticulum stress in epithelial-mesenchymal transition of alveolar epithelial cells: effects of misfolded surfactant protein. Am. J. Respir. Cell Mol. Biol. 2011;45:498–509. doi: 10.1165/rcmb.2010-0347OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nogee LM, Dunbar AE, 3rd, Wert SE, Askin F, Hamvas A, Whitsett JA. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N. Engl. J. Med. 2001;344:573–79. doi: 10.1056/NEJM200102223440805. [DOI] [PubMed] [Google Scholar]
- 91.Thomas AQ, Lane K, Phillips J, 3rd, Prince M, Markin C, et al. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am. J. Respir. Crit. Care Med. 2002;165:1322–28. doi: 10.1164/rccm.200112-123OC. [DOI] [PubMed] [Google Scholar]
- 92.Maguire JA, Mulugeta S, Beers MF. Multiple ways to die: delineation of the unfolded protein response and apoptosis induced by surfactant protein C BRICHOS mutants. Int. J. Biochem. Cell Biol. 2012;44:101–12. doi: 10.1016/j.biocel.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lawson WE, Cheng DS, Degryse AL, Tanjore H, Polosukhin VV, et al. Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proc. Natl. Acad. Sci. USA. 2011;108:10562–67. doi: 10.1073/pnas.1107559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Beri R, Liu G, Mueller A, Trejo H, Kamp D. Asbestos induces an endoplasmic reticulum stress response in alveolar epithelial cells. Am. J. Resp. Crit. Care Med. 2010;181:4930. (Abstr.) [Google Scholar]
- 95.Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137:413–31. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 96.Brady CA, Jiang D, Mello SS, Johnson TM, Jarvis LA, et al. Distinct p53 transcriptional programs dictate acute DNA-damage responses and tumor suppression. Cell. 2011;145:571–83. doi: 10.1016/j.cell.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kenzelmann Broz D, Attardi LD. In vivo analysis of p53 tumor suppressor function using genetically engineered mouse models. Carcinogenesis. 2010;31:1311–18. doi: 10.1093/carcin/bgp331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bakhanashvili M, Grinberg S, Bonda E, Simon AJ, et al. p53 in mitochondria enhances the accuracy of DNA synthesis. Cell Death Differ. 2008;15:1865–74. doi: 10.1038/cdd.2008.122. [DOI] [PubMed] [Google Scholar]
- 99.Lebedeva MA, Eaton JS, Shadel GS. Loss of p53 causes mitochondrial DNA depletion and altered mitochondrial reactive oxygen species homeostasis. Biochim. Biophys. Acta. 2009;178:328–34. doi: 10.1016/j.bbabio.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nelson A, Mendoza T, Hoyle GW, Brody AR, Fermin C, Morris GF. Enhancement of fibrogenesis by the p53 tumor suppressor protein in asbestos-exposed rodents. Chest. 2001;120(1 Suppl.):33–34S. doi: 10.1378/chest.120.1_suppl.s33. [DOI] [PubMed] [Google Scholar]
- 101.Mishra A, Liu J-Y, Brody AR, Morris GF. Inhaled asbestos fibers induce p53 expression in the rat lung. Am. J. Respir. Cell Mol. Biol. 1997;16:479–85. doi: 10.1165/ajrcmb.16.4.9115760. [DOI] [PubMed] [Google Scholar]
- 102.Burmeister B, Schwerdtle T, Poser I, Hoffmann E, Hartwig A, et al. Effects of asbestos on initiation of DNA damage, induction of DNA-strand breaks, p53 expression and apoptosis in primary, SV40-transformed and malignant human mesothelial cells. Mutat. Res. 2004;558:81–92. doi: 10.1016/j.mrgentox.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 103.Plataki M, Koutsopoulos AV, Darivianaki K, Delides G, Siafakas NM, et al. Expression of apoptotic and antiapoptotic markers in epithelial cells in idiopathic pulmonary fibrosis. Chest. 2005;127:266–74. doi: 10.1378/chest.127.1.266. [DOI] [PubMed] [Google Scholar]
- 104.Johnson NF, Jaramillo RJ. p53, Cip1, and Gadd153 expression following treatment of A549 cells with natural man-made vitreous fibers. Environ. Health Perspect. 1997;105:1143–45. doi: 10.1289/ehp.97105s51143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Levresse V, Renier A, Fleury-Feith J, Levy F, Moritz S, et al. Analysis of cell cycle disruptions in cultures of rat pleural mesothelial cells exposed to fibers. Am. J. Respir. Cell Mol. Biol. 1997;17:660–71. doi: 10.1165/ajrcmb.17.6.2854. [DOI] [PubMed] [Google Scholar]
