Abstract
The steroid hormone 17β-estradiol (E2) has profound effects on the uterus. However, with the E2-induced increase in uterine cell proliferation and metabolism comes increased production of reactive oxygen species (ROS). We examined the expression of an interactive network of oxidative stress response proteins including thioredoxin (Trx), Cu/Zn superoxide dismutase (SOD1), apurinic endonuclease (Ape1), and protein disulfide isomerase (PDI). We demonstrated that treatment of ovariectomized C57BL/6J female mice with E2 increased the mRNA and protein levels of Trx, but decreased SOD1 and Ape1 mRNA and protein expression. In contrast, E2 treatment increased PDI protein levels but had no effect on PDI transcript levels.Interestingly, E2 treatment also increased two markers of cellular damage, lipid peroxidation and protein carbonylation. Our studies suggest that the decreased expression of SOD1 and Ape1 caused by E2 treatment may in the long term result in disruption of ROS regulation and play a role in endometrial carcinogenesis.
Keywords: Cu/Zn superoxide dismutase, apurinic endonuclease, thioredoxin, protein disulfide isomerase, oxidative stress, reactive oxygen species, estrogen receptor α
INTRODUCTION
The steroid hormone 17β-estradiol (E2) plays critical roles in the reproductive tract, mammary gland, brain, and skeletal and cardiovascular systems (Couse and Korach 1999,Korach 1994,Subbiah 1998,Toran-Allerand 1996,Hall et al 2001). In order to bring about its effects in target cells, E2 binds to the intracellular estrogen receptor (ER) and interacts with specific DNA sequences, estrogen response elements, to modulate gene expression (Heldring et al 2007). Using transgenic mice, Korach and coworkers (Deroo et al 2004) demonstrated that the effects of E2 in the uterus are mediated by ERα. The importance of this receptor in uterine function is apparent in ERα null mice, which have uteri that are underdeveloped and unable to respond to E2 treatment (Walker and Korach 2004). In fact, microarray analysis has demonstrated that ERα regulates a wide range of genes in the uteri of wild type mice, but not in ERα null mice (Hewitt et al 2003). The uterine genes regulated by E2 in wild type mice include those involved in cell cycle progression, apoptosis, cellular signaling, and redox regulation (Hong et al 2004). The uterine redox system may be especially important during E2-induced cell proliferation (Deroo et al 2004), a time when cellular metabolism is increased and production of reactive oxygen species (ROS) rises.
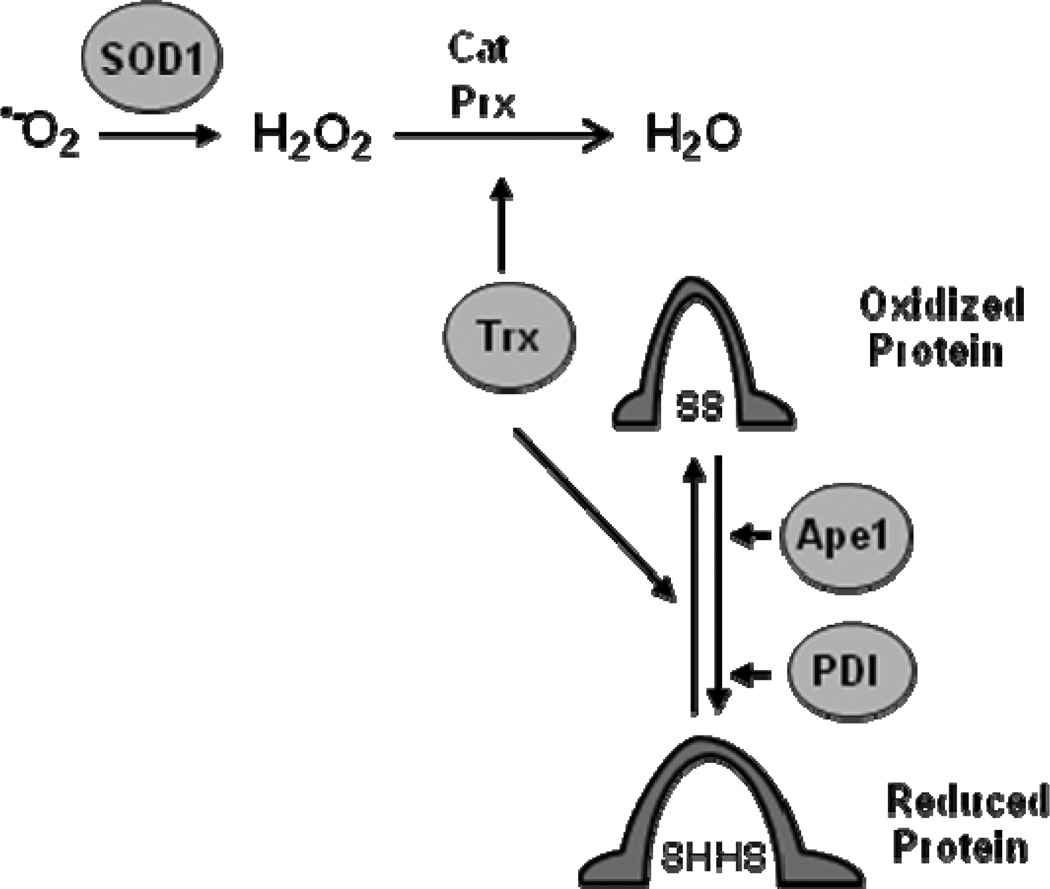
Oxidative stress can be brought about by the accumulation of ROS, which include superoxide, hydrogen peroxide, and hydroxyl radical. If ROS accumulate, the oxidative stress can damage proteins, lipids, and DNA (Halliwell 2007,Klaunig et al 2010). In order to avoid ROS-induced damage to cellular macromolecules, cells rely on a host of proteins to convert ROS to less harmful substances. Cu/Zn superoxide dismutase (SOD1) converts superoxide to hydrogen peroxide (Fig. 1), which is then converted to water by other cellular enzymes including catalase (Cat) and peroxiredoxins (Prx) (Zelko et al 2002). Thioredoxin (Trx) is required to maintain Prx in an active, reduced state (Arner and Holmgren 2000). If ROS levels accumulate and proteins become oxidized, Trx, protein disulfide isomerase (PDI) and apurinic endonuclease (Ape1) can reduce oxidized proteins and help to restore their activity (Nordberg and Arner 2001,Wilkinson and Gilbert 2004,Tell et al 2009). In addition to restoring protein activity, PDI serves as a molecular chaperone for numerous cellular proteins and Ape1 plays an essential role in DNA repair by recognizing apurinic sites and nicking the DNA backbone so that DNA repair can proceed (Noiva 1999,Hatahet and Ruddock 2007,Mol et al 2000). Thus, together these proteins form a functional network of proteins that maintain the intracellular environment and the integrity of cellular macromolecules.
Fig. 1. Oxidative stress response proteins help maintain protein structure and function.
SOD1 dismutates superoxide to form H2O2 and other cellular enzymes including catalase (Cat) and Trx, which activates peroxiredoxins (Prx), help eliminate H2O2. Cat and Prx convert H2O2 to H2O and O2. Together, Trx, Ape1, and PDI reduce cellular proteins to maintain their native protein structure and function. Adapted from Ref.(Webster et al 2001).
Interestingly, we identified SOD1, Ape1, Trx, and PDI in a complex with the DNA-bound ERα, showed that these proteins influence estrogen-responsive gene expression, and demonstrated that E2 increases SOD1 and Trx expression in human breast cancer cells and enhances SOD1 and Ape1 expression in brain slice cultures (Rao et al 2011,Schultz-Norton et al 2011,Rao et al 2009,Rao et al 2008,Schultz-Norton et al 2006a,Curtis et al 2009,Dietrich et al 2013) In addition, two groups previously reported that E2 increases Trx mRNA levels in the rodent uterus (Deroo et al 2004,Sahlin et al 1999). From these combined studies, we hypothesized that E2 might be involved in regulating expression of this interactive complex of oxidative stress response proteins in the uterus. To determine whether this was the case and whether alteration in their expression might result in damage of cellular macromolecules, we examined expression of SOD1, Ape1, PDI, and Trx and markers of protein and lipid damage in the uteri of ovariectomized, female mice that had or had not been exposed to E2.
