Abstract
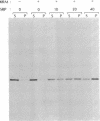
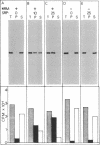
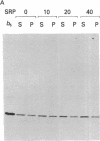
We have investigated the in vitro integration into dog pancreas microsomal membranes of three integral membrane proteins that were synthesized de novo in a wheat germ cell-free translation system: calcium ATPase of rabbit sarcoplasmic reticulum, MP26 of bovine lens fiber plasma membrane, and rat liver cytochrome b5. Biosynthetically these proteins show a common feature in that they are synthesized without a transient NH2-terminal signal sequence. Two of these proteins, ATPase and MP26, were shown to require the recently discovered signal-recognition particle (SRP) [Walter, P. & Blobel, G. (1982) Nature (London) 299, 691-698] for integration. By this criterion, therefore, they each contain at least one uncleaved signal sequence. Surprisingly, however, the uncleaved signal sequence(s) of these two proteins did not induce the characteristic SRP-mediated translation arrest that was previously shown for a cleaved signal sequence. Unlike ATPase and MP26, cytochrome b5 did not require SRP for integration into microsomal membrane. Thus, the distinction between an "insertion" sequence (specifying unassisted and opportunistic integration into any exposed membrane) and a "signal" sequence (directing integration into a specific membrane by a receptor-mediated mechanism) is a valid one. By assaying for SRP dependence, the two mechanisms of integration can now be experimentally distinguished.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Walter P., Blobel G. Signal recognition protein is required for the integration of acetylcholine receptor delta subunit, a transmembrane glycoprotein, into the endoplasmic reticulum membrane. J Cell Biol. 1982 May;93(2):501–506. doi: 10.1083/jcb.93.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendzko P., Prehn S., Pfeil W., Rapoport T. A. Different modes of membrane interactions of the signal sequence of carp preproinsulin and of the insertion sequence of rabbit cytochrome b5. Eur J Biochem. 1982 Mar;123(1):121–126. doi: 10.1111/j.1432-1033.1982.tb06507.x. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G. Intracellular protein topogenesis. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1496–1500. doi: 10.1073/pnas.77.3.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Dobberstein B., Blobel G. Functional interaction of plant ribosomes with animal microsomal membranes. Biochem Biophys Res Commun. 1977 Feb 21;74(4):1675–1682. doi: 10.1016/0006-291x(77)90637-4. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A. The spontaneous insertion of proteins into and across membranes: the helical hairpin hypothesis. Cell. 1981 Feb;23(2):411–422. doi: 10.1016/0092-8674(81)90136-7. [DOI] [PubMed] [Google Scholar]
- Enoch H. G., Fleming P. J., Strittmatter P. The binding of cytochrome b5 to phospholipid vesicles and biological membranes. Effect of orientation on intermembrane transfer and digestion by carboxypeptidase Y. J Biol Chem. 1979 Jul 25;254(14):6483–6488. [PubMed] [Google Scholar]
- Erickson A. H., Blobel G. Early events in the biosynthesis of the lysosomal enzyme cathepsin D. J Biol Chem. 1979 Dec 10;254(23):11771–11774. [PubMed] [Google Scholar]
- Gilmore R., Walter P., Blobel G. Protein translocation across the endoplasmic reticulum. II. Isolation and characterization of the signal recognition particle receptor. J Cell Biol. 1982 Nov;95(2 Pt 1):470–477. doi: 10.1083/jcb.95.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg E. L., Anderson D. J., Friedlander M., Gilula N. B. Comparative analysis of the major polypeptides from liver gap junctions and lens fiber junctions. J Cell Biol. 1982 Jan;92(1):53–59. doi: 10.1083/jcb.92.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentoft N., Dearborn D. G. Labeling of proteins by reductive methylation using sodium cyanoborohydride. J Biol Chem. 1979 Jun 10;254(11):4359–4365. [PubMed] [Google Scholar]
- Lizardi P. M., Engelberg A. Rapid isolation of RNA using proteinase K and sodium perchlorate. Anal Biochem. 1979 Sep 15;98(1):116–122. doi: 10.1016/0003-2697(79)90714-0. [DOI] [PubMed] [Google Scholar]
- Meyer D. I., Krause E., Dobberstein B. Secretory protein translocation across membranes-the role of the "docking protein'. Nature. 1982 Jun 24;297(5868):647–650. doi: 10.1038/297647a0. [DOI] [PubMed] [Google Scholar]