- 106.Paakko P, Ramet M, Vahakangas K, Korpela N, Soini U, et al. Crocidolite asbestos causes an induction of p53 and apoptosis in cultured A549 lung carcinoma cells. Apoptosis. 1998;3:203–12. doi: 10.1023/a:1009655007284. [DOI] [PubMed] [Google Scholar]
- 107.Matsuoka M, Igisu H, Morimoto Y. Phosphorylation of p53 protein in A549 human pulmonary epithelial cells exposed to asbestos fibers. Environ. Health Perspect. 2003;111:509–12. doi: 10.1289/ehp.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kopnin PB, Kravchenko IV, Furalyov VA, Pylev LN, Kopnin BP. Cell type–specific effects of asbestos on intracellular ROS levels, DNA oxidation and G1 cell cycle checkpoint. Oncogene. 2004;23:8834–40. doi: 10.1038/sj.onc.1208108. [DOI] [PubMed] [Google Scholar]
- 109.Nuorva K, Makitaro R, Huhti E, et al. p53 protein accumulation in lung carcinomas of patients exposed to asbestos and tobacco smoke. Am. J. Respir. Crit. Care Med. 1994;150:528–33. doi: 10.1164/ajrccm.150.2.8049841. [DOI] [PubMed] [Google Scholar]
- 110.Husgafvel-Oursiainen K, Kannio A, Oksa P, et al. Mutations, tissue accumulation and serum levels of p53 in patients with occupational cancers from asbestos and silica exposure. Environ. Mol. Mutagen. 1997;30:224–30. [PubMed] [Google Scholar]
- 111.Lin F, Liu Y, Keshava N, Li S. Crocidolite induces cell transformation and p53 gene mutation in BALB/c-3T3 cells. Teratog. Carcinog. Mutagen. 2000;20:273–81. doi: 10.1002/1520-6866(2000)20:5<273::aid-tcm3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 112.Yee H, Yie TA, Goldberg J, Wong KM, Rom WN. Immunohistochemical study of fibrosis and adenocarcinoma in dominant-negative p53 transgenic mice exposed to chrysotile asbestos and benzo[a]pyrene. J. Environ. Pathol. Toxicol. Oncol. 2008;27:267–76. doi: 10.1615/jenvironpatholtoxicoloncol.v27.i4.30. [DOI] [PubMed] [Google Scholar]
- 113.Nymark P, Lindholm PM, Korpela MV, Lahti L, Ruosaari S, et al. Gene expression profiles in asbestos-exposed epithelial and mesothelial cell lines. BMC Genomics. 2007;8:1–17. doi: 10.1186/1471-2164-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hevel JM, Olson-Buelow LC, Ganesan B, Stevens JR, Hardman JP, et al. Novel functional view of the crocidolite asbestos–treated A549 human lung epithelial transcriptome reveals an intricate network of pathways with opposing functions. BMC Genomics. 2008;9:376. doi: 10.1186/1471-2164-9-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Panduri V, Surapureddi S, Soberanes S, Weitzman SA, Chandel N, Kamp DW. p53 mediates amosite asbestos induced alveolar epithelial cell mitochondria-regulated apoptosis. Am. J. Respir. Cell Mol. Biol. 2006;34:443–52. doi: 10.1165/rcmb.2005-0352OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ralph SJ, Rodriguez-Enriquez S, Neuzil J, Saavedra E, Moreno-Sanchez R. The causes of cancer revisited: “mitochondrial malignancy” and ROS-induced oncogenic transformation—why mitochondria are targets for cancer therapy. Mol. Aspects Med. 2010;31:145–70. doi: 10.1016/j.mam.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 117.Gredilla R, Bohr VA, Stevnsner T. Mitochondrial DNA repair and association with aging—an update. Exp. Gerontol. 2010;45:478–88. doi: 10.1016/j.exger.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shibutani S, Takeshita M, Grollman AP. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxo-dG. Nature. 1991;349:431–34. doi: 10.1038/349431a0. [DOI] [PubMed] [Google Scholar]
- 119.Zhao XH, Jia G, Liu YQ, Yan L, Jin Y, Liu N. Association between polymorphisms of DNA repair gene XRCC1 and DNA damage in asbestos-exposed workers. Biomed. Environ. Sci. 2006;19:232–38. [PubMed] [Google Scholar]