MATERIALS AND METHODS
Mice
C57BL/6J female mice (13–16 weeks old) were obtained from Jackson Laboratory (Bar Harbor, ME) and maintained on a 12 h light/dark schedule with access to water and food ad libitum. Ovariectomized mice were maintained on phytoestrogen-free chow. Tissue was harvested from mice in accordance with guidelines of the University of Illinois at Urbana-Champaign Institutional Animal Care and Use Committee and Division of Animal Resources.
Ovariectomy and silastic tubing implantation
One week after arrival, mice were anesthetized by inhalation of 4% isoflurane, bilaterally ovariectomized, and implanted subcutaneously with silastic tubing (0.062 in/0.125 in, inner/outer diameter, 1 in length; Dow Corning, Midland, MI) that was plugged at both ends with medical adhesive (Dow Corning). Tubing contained 35 µl cottonseed oil (vehicle) or 35 µl of cottonseed oil with 180 µg/ml E2, which produces a low physiological level of circulating E2 (~25 pg/ml), which is equivalent to estrous levels of this hormone in mice (Dubal et al 2001,Wise et al 1981,Nelson et al 1992,Couse et al 1995). Seven days after ovariectomy and implantation of silastic tubing, oil- and E2-treated mice were anesthetized with isoflurane, euthanized by decapitation, and uteri were harvested for qualitative real time polymerase chain reaction (PCR), Western blot analysis or immunofluorescent imaging.
Immunofluorescent microscopy imaging
Uteri were fixed overnight at room temperature in 4% paraformaldehyde, processed, embedded in paraffin, and sectioned into 7 µm slices. Paraffin was removed, sections were rehydrated and incubated in antigen retrieval buffer (10 mM citric acid, pH 6) for 10 min at 95°C. Sections remained in the antigen retrieval buffer for 2 h as the solution cooled and were then washed 3× with PBS. Nonspecific binding was blocked by incubating sections in blocking buffer (PBS with 5% normal donkey serum and 0.05% Tween-20) for 10 min at room temperature. Sections were then incubated in blocking buffer with an ERα- (1:600, ab31312, Abcam Inc., Cambridge, MA), PR- (1:100, RM-9102, Thermo Scientific, Pittsburgh, PA), Ape1- (1:100, sc9919, Biotechnologies, Santa Cruz, CA), SOD1-(1:100, sc11407, Santa Cruz Biotechnologies), Trx- (1:200, ab86255, Abcam Inc.), or PDI- (1:100, 3501, Cell Signaling Technology, Danvers, MA) specific antibody overnight at 4°C. Tissue sections were then washed 3× with PBS-T (PBS containing 0.1% Tween-20) and incubated with DyLight 488-conjugated anti-rabbit, anti-goat, or anti-rabbit IgG (1:500, Jackson ImmunoResearch Laboratories Inc., West Grove, PA) for 1 h in the dark at room temperature, washed 3× with PBS-T, incubated with DAPI nucleic acid stain for 10 min at room temperature, washed 3× with PBS, and mounted with Pro-Long Gold antifade reagent (Life Technologies, Grand Island, NY). DAPI co-staining was included for each treatment to identify nuclei and ensure that similar numbers of cells were present. Control slices, which had not been exposed to primary antibody, were processed in parallel.
Image collection
All images were obtained with a 40× oil-immersion objective using a Leica DM 4000 B confocal microscope and Leica TCS SPE system and Application Suite Advanced Fluorescence software (Leica Microsystems, Inc., Bannockburn, IL). Detector gain and offset, laser power, and bandwidth of emission collection were kept constant for all treatments in each experiment and adjusted so that images had a full range of pixel intensities (0–255) and saturation was minimized.
Preparation of uterine extracts and Western blot analysis
Each uterus was combined with 500 µl RIPA buffer (Thermo Scientific) and 1× Protease Inhibitor Cocktail (Sigma, St. Louis, MO) and homogenized for 10 seconds at low speed with a Pro Homogenizer (ProScientific Inc., Oxford, CT). The buffer was adjusted to 400 mM NaCl with 5 M NaCl, placed on ice, and vortexed every 5 min for 15 min. The lysates were centrifuged at 14,000× g for 20 minutes at 4°C and the protein concentration of each supernatant was determined using the bicinchoninic acid (BCA) assay (Thermo Scientific) with bovine serum albumin (BSA) as a standard. 15 µg lysate was loaded onto each lane of a denaturing 4–20% gradient gel and fractionated. Proteins were transferred to a nitrocellulose membrane and the blot was probed with an SOD1- (sc11407, Santa Cruz Biotechnologies), Ape1- (ab194, Abcam Inc.), PDI- (3501, Cell Signaling), and glyceraldehyde-3-PDH- (GAPDH, A86340H, Biodesign, Saco, ME) specific antiboy. Western blots were imaged and quantitated with a Licor Odyssey Infrared Imaging System.
RNA isolation and quantitation
Total RNA was isolated and purified from uteri using QIA shredder (Qiagen, Valencia, CA) and RNeasy Mini Kit (Qiagen) according to the manufacurer’s instructions and treated with DNase I (Qiagen) to remove contaminating genomic DNA. RNA concentrations were determined and cDNA was synthesized using the iScript™ cDNA Synthesis Kit (Bio-Rad, Hercules, CA) as described by the manufacturer. 1µl of cDNA was combined with iQ™ SYBR® Green Supermix (Bio-Rad) and 0.9 µM each of forward and reverse primers and placed in a Bio-Rad iQ5 multicolor Real-Time PCR Detection System. Mouse Trx (5’-GCTCCGCCCTATTTCTATAAAG-3’ and 5’-GCGTCTCAGCAGAACCAG-3’), SOD1 (5’-CGTCCGTCGGCTTCTCGTCTTG-3’ and 5’-GTCGCCCTTCAGCACGCACAC-3’), PDI (5’-CTGCTGTTCCTGCCCAAGAGTG-3’ and 5’-TGGTTATCAGTGTGGTCGCTATCG-3’), Ape1 (5’-AGCCAGAGACCAAGAAGAG-3’ and 5’-CCACATTCCAGGAGCATATC-3’), and hypoxanthine-guanine phosphoribosyltransferase, HPRT, (5’-CTTACCTCACTGCTTTCC-3’ and 5’-GTTCATCATCGCTAATCAC-3’) primers were used for amplification. Primer efficiencies were between 95% to 110%. Relative gene expression data was determined using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method.
Lipid peroxidation assay
Uteri (~30 mg tissue) were homogenized on ice in 1 ml of 3’4-methylenedioxyamphetamine (MDA) Lysis Buffer (Abcam Inc.) with 3 µl butylated hydroxytoluene (BHT, 100×, Abcam Inc.) and centrifuged at 13,000 × g for 10 min at 4° C to remove insoluble material. 200 µl supernatant or standards containing 0, 4, 8 12, 16 or 20 nmol MDA were combined with 600 µl of thiobarbituric acid solution composed of 375 µl 20% acetic acid solution (pH 3.5) and 225 µl of 1.33% thiobarbituric acid and incubated at 95°C for 60 min. After incubation, 200 µl of each sample or standard (0, 4, 8, 12, 16, 20 nmol MDA) were transferred to a 96 well plate in triplicate and read at 532 nm by a SpectraMax 340 PC Microplate Reader.
Protein cabonylation assay
Protein carbonylation was quantified using the OxiSelect™ Protein Carbonyl ELISA Kit (STA-310, Cell Biolabs, San Diego, CA) as decribed by the manufacturer. In addition, protein samples were fractionated on a denaturing gel, electroblotted onto a nitrocellulose membrane, incubated with 2,4-dinitrophenylhydrazine (DNPH), and the derivatized proteins were detected with an anti-dinitrophenyl (anti-DNP) antibody. A replicate gel was run in parellel and subjective to Western blot analysis with a GAPDH specific antibody to normalize for differences in sample loading.