- Mostov K. E., DeFoor P., Fleischer S., Blobel G. Co-translational membrane integration of calcium pump protein without signal sequence cleavage. Nature. 1981 Jul 2;292(5818):87–88. doi: 10.1038/292087a0. [DOI] [PubMed] [Google Scholar]
- Nicholson B. J., Takemoto L. J., Hunkapiller M. W., Hood L. E., Revel J. P. Differences between liver gap junction protein and lens MIP 26 from rat: implications for tissue specificity of gap junctions. Cell. 1983 Mar;32(3):967–978. doi: 10.1016/0092-8674(83)90081-8. [DOI] [PubMed] [Google Scholar]
- Okada Y., Frey A. B., Guenthner T. M., Oesch F., Sabatini D. D., Kreibich G. Studies on the biosynthesis of microsomal membrane proteins. Site of synthesis and mode of insertion of cytochrome b5, cytochrome b5 reductase, cytochrome P-450 reductase and epoxide hydrolase. Eur J Biochem. 1982 Feb;122(2):393–402. doi: 10.1111/j.1432-1033.1982.tb05894.x. [DOI] [PubMed] [Google Scholar]
- Paul D. L., Goodenough D. A. In vitro synthesis and membrane insertion of bovine MP26, an integral protein from lens fiber plasma membrane. J Cell Biol. 1983 Mar;96(3):633–638. doi: 10.1083/jcb.96.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachubinski R. A., Verma D. P., Bergeron J. J. Synthesis of rat liver microsomal cytochrome b5 by free ribosomes. J Cell Biol. 1980 Mar;84(3):705–716. doi: 10.1083/jcb.84.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reithmeier R. A., MacLennan D. H. The NH2 terminus of the (Ca2+ + Mg2+)-adenosine triphosphatase is located on the cytoplasmic surface of the sarcoplasmic reticulum membrane. J Biol Chem. 1981 Jun 25;256(12):5957–5960. [PubMed] [Google Scholar]
- Rizzolo L. J., Maire M., Reynolds J. A., Tanford C. Molecular weights and hydrophobicity of the polypeptide chain of sarcoplasmic reticulum calcium(II) adenosine triphosphatase and of its primary tryptic fragments. Biochemistry. 1976 Aug 10;15(16):3433–3437. doi: 10.1021/bi00661a006. [DOI] [PubMed] [Google Scholar]
- Rizzolo L. J., Tanford C. Denaturation of the tryptic fragments of the calcium (II) adenosine triphosphatase from sarcoplasmic reticulum by guanidinium hydrochloride. Biochemistry. 1978 Sep 19;17(19):4044–4048. doi: 10.1021/bi00612a027. [DOI] [PubMed] [Google Scholar]
- Tajima S., Sato R. Topological studies of the membrane-binding segment of cytochrome b5 embedded in phosphatidylcholine vesicles. J Biochem. 1980 Jan;87(1):123–134. doi: 10.1093/oxfordjournals.jbchem.a132717. [DOI] [PubMed] [Google Scholar]
- Takagaki Y., Radhakrishnan R., Gupta C. M., Khorana H. G. The membrane-embedded segment of cytochrome b5 as studied by cross-linking with photoactivatable phospholipids. J Biol Chem. 1983 Aug 10;258(15):9128–9135. [PubMed] [Google Scholar]
- Takagaki Y., Radhakrishnan R., Wirtz K. W., Khorana H. G. The membrane-embedded segment of cytochrome b5 as studied by cross-linking with photoactivatable phospholipids. II. The nontransferable form. J Biol Chem. 1983 Aug 10;258(15):9136–9142. [PubMed] [Google Scholar]
- Walter P., Blobel G. Purification of a membrane-associated protein complex required for protein translocation across the endoplasmic reticulum. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7112–7116. doi: 10.1073/pnas.77.12.7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature. 1982 Oct 21;299(5885):691–698. doi: 10.1038/299691a0. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum III. Signal recognition protein (SRP) causes signal sequence-dependent and site-specific arrest of chain elongation that is released by microsomal membranes. J Cell Biol. 1981 Nov;91(2 Pt 1):557–561. doi: 10.1083/jcb.91.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum. II. Signal recognition protein (SRP) mediates the selective binding to microsomal membranes of in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):551–556. doi: 10.1083/jcb.91.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Ibrahimi I., Blobel G. Translocation of proteins across the endoplasmic reticulum. I. Signal recognition protein (SRP) binds to in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):545–550. doi: 10.1083/jcb.91.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Fischman D. A., Steck T. L. Selective solubilization of proteins and phospholipids from red blood cell membranes by nonionic detergents. J Supramol Struct. 1973;1(3):233–248. doi: 10.1002/jss.400010308. [DOI] [PubMed] [Google Scholar]