Abstract
Patch tests were introduced as a diagnostic tool in the late nineteenth century. Since then, they have improved considerably becoming what they are today. Patch tests are used in the diagnostic investigation of contact dermatitis worldwide. Batteries or series previously studied and standardized should be used in patch testing. The methodology is simple, but it requires adequate training for the results to be correctly interpreted and used. Despite having been used for over a century, it needs improvement like all other diagnostic techniques in the medical field.
Keywords: Dermatitis, allergic contact; Dermatitis, contact; Diagnosis; Methods; Patch tests; Skin tests
Abstract
Os testes de contato foram introduzidos, como ferramenta diagnóstica, no final do século XIX. Desde então passaram por diversos aprimoramentos tornando-os o que são hoje. Eles são utilizados na investigação diagnóstica das dermatites de contato em diferentes partes do mundo. Devem ser aplicados com a utilização de baterias ou séries previamente estudadas e padronizadas. A metodologia é simples, mas requer treinamento adequado para sua interpretação e bom aproveitamento dos resultados obtidos. Apesar de ser utilizado há mais de um século, necessita de aprimoramentos como todas as outras técnicas utilizadas para investigação diagnóstica na área médica.
INTRODUCTION
Patch tests are tools used in the identification of the etiologic agent (s) of allergic contact dermatitis. It is a scientific method of investigation, with internationally defined rules and well-established foundations, which are under continuous review and updating. The reading and interpretation of test results, whether positive or negative, are a complex process that requires training and experience, considering their relevance and associating it with the clinical history of contact dermatitis (CD).
HISTORY
CD has been recognized since ancient times and has been present throughout mankind's history. There are reports of intense itching after contact with trees (pines) dating from I d.C; reactions to some contactants were suspected in some cases of dermatitis in the XIX century, even before the term "allergy" was coined by von Pirquet in 1906.
In the seventeenth, eighteenth and nineteenth centuries, some researchers empirically used agents that triggered dermatosis in their patients with the intent of causing a skin reaction and thus correlate it with the causative agent. Still in the nineteenth century, the German physician Joseph Jadassohn created patch testing, using it for the first time as a diagnostic tool. On September 23, 1895, at the 5th Congress of the German Society of Dermatology, Jadassohn disclosed his findings in Graz, Austria. This was universally considered the date of birth of patch testing, which he called "Funktionelle Hautprüfung". In the early twentieth century, as a professor of dermatology at the University of Breslau (1917-1932), Jadassohn recognized the process of late hypersensitivity reactions, described contact dermatitis associated with mercurial agents and reproduced eczema on skin areas that were previously healthy in sensitized patients, strengthening the theoretical bases of contact dermatitis.1,2
In the same period, the French entomologist Jean-Henri Fabre conducted studies on urticarial reactions associated with the effect of caterpillars on his own skin. Even though his initial intention was not medical, he was considered a genius at the time, as he reproduced contact urticaria and motivated, based on this experiment, testing of other potentially irritating agents such as parts of plants and animals.2
Bruno Bloch, Jadassohn's pupil, continued his work and, for the first time, found a way to grade the intensity of the reaction to patch tests, as well as suggest a standardized battery of tests. Bloch's ideas were developed and disseminated by another important researcher, Paul Bonnevie. In an unprecedented way, he proposed a standardized series of testing substances to establish the etiology of CD. Still in the 1930s, the American dermatologist Marion Sulzberger, then professor at the University of New York, took this knowledge to the new world. After World War II, occupational dermatosis and CD became the focus of medical studies throughout the world, with the emergence of the first specialized clinics. It then became essential to standardize these tests, since different researchers used different techniques of application, concentration, vehicles, and in some cases, different allergens to detect the cause of allergy. Therefore, in 1962 the Scandinavian Committee for Standardization of Routine Patch Testing was created; in 1967 it was expanded to the International Contact Dermatitis Research Group (ICDRG), which was a significant step toward the standardization of patch testing.2,3,4
IMUNOLOGIC FUNDAMENTALS
CD or contact eczema is a skin condition caused by external agents (allergens), which, in contact with the skin, trigger an inflammatory reaction. In general, it clinically manifests as an eczematous dermatosis.