Statistics
Data from at least three independent experiments were combined and are expressed as the mean ± SEM. Statistical analysis was performed using unpaired t tests where a p value of ≤ 0.05 was considered statistically significant.
RESULTS
E2 alters uterine localization of ERα and progesterone receptor (PR)
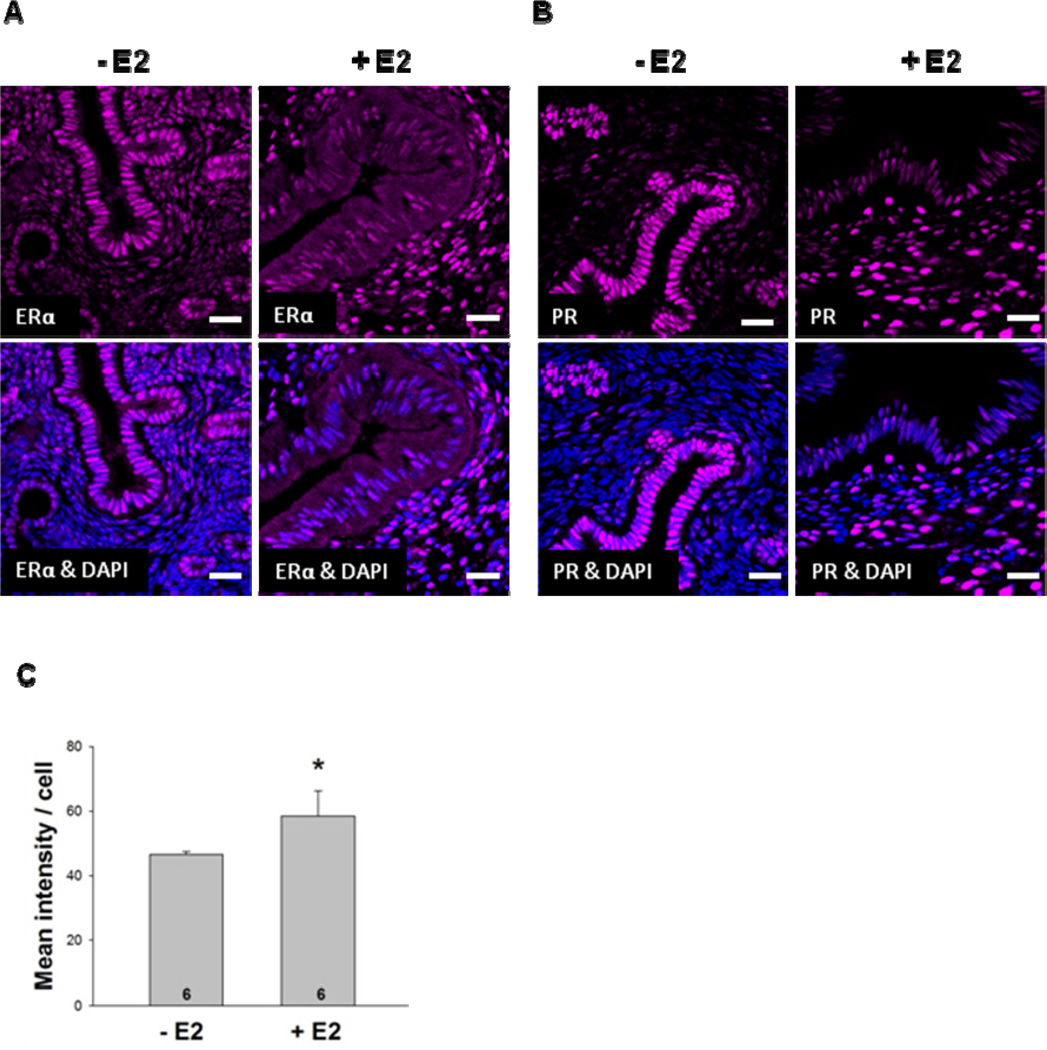
We first examined the expression of uterine ERα in C57BL/6J female mice that had been ovariectomized and implanted with silastic tubing containing oil or E2. After 7 days, the uteri from mice that had been treated with E2 were dramatically larger than uteri from mice that had been treated with oil (Supplemental Fig. 1). Immunofluorescent staining of uteri from oil-treated mice demonstrated that ERαprotein expression was most prominent in luminal and glandular epithelial cells but was also expressed in the stroma (Fig. 2A). When mice were exposed to E2 for 7 days, ERα protein expression in epithelial cells was greatly diminished and was more highly expressed in the stroma.
Fig. 2. E2 regulates PR expression.
Ovariectomized female mice were treated with oil or E2 for 7 days. (A) ERα and (B) PR expression was examined in uterine sections using immunofluorescent microscopy. (C) Quantification of PR staining of oil- and E2-treated mice demonstrated that PR expression was significantly increased in the uteri of E2-treated animals (*p<0.05). DAPI counter staining was included to identify cell nuclei. Scale bars indicate 25 µm. Six mice were included in the oil- and E2-treated groups. Representative images are shown.
To determine whether our E2 treatment was effective in enhancing estrogen-responsive gene expression in the uterus, the level of the PR was examined (Fig. 2B). Like ERα, PR protein was highly expressed in uterine epithelial cells of oil-treated mice, but was far less abundant in the stroma. When mice were treated with E2, PR expression was significantly diminished in the epithelial cells and there was a dramatic increase in PR protein expression in the stroma. These studies demonstrate that although PR expression in epithelial cells was not dependent on hormone exposure, E2 was required to increase PR expression in the stroma. Furthermore, the overall increased expression of PR protein in the uteri of E2-treated mice (Fig. 2C) was due to increased PR expression in the stroma. These findings are consistent with previous reports of ovariectomized, oil- and E2-treated animals (Sahlin et al 2006) and studies of ERα knock out mice, which demonstrated that PR expression is dependent on ERα(Kurita et al 2001).
Uterine expression of oxidative stress response proteins in oil- and E2- treated ovariectomized mice
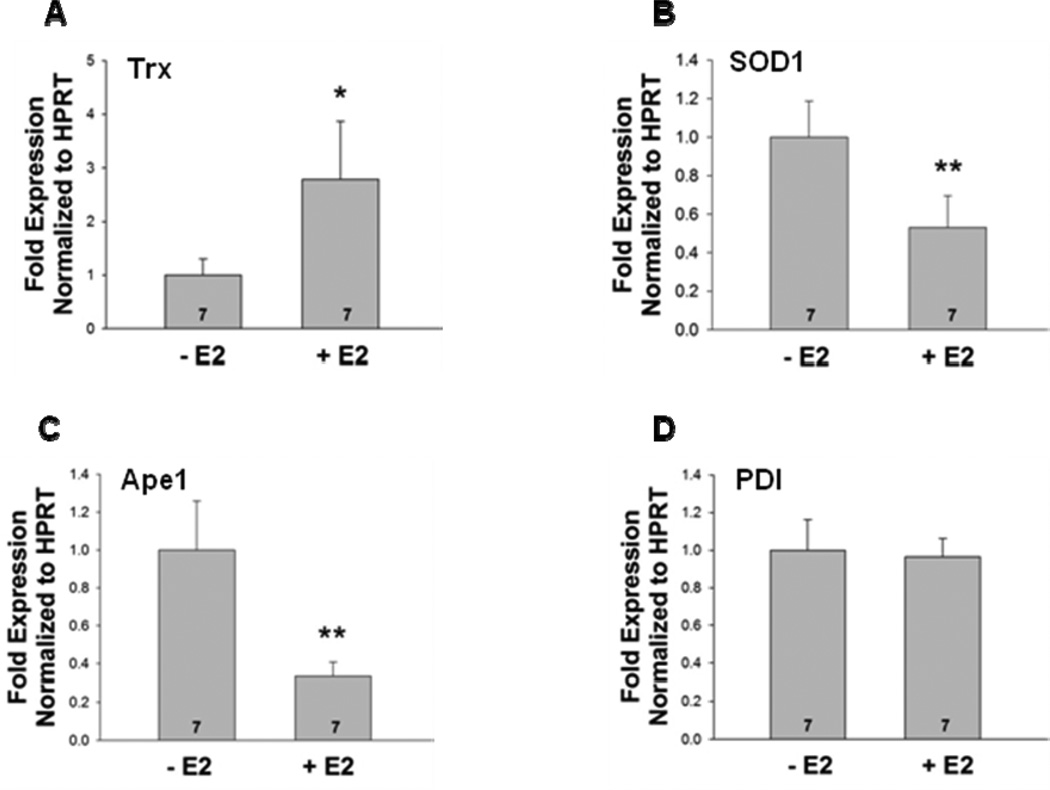
An earlier study by Deroo et al (Deroo et al 2004) reported that treatment of ovariectomized female mice with E2 significantly increased uterine Trx mRNA levels. We also observed an increase in Trx transcript levels in E2-treated mice (Fig. 3). In contrast, E2 caused a decline in SOD1 and Ape1 mRNA levels, but did not affect PDI levels.