- 120.Dianzani I, Gibello L, Biava A, Giordano M, Bertolotti M, et al. Polymorphisms in DNA repair genes as risk factors for asbestos-related malignant mesothelioma in a general population study. Mutat. Res. 2006;599:124–34. doi: 10.1016/j.mrfmmm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 121.Dusinska M, Dzupinkova Z, Wsolova L, Harrington V, Collins AR. Possible involvement of XPA in repair of oxidative DNA damage deduced from analysis of damage, repair and genotype in a human population study. Mutagenesis. 2006;21:205–11. doi: 10.1093/mutage/gel016. [DOI] [PubMed] [Google Scholar]
- 122.Pietruska JR, Johnston T, Zhitkovich A, Kane AB. XRCC1 deficiency sensitizes human lung epithelial cells to genotoxicity by crocidolite asbestos and Libby amphibole. Environ. Health Persp. 2010;118:1707–13. doi: 10.1289/ehp.1002312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bjoras M, Luna L, Johnsen B, Hoff E, Haug T, et al. Opposite base-dependent reactions of a human base excision repair enzyme on DNA containing 7,8-dihydro-8-oxoguanine and abasic sites. EMBO J. 1997;16:6314–22. doi: 10.1093/emboj/16.20.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Aburatani H, Hippo Y, Ishida T, Takashima R, Matsuba C, et al. Cloning and characterization of mammalian 8-hydroxyguanine-specific DNA glycosylase/apurinic, apyrimidinic lyase, a functional mutM homologue. Cancer Res. 1997;57:2151–56. [PubMed] [Google Scholar]
- 125.Bohr VA, Stevnsner T, de Souza-Pinto NC. Mitochondrial DNA repair of oxidative damage in mammalian cells. Gene. 2002;286:127–34. doi: 10.1016/s0378-1119(01)00813-7. [DOI] [PubMed] [Google Scholar]
- 126.Mirbahai L, Kershaw RM, Green RM, Hayden RE, Meldrum RA, Hodges NJ. Use of a molecular beacon to track the activity of the base excision repair protein OGG1 in live cells. DNA Repair. 2010;9:144–52. doi: 10.1016/j.dnarep.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 127.Dobson AW, Xu Y, Kelley MR, LeDoux SP, Wilson GL. Enhanced mitochondrial DNA repair and cellular survival after oxidative stress by targeting the human 8-oxoguanine glycosylase repair enzyme to mitochondria. J. Biol. Chem. 2000;275:37518–23. doi: 10.1074/jbc.M000831200. [DOI] [PubMed] [Google Scholar]
- 128.Ruchko M, Gorodnya O, LeDoux SP, Alexeyev MF, Al-Mehdi AB, Gillespie MN. Mitochondrial DNA damage triggers mitochondrial dysfunction and apoptosis in oxidant-challenged lung endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;288:530–35. doi: 10.1152/ajplung.00255.2004. [DOI] [PubMed] [Google Scholar]
- 129.Rachek LI, Grishko VI, Ledoux SP, Wilson GL. Role of nitric oxide–induced mtDNA damage in mitochondrial dysfunction and apoptosis. Free Radic. Biol. Med. 2006;40:754–62. doi: 10.1016/j.freeradbiomed.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 130.Harrison JF, Rinne ML, Kelley MR, Druzhyna NM, Wilson GL, Ledoux SP. Altering DNA base excision repair: use of nuclear and mitochondrial-targeted N-methylpurine DNA glycosylase to sensitize astroglia to chemotherapeutic agents. Glia. 2007;55:1416–25. doi: 10.1002/glia.20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ruchko MV, Gorodnya OM, Zuleta A, Pastukh VM, Gillespie MN. The DNA glycosylase Ogg1 defends against oxidant-induced mtDNA damage and apoptosis in pulmonary artery endothelial cells. Free Radic. Biol. Med. 2010;50:1107–13. doi: 10.1016/j.freeradbiomed.2010.10.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hashiguchi K, Stuart JA, de Souza-Pinto NC, Bohr VA. The C-terminal α-O helix of human Ogg1 is essential for 8-oxoguanine DNA glycosylase activity: The mitochondrial β-Ogg1 lacks this domain and does not have glycosylase activity. Nucleic Acids Res. 2004;32:5596–608. doi: 10.1093/nar/gkh863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Singh KK, Desouki MM, Franklin RB, Costello LC. Mitochondrial aconitase and citrate metabolism in malignant and nonmalignant human prostate tissues. Mol. Cancer. 2006;5:14. doi: 10.1186/1476-4598-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bulteau AL, Ikeda-Saito M, Szweda LI. Redox-dependent modulation of aconitase activity in intact mitochondria. Biochemistry. 2003;42:14846–55. doi: 10.1021/bi0353979. [DOI] [PubMed] [Google Scholar]