Allergens are substances with physicochemical properties that allow them to cross the skin barrier, such as low molecular weight - less than 500 Da - and lipophilicity, which allows them to awaken the immune system of susceptible individuals. Recent studies emphasize the importance of the interaction between the skin barrier and the immune system for the understanding of the pathophysiology of contact dermatitis. Disruption of the integrity of the epidermal barrier appears to be the first step in the events following contact with the allergen.5 This fact may partially explain the increased incidence of this disease in the elderly and atopic individuals, due to a decreased lipid mantle and consequent alteration of the skin permeability. Once in contact with the internal medium, allergens are able to bond with endogenous proteins constituting protein-hapten complexes, thus leading to a skin immune response. The skin immune system is formed by the skin-associated lymphoid tissue (SALT), dermal microvasculature (DMU), dermal immune system (DIS) and functioning skin immune system (FSIS). The SALT is responsible for the induction of immunity and tolerance; it is formed by Langerhans Cells (LC), keratinocytes, endothelial cells and draining skin lymph nodes. The DMU shows endothelial cells, dendritic cells and leukocytes (monocytes, macrophages, T lymphocytes and mast cells), responsible for skin inflammatory reactions.
ACD develops as an acquired immune-mediated inflammatory reaction called type IV as described by Gell and Coombs, in which an endogenous protein is considered "non-self" as it binds to the hapten. The complete antigen (hapten-protein conjugate) has a molecular weight of over 5000 Da; it is processed and taken to antigen-presenting cells, such as Langerhans cells (LC) or dendritic cells (DC) so that they act internalizing, processing and transporting specific surface antigens bound to MHC molecules reaching T lymphocytes in regional lymph nodes via afferent lymphatic vessels. This is the sensitization phase, or afferent pathway, and occurs in approximately 10 days.
Disruption of the epidermal barrier also causes the release of substances capable of inducing the maturation of LC / DC, as well as assisting the polarization of naive T cells.
The main cytokines involved in this phase are Il-1, IL-18, which stimulate the release of TNF-α and GM-CSF (colony stimulating factor for macrophages) from keratinocytes and dermal cells. In the lymph node, LC / DC interact with naive T cells which, after activated, start producing different cytokines, including IL-2, considered growth factor of LT. Depending on the immunological environment (amount of allergen, soluble mediators) naive T cells differentiate forming effector T cells, mainly Th1 cells, in the case of skin.6
These effector cells propagate via efferent lymphatic vessels and thoracic duct, reaching the peripheral circulation. They may enter lymphoid tissues and settle in paracortical areas through its ligands CCL19 and CCL21. However, increased expression of molecules facilitates their spontaneous migration to the skin.7,8
Once sensitized, individuals can develop allergic contact dermatitis (ACD). After a new contact with the allergen, in the elicitation phase, there is induction of the inflammatory reaction, with maximum activity within 2 -3 days, and whose intensity gradually decreases if the causative agent is removed.
Further events are similar to what occurs in the sensitization phase, but with a less prominent activity of LC, mast cells, keratinocytes and macrophages, since effector T lymphocytes migrate more easily to the site of contact.
At that stage, cytokines released by keratinocytes after contact with the antigen facilitate the migra-tion of T memory cells.
There are many discussions in the literature about the phenotype of lymphocytes present at this stage of ACD. The inflammatory infiltrate is full of CD4 + T cells; however, CD 8 + T cells mediate the inflammatory process through their cytotoxic activity, trying to destroy the antigen. The phenotype of T lymphocytes found in the inflammatory infiltrate depends on when it is studied because it is a dynamic process. Okazaki et al. studied this process dynamics and demonstrated that lymphocytes found in the early process are LT CD8 producers of IFN-γ, followed by LT CD4. The largest proportion of LT CD8 was found 12 hours after contact and of CD4, 24 hours after contact.9
Two events may occur in the resolution phase: chronicity or total resolution of symptoms. Factors leading to the chronicity of the process are not yet well established. Early withdrawal of the causative agent leads to rapid resolution of the dermatitis. On the other hand, chronic exposure determines permanent damage to the tissue, but immunoregulatory factors prevent excessive cytotoxicity.
In the late stage of ACD, keratinocytes, macrophage infiltrate and T cells begin to produce IL-10.This interleukin has an anti-inflammatory activity and induces suppression of the activity of antigen-presenting cells and macrophages. Activated keratinocytes release PGE2 and TGF-β, which eventually reduce even more the production of pro-inflammatory interleukins.
The function of the patch test is to produce, in a controlled manner, the elicitation phase of CD, and thus determine the etiological agent of this dermatitis.
INDICATIONS
Patch tests are indicated: 1) for patients with a diagnostic hypothesis of CD, 2) patients with other skin conditions that may be aggravated by CD (atopic dermatitis, seborrheic dermatitis and stasis, nummular eczema, psoriasis, and dyshidrosis), 3) patients which chronic eczema without an established etiology, 4) suspected cases of occupational contact dermatitis.10,11
The patch test can also be used to investigate drug reactions that manifest with skin lesions resulting from a late hypersensitivity mechanism, such as maculopapular rash, DRESS (drug, rush, eosinophilia, systemic symptoms), fixed drug eruption.