Fig. 3. E2 treatment differentially regulates Trx, SOD1, Ape1, and PDI mRNA levels.
RNA was isolated from the uteri of ovariectomized female mice that had been treated with oil or E2. cDNA was synthesized and (A) Trx, (B) SOD1, (C) Ape1, and (D) PDI mRNA levels were determined using quantitative real time PCR. Each sample was normalized to the amount of hypoxanthine phosphorybosyl transferase (HPRT) mRNA present. E2 induced significant differences in the mRNA expression of Trx (*, p<0.05), SOD1 (**, p<0.01), and Ape1 (**, p<0.01), but not PDI (p=0.63). Seven mice were included in the oil- and E2-treated groups as indicated at the base of each treatment group bar.
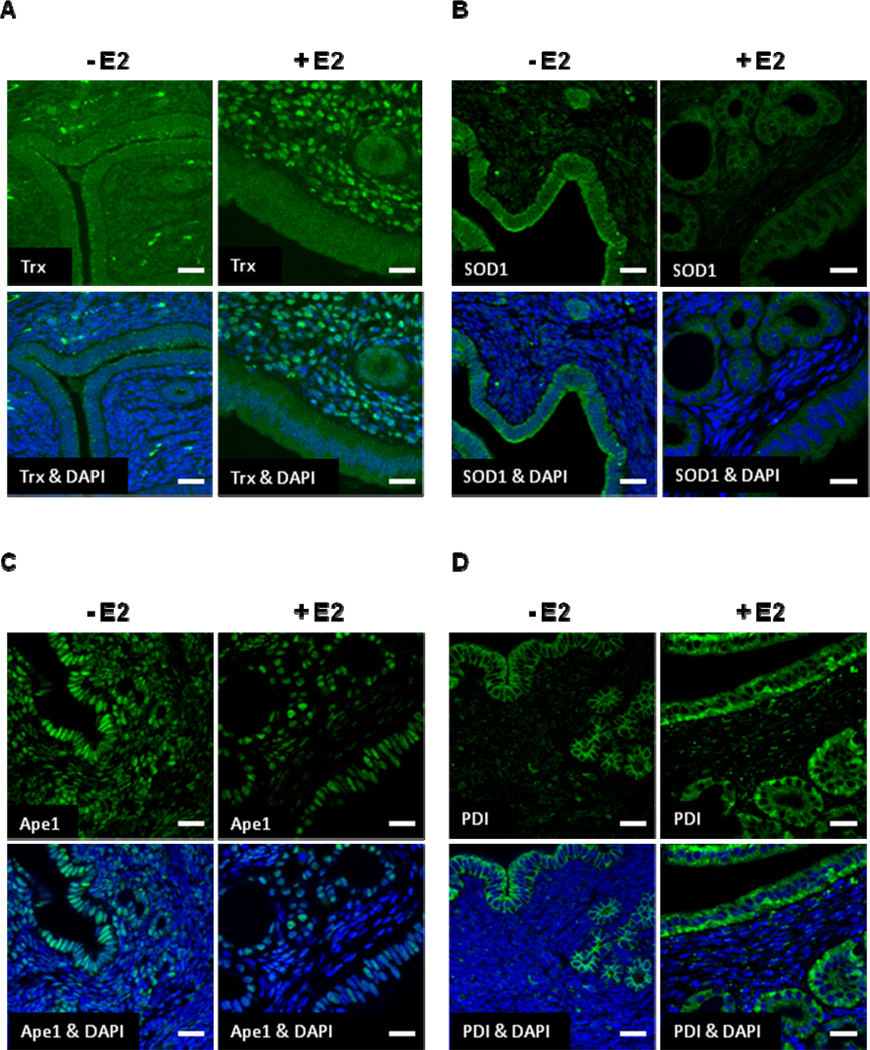
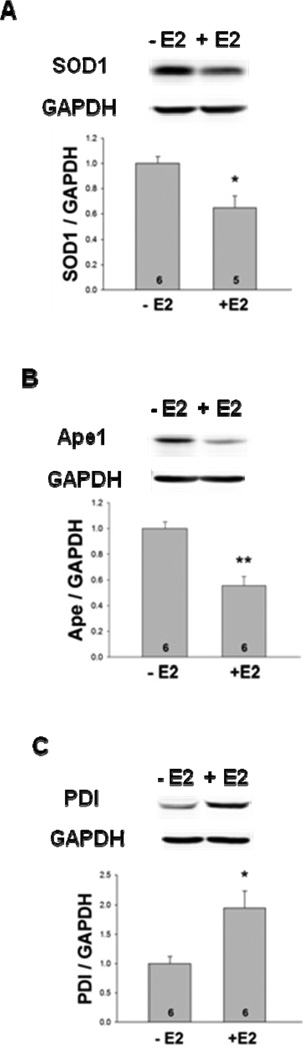
While mRNA and protein levels are oftentimes similarly affected by E2 treatment, this is not always the case. Thus, we assessed the level of uterine Trx, SOD1, Ape1, and PDI protein in ovariectomized female mice that had been treated with oil or E2 using immunofluorescent microscopy. While little Trx was expressed in the luminal or glandular epithelial cells regardless of hormone treatment (Fig. 4A), robust nuclear staining was observed in the stroma of mice that had been treated with E2. Quantitation of multiple fields of 6 slides each from oil- and E2-treated mice demonstrated that only 10% of the total DAPI-stained cells in the stroma of oil-treated animals expressed Trx, but that 60% of the total DAPI-stained cells in the stroma of E2-treated animals expressed Trx. Thus, E2 had a profound effect on Trx expression in the stroma. SOD1 was expressed primarily in the cytoplasm of luminal and glandular epithelial cells in the absence of E2 (Fig. 4B). SOD1 expression was decreased and was particularly obvious in uterine epithelial cells when mice were treated with E2. Ape1 was highly expressed in the nuclei of epithelial and stromal cells in the absence of E2 (Fig. 4C). However, when mice were treated with E2, Ape1 expression was reduced in epithelial cells and in the stroma. PDI was more highly expressed in the cytoplasm of uterine epithelial cells than in the stroma of oil-treated mice (Fig. 4D). In contrast to our RT-PCR data, however, when mice were treated with E2, PDI expression was significantly increased in luminal and glandular epithelial cells. Thus, in spite of the fact that uterine PDI mRNA levels were unaffected by E2 treatment, there was an increase in uterine PDI protein in E2-treated mice.
Fig. 4. E2 Influences Trx, SOD1, Ape1, and PDI protein expression in uterine epithelial and stromal cells.