- 135.Williams MD, Van Remmen H, Conrad CC, Huang TT, Epstein CJ, Richardson A. Increased oxidative damage is correlated to altered mitochondrial function in heterozygous manganese superoxide dismutase knockout mice. J. Biol. Chem. 1998;273:28510–15. doi: 10.1074/jbc.273.43.28510. [DOI] [PubMed] [Google Scholar]
- 136.Yan LJ, Levine RL, Sohal RS. Oxidative damage during aging targets mitochondrial aconitase. Proc. Natl. Acad. Sci. USA. 1997;94:11168–72. doi: 10.1073/pnas.94.21.11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Park LC, Albers DS, Xu H, Lindsay JG, Beal MF, Gibson GE. Mitochondrial impairment in the cerebellum of the patients with progressive supranuclear palsy. J. Neurosci. Res. 2001;66:1028–34. doi: 10.1002/jnr.10062. [DOI] [PubMed] [Google Scholar]
- 138.Bradley JL, Blake JC, Chamberlain S, Thomas PK, Cooper JM, Schapira AH. Clinical, biochemical and molecular genetic correlations in Friedreich's ataxia. Hum. Mol. Genet. 2000;9:275–82. doi: 10.1093/hmg/9.2.275. [DOI] [PubMed] [Google Scholar]
- 139.Tabrizi SJ, Cleeter MW, Xuereb J, Taanman JW, Cooper JM, Schapira AH. Biochemical abnormalities and excitotoxicity in Huntington's disease brain. Ann. Neurol. 1999;45:25–32. doi: 10.1002/1531-8249(199901)45:1<25::aid-art6>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 140.Chen XJ, Wang X, Kaufman BA, Butow RA. Aconitase couples metabolic regulation to mitochondrial DNA maintenance. Science. 2005;307:714–17. doi: 10.1126/science.1106391. [DOI] [PubMed] [Google Scholar]
- 141.Bulteau AL, O'Neill HA, Kennedy MC, Ikeda-Saito M, Isaya G, Szweda LI. Frataxin acts as an iron chaperone protein to modulate mitochondrial aconitase activity. Science. 2004;305:242–45. doi: 10.1126/science.1098991. [DOI] [PubMed] [Google Scholar]
- 142.Bacsi A, Chodaczek G, Hazra TK, Konkel D, Boldogh I. Increased ROS generation in subsets of OGG1 knockout fibroblast cells. Mech. Ageing Dev. 2007;128:637–49. doi: 10.1016/j.mad.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Youn CK, Song PI, Kim MH, Kim JS, Hyun JW, et al. Human 8-oxoguanine DNA glycosylase suppresses the oxidative stress induced apoptosis through a p53-mediated signaling pathway in human fibroblasts. Mol. Cancer Res. 2007;5:1083–98. doi: 10.1158/1541-7786.MCR-06-0432. [DOI] [PubMed] [Google Scholar]
- 144.Xie Y, Yang H, Miller JH, Shih DM, Hicks GG, et al. Cells deficient in oxidative DNA damage repair genes My hand Ogg1 are sensitive to oxidants with increased G2/M arrest and multinucleation. Carcinogenesis. 2008;29:722–28. doi: 10.1093/carcin/bgn033. [DOI] [PubMed] [Google Scholar]
- 145.Bota DA, Davies KJA. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat. Cell Biol. 2002;4:674–80. doi: 10.1038/ncb836. [DOI] [PubMed] [Google Scholar]
- 146.Bota DA, Ngo JK, Davies KJ. Downregulation of the human Lon protease impairs mitochondrial structure and function and causes cell death. Free Radic. Biol. Med. 2005;38:665–77. doi: 10.1016/j.freeradbiomed.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 147.Granot Z, Kobiler O, Melamed-Book N, Eimerl S, Bahat A, et al. Turnover of mitochondrial steroidogenic acute regulatory (StAR) protein by Lon protease: the unexpected effect of proteasome inhibitors. Mol. Endocrinol. 2007;21:2164–77. doi: 10.1210/me.2005-0458. [DOI] [PubMed] [Google Scholar]
- 148.Roepke M, Diestel A, Bajbouj K, Walluscheck D, Schonfeld P, et al. Lack of p53 augments thymoquinone-induced apoptosis and caspase activation in human osteosarcoma cells. Cancer Biol. Ther. 2007;6:160–69. doi: 10.4161/cbt.6.2.3575. [DOI] [PubMed] [Google Scholar]
- 149.Tsui K-H, Feng T-H, Lin Y-F, Chang P-L, Juang H-H. p53 downregulates the gene expression of mitochondrial aconitase in human prostate carcinoma cells. Prostate. 2011;71:62–70. doi: 10.1002/pros.21222. [DOI] [PubMed] [Google Scholar]