Although there is not a formal contraindication, patch tests should be avoided in pregnant women. Although the absorption of substances is minimal and does not compromise the fetus, immunological changes typical of pregnancy interfere with the response to patch testing.
METHODOLOGY
Before starting the application process, patients should be informed of: 1) test objectives 2) the prohibition of wetting their back 3) the prohibition of performing activities that result in excessive sweating 4) probable local symptoms, such as itching 5) the prohibition of exposing themselves to UV radiation up to 15 days prior to testing, as it has an immunosuppressive effect.
Battery or series and types of chambers
The methodology for application of the tests must meet a number of important criteria.
The first refers to the material being tested.
The battery components are prepared to better penetrate the skin, without local irritation.
Each allergen should be prepared in a suitable vehicle, and no vehicle is considered optimal for all of them. The most used is petrolatum, which allows good occlusion, keeps the allergens stable, and is low cost. Other possible vehicles are water, solvents (acetone, ethanol, methylethylcetone) and hydrophilic gel.
When liquid substances are applied to the skin, there is the need to use paper filters in the preparation of the test.
Chambers, that is, the material in which the substances to be tested are placed, must also be assessed. There are different types of chambers available; however, the most used in Brazil is Finn Chamber (Epitest, Finland). It consists of circular aluminum chambers, on a Scanpor (acrylate-based adhesive). Other chambers used are van der Bend (Netherlands) and IQ chambers (Chemotechnique, Sweden) (Figure 1). Chambers are designed to avoid sensitization reactions to their own material.
FIGURE 1.
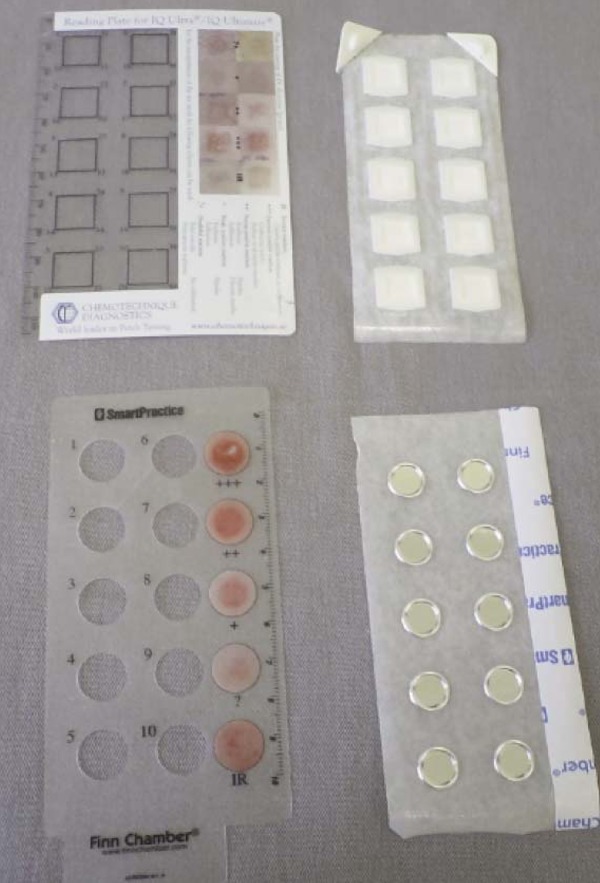
Examples of chambers: A) IQ chamber B) Finn chamber
The battery or series to be used should be standardized so that the results are reproducible and comparable with a high level of safety.
The Brazilian Standard Battery (FDA Allergenic - Rio de Janeiro, Brazil) consists of 30 substances; of these, only formaldehyde is formulated in an aqueous vehicle. It was studied and standardized by the Brazilian Study Group of Contact Dermatitis (BSGCD), with results published in 2000.12 Since then, it has been used as a reference in many studies done in Brazil. The same group later developed another series named Cosmetics Battery, with additional antigens (Charts 1 and 2).
CHART 1.