Ovariectomized female mice were treated with oil or E2 for 7 days and (A) Trx, (B) SOD1, (C) Ape1, and (D) PDI expression was examined in uterine sections using immunofluorescent microscopy. DAPI counter staining was included to identify cell nuclei. Scale bars indicate 25 µm. Six mice were included in the oil- and E2-treated groups. Representative images are shown.
Western blot analysis was used to quantitatively determine the uterine protein levels of SOD1, Ape1, and PDI in whole cell extracts. As seen in Fig. 5, E2 significantly decreased SOD1 and Ape1 protein levels and, in agreement with our immunofluorescent microscopy studies, E2 significantly increased PDI protein expression. The expression of each protein was normalized to GAPDH to compensate for any loading discrepancies. Although six different Trx–specific antibodies were utilized, we were unable to detect Trx by Western blot analysis. Our immunofluorescent microscopy had, however, clearly demonstrated that E2 dramatically increased Trx protein expression in uterine stromal cells (Fig. 4A).
Fig. 5. E2 treatment differentially alters SOD1, Ape1, and PDI protein levels.
Whole cell extracts were prepared from the uteri of ovariectomized female mice that had been treated with oil or E2 and subjected to quantitative Western blot analysis. The levels of (A) SOD1 (*, p<0.05), (B) Ape1 (**, p<0.01), and (C) PDI (*, p<0.05) protein were significantly different in oil- and E2-treated mice. Expression of each protein was normalized to the amount of GAPDH present. Six mice were included in the oil- and E2-treated groups as indicated at the base of each treatment group bar.
Oxidative stress response in oil- and E2- treated ovariectomized mice
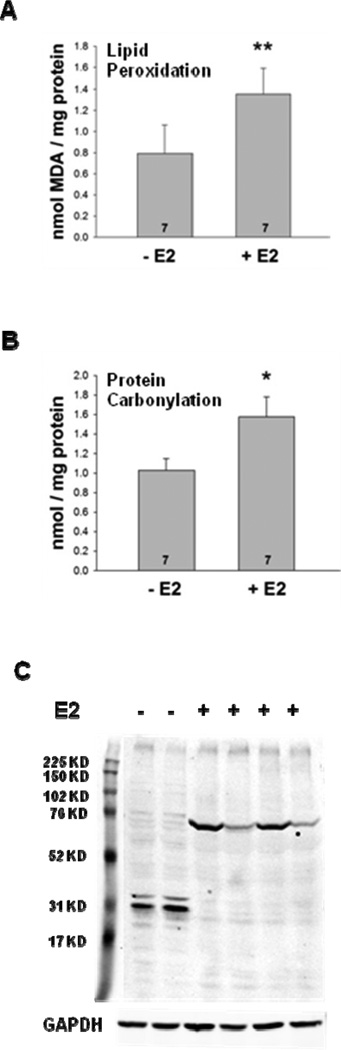
If not appropriately dissipated, accumulated ROS can lead to protein, lipid, and DNA damage (Halliwell 2007,Klaunig et al 2010). To determine whether cellular macromolecules were affected by E2 treatment, quantitative analyses of lipid peroxidation and protein carbonylation were performed. As seen in Fig. 6A, E2 treatment increased uterine lipid peroxidation 1.7-fold (**, p<0.01). Similarly, E2 treatment elicited a significant increase in uterine protein carbonylation from 1.03 ± 0.12 nmol/mg protein in oil-treated mice to 1.58 ± 0.2 nmol/mg protein in E2-treated mice (*, p<0.05), which represents a 1.5-fold increase (Fig. 6B). To further analyze the effects of E2 on protein carbonylation, uterine extracts were prepared and Western blot analysis was used to examine the E2-induced protein carbonylation patterns. In fact, protein carbonylation was distinctly different when mice were treated with oil or E2 (Fig. 6C).
Fig. 6. E2 treatment increases lipid peroxidation and protein carbonylation in the uterus.
Ovariectomized female mice were treated with oil or E2. (A) Lipid peroxidation and (B) protein carbonylation were assessed as described in Materials and Methods. Significant increases in the levels of lipid peroxidation (**, p<0.01) and protein carbonylation (*, p<0.05) were observed in the E2-treated mice. Seven mice were included in the oil- and E2-treated groups as indicated at the base of each treatment group bar. (C) The spectrum of carbonylated proteins in whole cell uterine extracts from oil- and E2-treated mice was examined by Western blot analysis. GAPDH served as a loading control.
DISCUSSION
Our studies have demonstrated that E2 treatment of ovariectomized female mice differentially alters the expression of oxidative stress response proteins in the uterus and increases two markers of oxidative stress, lipid peroxidation and protein carbonylation.
Differential regulation of uterine oxidative stress response proteins by E2
Trx is an antioxidant protein that facilitates the reduction of oxidized cellular proteins through cysteine thiol-disulfide exchange and is required for the reduction of PDI, Ape1, and numerous transcription factors including nuclear factor-κB and activator protein 1 (Nordberg and Arner 2001,Holmgren and Lu 2010,Hirota et al 1999,Das and Muniyappa 2010). We have now shown that although little Trx was expressed in uterine epithelial cells, robust nuclear staining was observed in the stroma of E2-treated mice demonstrating that uterine stromal cells are responsible for the E2-mediated increase in uterine Trx mRNA expression observed in RT-PCR. In fact, an E2-mediated increase in Trx expression has been observed in primary stromal cell cultures from human endometrium and rat uteri (Sahlin et al 1999,Maruyama et al 1999) suggesting that E2 acts directly on uterine stromal cells to increase Trx expression. The accumulation of Trx in nuclei (Fig. 4) has also been observed after UVB irradiation and ischemia (Hirota et al 1999,Malik et al 2006) and may help to ensure that the activity of the transcription factors required for the E2-mediated increase in gene expression is maintained and may be especially important in cells that have sustained oxidative damage.
PDI catalyzes the formation, reduction, and isomerization of disulfide bonds and functions as a molecular chaperone to help maintain the structure and function of cellular proteins including ERα (Wilkinson and Gilbert 2004,Schultz-Norton et al 2006b). Although it appeared that PDI mRNA levels were unaffected by E2 treatment, immunofluorescent microscopy and quantative Western blot analysis demonstrated that E2 treatment signficantly increased uterine PDI protein levels. This disparity may in part be attributed to the long half life (7 days) of this protein (Ohba et al 1981,Schultz-Norton et al 2006c). Since E2-treated mice had significantly higher levels of PDI protein than oil-treated mice, one might anticipate that the increased level of PDI would help to maintain the structural integrity of zinc finger proteins. PDI may be especially important in maintaining the structural organization of the two zinc fingers residing in the ERα DNA-binding domain (Schultz-Norton et al 2006c) and in sustaining hormone action in the uterus. In addition to its role in maintaining protein structure and function, PDI also serves as an intracellular binding protein for E2 and, although the affinity of PDI for E2 is modest, it may help to maintain higher local concentrations of E2 (Fu et al 2008).
The importance of SOD1 in uterine function is apparent in the implantation defects observed in SOD1 null female mice (Noda et al 2012). SOD1 is a key antioxidant enzyme responsible for decreasing superoxide levels in cells (Zelko et al 2002). Thus, the E2-mediated reduction of SOD1 expression combined with the E2-mediated increase in cell proliferation, metabolism, and superoxide production could impair the ability of uterine cells to reduce superoxide levels and lead to damage of cellular macromolecules. In fact, increased protein carbonylation and lipid peroxidation were observed in E2-treated mice (Fig. 6).
Ape1 plays an essential role in DNA repair and helps to maintain the activity of a number of transcription factors including Fos, Jun, nuclear factor-κB, hypoxia-inducible factor (HIF) 1 alpha, HIF-like factor, and p53 in an active, reduced state (Kelley et al 2012,Bhakat et al 2009). The diminished expression of uterine Ape1 observed in E2-treated mice could limit DNA repair and reduction of oxidized transcription factors, which could be problematic given the increased DNA replication and transcription that occurs with E2 treatment.
Although there were clear differences in the effect of E2 on expression of the oxidative stress response proteins, the mechanisms by which E2 modulates their expression have not been defined. Importantly, Hewitt et al (Hewitt et al 2012) recently reported that ERα is associated with 5000 DNA regions in the uteri of ovariectomized, vehicle-treated female mice, but that ERα is associated with more than 17,000 DNA regions in the uteri of E2-treated mice. Additionally, they identified ERα-associated gene regions flanking each of the oxidative stress response protein genes we examined (S Hewitt and K Korach, personal communication). Although ERα is associated with each of the oxidative stress response gene regions in the absence of E2, more ERα is associated with these same gene regions in the presence of E2. Thus, it appears that both unoccupied and E2-occupied ERα are involved in regulating expression of uterine oxidative stress response protein genes. Because individual EREs alter ERα conformation and recruitment of coregulatory proteins (Wood et al 1998,Wood et al 2001,Loven et al 2001,Schultz et al 2002), each gene is specifically regulated by the coregulatory proteins bound to the receptor and trans-acting factors associated with cis regulatory elements.
Implications of long term exposure of the uterus to E2
While E2-induced expression of Trx and PDI proteins in the uterus might be useful in maintaining the structure and function of cellular proteins, the decreased expression of SOD1 and Ape1 proteins could have deleterious effects over time. Because SOD1 is responsible for initiating ROS reduction (Fig. 1), an SOD1 deficiency could significantly alter the intracellular environment, increase oxidative stress, and lead to increased damage to proteins, lipids, and DNA. Furthermore, the decrease in Ape1, which plays an integral role in reducing proteins and repairing DNA, could lead to altered protein structure/function and DNA mutation.
The dysregulation of oxidative stress response proteins has been linked to a number of diseases including cancer. Altered Trx, SOD1, and Ape1 expression have been associated with various human cancers (Shan et al 2010,Sun et al 2012,Simons et al 2009,Cha et al 2009,Sun and Rigas 2008,Arner and Holmgren 2006,Weydert et al 2006,Robertson et al 2001,Zhang et al 2009). Furthermore, we previously showed that expression of SOD1, Ape1, Trx, and PDI is increased in invasive breast cancer compared to normal mammary tissue (Curtis et al 2010) and suggested that this increased expression might promote cancer cell survival.
The role of E2 in promoting endometrial cancer has been recognized for decades and both endogenous and exogenous sources of E2 have been implicated in increasing the incidence of this disease (Tinelli et al 2008). However, the mechanisms by which E2 increases uterine carcinogenesis have remained unclear. Our studies, combined with an emerging body of evidence, suggest that oxidative stress may be an underlying cause of E2-mediated endometrial carcinogenesis (Pejic et al 2009,Punnonen et al 1993,Ohwada et al 1996). In fact, SOD1 activity is significantly reduced in endometrial hyperplasia and cancer compared to normal endometrium (Pejic et al 2009,Punnonen et al 1993).
We have now shown that unopposed E2 treatment influences the expression of an interactive network of oxidative stress response proteins in the uterus and leads to increased damage to cellular macromolecules. In fact, long-term E2 treatment has been associated with pathological changes (Highman et al 1978,Greenman et al 1984,Gunin et al 2001). Our studies suggest that in the short term, E2-mediated changes may be involved in normal uterine remodeling, but that long-term unabated exposure of the uterus to E2 may over time promote endometrial carcinogenesis.
Supplementary Material
Silastic tubing containing oil or E2 was implanted in ovariectomized female mice. After 7 days the animals were sacrificed, uteri were removed, and uterine extracts were prepared. A representative image from oil or E2 treated animals demonstrates that E2 has a profound effect on uterine size. The uteri from mice that had been treated with E2 were dramatically larger than uteri from mice that had been treated with oil for 7 days.
Silastic tubing containing oil or E2 was implanted in ovariectomized female mice. After 7 days the animals were sacrificed, uteri were removed, RNA was extracted, and cDNA was prepared for quantitative real time PCR. No change in HPRT CT values was found in the uteri of oil- and E2-treated mice.
Treatment of ovariectomized C57BL/6J female mice with E2 increased the protein expression of Trx and PDI, but decreased SOD1 and Ape1 expression in uterus.
E2 treatment also increased two markers of cellular damage, lipid peroxidation and protein carbonylation.
Our studies suggest that the altered expression of oxidative stress response proteins caused by E2 treatment may in the long term lead to pathological changes in uterine cells that result in disruption of reactive oxygen species (ROS) regulation and play a role in endometrial carcinogenesis.
ACKNOWLEDGEMENTS
We are indebted to Ken Korach and Sylvia Hewitt (NIEHS, North Carolina), who provided detailed information about the location of ERα-associated gene regions in the uterus and we thank members of the Nardulli lab for technical assistance and helpful discussions. This work was supported by NIH Grant DK 053884 (to AMN). AKD was supported by a predoctoral fellowship from the NIEHS Reproductive Toxicology Training Grant T32 ES007326.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
L.Y., A.D., and A.N. have nothing to declare.
References
- Arner ES, Holmgren A. The thioredoxin system in cancer. Semin. Cancer Biol. 2006;16:420–426. doi: 10.1016/j.semcancer.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Arner ES, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- Bhakat KK, Mantha AK, Mitra S. Transcriptional regulatory functions of mammalian AP-endonuclease (APE1/Ref-1), an essential multifunctional protein. Antioxid. Redox Signal. 2009;11:621–638. doi: 10.1089/ars.2008.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha MK, Suh KH, Kim IH. Overexpression of peroxiredoxin I and thioredoxin1 in human breast carcinoma. J. Exp. Clin. Cancer Res. 2009;28 doi: 10.1186/1756-9966-28-93. 93-9966-28-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Curtis SW, Washburn TF, Lindzey J, Golding TS, Lubahn DB, Smithies O, Korach KS. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol. Endocrinol. 1995;9:1441–1454. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr. Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- Curtis CD, Thorngren DL, Nardulli AM. Immunohistochemical analysis of oxidative stress and DNA repair proteins in normal mammary and breast cancer tissues. BMC Cancer. 2010;10 doi: 10.1186/1471-2407-10-9. 9-2407-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CD, Thorngren DL, Ziegler YS, Sarkeshik A, Yates JR, Nardulli AM. Apurinic/apyrimidinic endonuclease 1 alters estrogen receptor activity and estrogen-responsive gene expression. Mol. Endocrinol. 2009;23:1346–1359. doi: 10.1210/me.2009-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das KC, Muniyappa H. c-Jun-NH2 terminal kinase (JNK)-mediates AP-1 activation by thioredoxin: phosphorylation of cJun, JunB, and Fra-1. Mol. Cell. Biochem. 2010;337:53–63. doi: 10.1007/s11010-009-0285-0. [DOI] [PubMed] [Google Scholar]
- Deroo BJ, Hewitt SC, Peddada SD, Korach KS. Estradiol regulates the thioredoxin antioxidant system in the mouse uterus. Endocrinology. 2004;145:5485–5492. doi: 10.1210/en.2004-0471. [DOI] [PubMed] [Google Scholar]
- Dietrich AK, Humphreys GI, Nardulli AM. 17beta-Estradiol increases expression of the oxidative stress response and DNA repair protein apurinic endonuclease (Ape1) in the cerebral cortex of female mice following hypoxia. J. Steroid Biochem. Mol. Biol. 2013 doi: 10.1016/j.jsbmb.2013.07.007. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc. Natl. Acad. Sci. U. S. A. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Wang P, Zhu BT. Protein disulfide isomerase is a multifunctional regulator of estrogenic status in target cells. J. Steroid Biochem. Mol. Biol. 2008;112:127–137. doi: 10.1016/j.jsbmb.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Greenman DL, Highman B, Kodell RL, Morgan KT, Norvell M. Neoplastic and nonneoplastic responses to chronic feeding of diethylstilbestrol in C3H mice. J. Toxicol. Environ. Health. 1984;14:551–567. doi: 10.1080/15287398409530605. [DOI] [PubMed] [Google Scholar]
- Gunin AG, Mashin IN, Zakharov DA. Proliferation, mitosis orientation and morphogenetic changes in the uterus of mice following chronic treatment with both estrogen and glucocorticoid hormones. J. Endocrinol. 2001;169:23–31. doi: 10.1677/joe.0.1690023. [DOI] [PubMed] [Google Scholar]
- Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J. Biol. Chem. 2001;276:36869–36872. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem. J. 2007;401:1–11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- Hatahet F, Ruddock LW. Substrate recognition by the protein disulfide isomerases. Febs J. 2007;274:5223–5234. doi: 10.1111/j.1742-4658.2007.06058.x. [DOI] [PubMed] [Google Scholar]
- Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiol. Rev. 2007;87:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Deroo BJ, Hansen K, Collins J, Grissom S, Afshari CA, Korach KS. Estrogen receptor-dependent genomic responses in the uterus mirror the biphasic physiological response to estrogen. Mol. Endocrinol. 2003;17:2070–2083. doi: 10.1210/me.2003-0146. [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Li L, Grimm SA, Chen Y, Liu L, Li Y, Bushel PR, Fargo D, Korach KS. Research resource: whole-genome estrogen receptor alpha binding in mouse uterine tissue revealed by ChIP-seq. Mol. Endocrinol. 2012;26:887–898. doi: 10.1210/me.2011-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highman B, Norvell MJ, Shellenberger TE. Pathological changes in female C3H mice continuously fed diets containing diethylstilbestrol or 17beta--estradiol. J. Environ. Pathol. Toxicol. 1978;1:1–30. [PubMed] [Google Scholar]
- Hirota K, Murata M, Sachi Y, Nakamura H, Takeuchi J, Mori K, Yodoi J. Distinct roles of thioredoxin in the cytoplasm and in the nucleus. A two-step mechanism of redox regulation of transcription factor NF-kappaB. J. Biol. Chem. 1999;274:27891–27897. doi: 10.1074/jbc.274.39.27891. [DOI] [PubMed] [Google Scholar]
- Holmgren A, Lu J. Thioredoxin and thioredoxin reductase: current research with special reference to human disease. Biochem. Biophys. Res. Commun. 2010;396:120–124. doi: 10.1016/j.bbrc.2010.03.083. [DOI] [PubMed] [Google Scholar]
- Hong SH, Nah HY, Lee JY, Gye MC, Kim CH, Kim MK. Analysis of estrogen-regulated genes in mouse uterus using cDNA microarray and laser capture microdissection. J. Endocrinol. 2004;181:157–167. doi: 10.1677/joe.0.1810157. [DOI] [PubMed] [Google Scholar]
- Kelley MR, Georgiadis MM, Fishel ML. APE1/Ref-1 role in redox signaling: translational applications of targeting the redox function of the DNA repair/redox protein APE1/Ref-1. Curr. Mol. Pharmacol. 2012;5:36–53. doi: 10.2174/1874467211205010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaunig JE, Kamendulis LM, Hocevar BA. Oxidative stress and oxidative damage in carcinogenesis. Toxicol. Pathol. 2010;38:96–109. doi: 10.1177/0192623309356453. [DOI] [PubMed] [Google Scholar]
- Korach KS. Insights from the study of animals lacking functional estrogen receptor. Science. 1994;266:1524–1527. doi: 10.1126/science.7985022. [DOI] [PubMed] [Google Scholar]
- Kurita T, Lee K, Saunders PT, Cooke PS, Taylor JA, Lubahn DB, Zhao C, Makela S, Gustafsson JA, Dahiya R, Cunha GR. Regulation of progesterone receptors and decidualization in uterine stroma of the estrogen receptor-alpha knockout mouse. Biol. Reprod. 2001;64:272–283. doi: 10.1095/biolreprod64.1.272. [DOI] [PubMed] [Google Scholar]
- Loven MA, Wood JR, Nardulli AM. Interaction of estrogen receptors alpha and beta with estrogen response elements. Mol. Cell. Endocrinol. 2001;181:151–163. doi: 10.1016/s0303-7207(01)00491-9. [DOI] [PubMed] [Google Scholar]
- Malik G, Gorbounov N, Das S, Gurusamy N, Otani H, Maulik N, Goswami S, Das DK. Ischemic preconditioning triggers nuclear translocation of thioredoxin and its interaction with Ref-1 potentiating a survival signal through the PI-3-kinase-Akt pathway. Antioxid. Redox Signal. 2006;8:2101–2109. doi: 10.1089/ars.2006.8.2101. [DOI] [PubMed] [Google Scholar]
- Maruyama T, Sachi Y, Furuke K, Kitaoka Y, Kanzaki H, Yoshimura Y, Yodoi J. Induction of thioredoxin, a redox-active protein, by ovarian steroid hormones during growth and differentiation of endometrial stromal cells in vitro. Endocrinology. 1999;140:365–372. doi: 10.1210/endo.140.1.6455. [DOI] [PubMed] [Google Scholar]
- Mol CD, Izumi T, Mitra S, Tainer JA. DNA-bound structures and mutants reveal abasic DNA binding by APE1 and DNA repair coordination [corrected] Nature. 2000;403:451–456. doi: 10.1038/35000249. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Felicio LS, Osterburg HH, Finch CE. Differential contributions of ovarian and extraovarian factors to age-related reductions in plasma estradiol and progesterone during the estrous cycle of C57BL/6J mice. Endocrinology. 1992;130:805–810. doi: 10.1210/endo.130.2.1733727. [DOI] [PubMed] [Google Scholar]
- Noda Y, Ota K, Shirasawa T, Shimizu T. Copper/zinc superoxide dismutase insufficiency impairs progesterone secretion and fertility in female mice. Biol. Reprod. 2012;86:1–8. doi: 10.1095/biolreprod.111.092999. [DOI] [PubMed] [Google Scholar]
- Noiva R. Protein disulfide isomerase: the multifunctional redox chaperone of the endoplasmic reticulum. Semin. Cell Dev. Biol. 1999;10:481–493. doi: 10.1006/scdb.1999.0319. [DOI] [PubMed] [Google Scholar]
- Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001;31:1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- Ohba H, Harano T, Omura T. Biosynthesis and turnover of a microsomal protein disulfide isomerase in rat liver. J. Biochem. 1981;89:901–907. doi: 10.1093/oxfordjournals.jbchem.a133273. [DOI] [PubMed] [Google Scholar]
- Ohwada M, Suzuki M, Sato I, Tsukamoto H, Watanabe K. Glutathione peroxidase activity in endometrium: effects of sex hormones and cancer. Gynecol. Oncol. 1996;60:277–282. doi: 10.1006/gyno.1996.0038. [DOI] [PubMed] [Google Scholar]
- Pejic S, Todorovic A, Stojiljkovic V, Kasapovic J, Pajovic SB. Antioxidant enzymes and lipid peroxidation in endometrium of patients with polyps, myoma, hyperplasia and adenocarcinoma. Reprod. Biol. Endocrinol. 2009;7:149. doi: 10.1186/1477-7827-7-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punnonen R, Kudo R, Punnonen K, Hietanen E, Kuoppala T, Kainulainen H, Sato K, Ahotupa M. Activities of antioxidant enzymes and lipid peroxidation in endometrial cancer. Eur. J. Cancer. 1993;29A:266–269. doi: 10.1016/0959-8049(93)90190-q. [DOI] [PubMed] [Google Scholar]
- Rao AK, Dietrich AK, Ziegler YS, Nardulli AM. 17beta-Estradiol-mediated increase in Cu/Zn superoxide dismutase expression in the brain: a mechanism to protect neurons from ischemia. J. Steroid Biochem. Mol. Biol. 2011;127:382–389. doi: 10.1016/j.jsbmb.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao AK, Ziegler YS, McLeod IX, Yates JR, Nardulli AM. Thioredoxin and thioredoxin reductase influence estrogen receptor alpha-mediated gene expression in human breast cancer cells. J. Mol. Endocrinol. 2009;43:251–261. doi: 10.1677/JME-09-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao AK, Ziegler YS, McLeod IX, Yates JR, Nardulli AM. Effects of Cu/Zn superoxide dismutase on estrogen responsiveness and oxidative stress in human breast cancer cells. Molecular Endocrinololgy. 2008;22:1113–1124. doi: 10.1210/me.2007-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KA, Bullock HA, Xu Y, Tritt R, Zimmerman E, Ulbright TM, Foster RS, Einhorn LH, Kelley MR. Altered expression of Ape1/ref-1 in germ cell tumors and overexpression in NT2 cells confers resistance to bleomycin and radiation. Cancer Res. 2001;61:2220–2225. [PubMed] [Google Scholar]
- Sahlin L, Masironi B, Akerberg S, Eriksson H. Tissue- and hormone-dependent progesterone receptor distribution in the rat uterus. Reprod. Biol. Endocrinol. 2006;4:47. doi: 10.1186/1477-7827-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlin L, Wang H, Masironi B, Holmgren A, Eriksson H. Regulation of thioredoxin mRNA in the rat uterus by gonadal steroids. J. Steroid Biochem. Mol. Biol. 1999;68:203–209. doi: 10.1016/s0960-0760(99)00031-x. [DOI] [PubMed] [Google Scholar]
- Schultz JR, Loven MA, Senkus Melvin VM, Edwards DP, Nardulli AM. Differential modulation of DNA conformation by estrogen receptors {alpha} and {beta} J. Biol. Chem. 2002;277:8702–8707. doi: 10.1074/jbc.M108491200. [DOI] [PubMed] [Google Scholar]
- Schultz-Norton JR, McDonald WH, Yates JR, Nardulli AM. Protein disulfide isomerase serves as a molecular chaperone to maintain estrogen receptor alpha structure and function. Mol. Endocrinol. 2006a;20:1982–1995. doi: 10.1210/me.2006-0006. [DOI] [PubMed] [Google Scholar]
- Schultz-Norton JR, McDonald WH, Yates JR, Nardulli AM. Protein disulfide isomerase serves as a molecular chaperone to maintain estrogen receptor alpha structure and function. Mol. Endocrinol. 2006b;20:1982–1995. doi: 10.1210/me.2006-0006. [DOI] [PubMed] [Google Scholar]
- Schultz-Norton JR, McDonald WH, Yates JR, Nardulli AM. Protein disulfide isomerase serves as a molecular chaperone to maintain estrogen receptor alpha structure and function. Mol. Endocrinol. 2006c;20:1982–1995. doi: 10.1210/me.2006-0006. [DOI] [PubMed] [Google Scholar]
- Schultz-Norton JR, Ziegler YS, Nardulli AM. ERalpha-associated protein networks. Trends Endocrinol. Metab. 2011;22:124–129. doi: 10.1016/j.tem.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Shan W, Zhong W, Zhao R, Oberley TD. Thioredoxin 1 as a subcellular biomarker of redox imbalance in human prostate cancer progression. Free Radic. Biol. Med. 2010;49:2078–2087. doi: 10.1016/j.freeradbiomed.2010.10.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons AL, Parsons AD, Foster KA, Orcutt KP, Fath MA, Spitz DR. Inhibition of glutathione and thioredoxin metabolism enhances sensitivity to perifosine in head and neck cancer cells. J. Oncol. 2009;2009:519563. doi: 10.1155/2009/519563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbiah MTR. Mechanisms of cardioprotection by estrogens. Proc Soc Exp Biol Med. 1998;217:23–29. doi: 10.3181/00379727-217-44201. [DOI] [PubMed] [Google Scholar]
- Sun Y, Rigas B. The thioredoxin system mediates redox-induced cell death in human colon cancer cells: implications for the mechanism of action of anticancer agents. Cancer Res. 2008;68:8269–8277. doi: 10.1158/0008-5472.CAN-08-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Rowehl LM, Huang L, Mackenzie GG, Vrankova K, Komninou D, Rigas B. Phospho-ibuprofen (MDC-917) suppresses breast cancer growth: an effect controlled by the thioredoxin system. Breast Cancer Res. 2012;14:R20. doi: 10.1186/bcr3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tell G, Quadrifoglio F, Tiribelli C, Kelley MR. The many functions of APE1/Ref-1: not only a DNA repair enzyme. Antioxid. Redox Signal. 2009;11:601–620. doi: 10.1089/ars.2008.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinelli A, Vergara D, Martignago R, Leo G, Malvasi A, Tinelli R. Hormonal carcinogenesis and socio-biological development factors in endometrial cancer: A clinical review. Acta Obstet. Gynecol. Scand. 2008;87:1101–1113. doi: 10.1080/00016340802160079. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand C. Mechanisms of estrogen action during neural development: mediation by interactions with the neurotophins and their receptors? J. Steroid Biochem. Mol. Biol. 1996;56:169–178. doi: 10.1016/0960-0760(95)00234-0. [DOI] [PubMed] [Google Scholar]
- Walker VR, Korach KS. Estrogen receptor knockout mice as a model for endocrine research. ILAR J. 2004;45:455–461. doi: 10.1093/ilar.45.4.455. [DOI] [PubMed] [Google Scholar]
- Webster KA, Prentice H, Bishopric NH. Oxidation of zinc finger transcription factors: physiological consequences. Antioxid. Redox Signal. 2001;3:535–548. doi: 10.1089/15230860152542916. [DOI] [PubMed] [Google Scholar]
- Weydert CJ, Waugh TA, Ritchie JM, Iyer KS, Smith JL, Li L, Spitz DR, Oberley LW. Overexpression of manganese or copper-zinc superoxide dismutase inhibits breast cancer growth. Free Radic. Biol. Med. 2006;41:226–237. doi: 10.1016/j.freeradbiomed.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Wilkinson B, Gilbert HF. Protein disulfide isomerase. Biochim. Biophys. Acta. 2004;1699:35–44. doi: 10.1016/j.bbapap.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Wise PM, Camp-Grossman P, Barraclough CA. Effects of estradiol and progesterone on plasma gonadotropins, prolactin, and LHRH in specific brain areas of ovariectomized rats. Biol. Reprod. 1981;24:820–830. doi: 10.1095/biolreprod24.4.820. [DOI] [PubMed] [Google Scholar]
- Wood JR, Greene GL, Nardulli AM. Estrogen response elements function as allosteric modulators of estrogen receptor conformation. Mol. Cell. Biol. 1998;18:1927–1934. doi: 10.1128/mcb.18.4.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JR, Likhite VS, Loven MA, Nardulli AM. Allosteric modulation of estrogen receptor conformation by different estrogen response elements. Mol. Endocrinol. 2001;15:7, 1114–1126. doi: 10.1210/mend.15.7.0671. [DOI] [PubMed] [Google Scholar]
- Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002;33:337–349. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang J, Xiang D, Wang D, Xin X. Alterations in the expression of the apurinic/apyrimidinic endonuclease-1/redox factor-1 (APE1/Ref-1) in human ovarian cancer and indentification of the therapeutic potential of APE1/Ref-1 inhibitor. Int. J. Oncol. 2009;35:1069–1079. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Silastic tubing containing oil or E2 was implanted in ovariectomized female mice. After 7 days the animals were sacrificed, uteri were removed, and uterine extracts were prepared. A representative image from oil or E2 treated animals demonstrates that E2 has a profound effect on uterine size. The uteri from mice that had been treated with E2 were dramatically larger than uteri from mice that had been treated with oil for 7 days.
Silastic tubing containing oil or E2 was implanted in ovariectomized female mice. After 7 days the animals were sacrificed, uteri were removed, RNA was extracted, and cDNA was prepared for quantitative real time PCR. No change in HPRT CT values was found in the uteri of oil- and E2-treated mice.