Standard Brazilian series
| Substance | Concentration | Vehicle | Substance | Concentration | Vehicle |
| Anthraquinone | 2.0% | Solid vas. | Neomycin | 20.0% | Solid vas. |
| Balsam of Peru | 25.0% | Solid vas. | Nitrofurazone | 1.0% | Solid vas. |
| Benzocaine | 5.0% | Solid vas. | Parabens2 | 12.0% | Solid vas. |
| Potassium bichromate | 0.5% | Solid vas. | Paraphenylenediamine | 1.0% | Solid vas. |
| P-tertiary butyl phenol | 3.0% | Solid vas. | Perfume-mix3 | 8.0% | Solid vas. |
| Carba mix1 | 3.0% | Solid vas. | PPD-mix4 | 0.6% | Solid vas. |
| Cobalt Chloride | 1.0% | Solid vas. | Promethazine | 1.0% | Solid vas. |
| Colophony | 20.0% | Solid vas. | Propylene glycol | 1.0% | Solid vas. |
| Ethylenediamine | 1.0% | Solid vas. | Quaternium 15 | 2.0% | Solid vas. |
| Formaldehyde | 2.0% | Water | Quinoline- mix5 | 5.0% | Solid vas. |
| Hydroquinone | 1.0% | Solid vas. | Epoxy-resin | 1.0% | Solid vas. |
| Irgasan | 1.0% | Solid vas. | Nickel sulfide | 5.0% | Solid vas. |
| Kathon CG | 0.5% | Solid vas. | Turpentine | 10.0% | Solid vas. |
| Lanolin | 20.0% | Solid vas. | Thimerosol | 0.1% | Solid vas. |
| Mercaptobenzothiazole | 1.0% | Solid vas. | Thiuram-mix6 | 1.0% | Solid vas. |
Source: Brazilian Contact Dermatitis Study Group, 2002.12
diphenylguanidine
Butyl, ethyl, propyl, methyl parabens, 3% each
Eugenol, isoeugenol, cinnamic alcohol, cinnamic aldehyde, greraniol, hydroxycitronellal, alpha-amyl cinnamic alcohol, oakmoss absolute, 1% each
N-phenyl-N-cyclohexyl-p-phenylenediamine, N-iso-N-phenyl-pphenylenediamine, N, N-diphenyl-p-phenylenediamine, 0.2% each
clioquinol clorquinadol, 3% each
tetramethylthiuram disulfide, tetramethylthiuram alkaline monosulphate, tetraethylthiuram disulfide, dipentamethylenethiuram mosulfite 0.25% each
CHART 2.
Cosmetics series
| Substance | Concentration | Vehicle |
| Germal 115 | 2.0% | Solid vas. |
| BHT | 2.0% | Solid vas. |
| Toluenesulphonamide- formaldehyde resin | 10.0% | Solid vas. |
| Triethanolamine | 2.5% | Solid vas. |
| Bronopol | 0.5% | Solid vas. |
| Chloracetamide | 0.2% | Solid vas. |
| Sorbic Acid | 2.0% | Solid vas. |
| Ammonium thioglycolate | 2.5% | Solid vas. |
| Chlorhexidine | 100% | Water |
| Amerchol | 0.5% | Water |
Source: EA Silva et al., 2012. 14
Besides the standardization of the antigens tested, the BSGCD battery considers the positioning of substances an important factor in the prevention of false positives. Duarte et al. have shown that substances with similar chemical structures that may crossreact and co-sensitizing substances should not be tested next to each other, as they may affect the response to other substances.13 Therefore, in the standard and cosmetics batteries, substances are ordered according to their chemical structures, not allowing cross-reactivity and co-sensitization.14
Application technique
Tests should be applied to the upper back, because of the extensive area, facilitating the placement of various substances. Another area that can be used is the upper arms and possibly the upper thighs. Hairy areas should be avoided due to low adhesion. However, if necessary, hair may be removed using razor blades in the direction of hair growth. This intervention can lead to difficulties in the interpretation of the results, as folliculitis may occur on site. In case of oily skin, mild cleansing with ethanol or solvents, only to remove excess oil, may be done.
After application of the chambers, adhesive tape can be used to prevent detachment and loss of adhesion of the tests, which facilitate false negative results. This step is important in tropical and subtropical areas due to increased sweating.15
The withdrawal of the tests is done 48 hours after application, in a well-lit place and using specific plaques for the type of chamber used, with holes corresponding to the location of allergens. The back should be marked with an indelible pen, allowing future readings.
Readings should be done by the attending physician, and positive results and their intensity should be recorded in a proper place or in the patient's medical record. It is appropriate to wait 15 to 20 minutes after removal of the test to do the reading, because immediately after the detachment of the adhesive, the site can be erythematous and sometimes edematous due to local vasodilatation.
The patient should return for a new reading 7296 hours after application of the tests. This new reading is fundamental because a sensitization reaction may occur more than 72 hours after contact. Furthermore, positive results of readings done 48 hours after application of the tests can become negative within 72-96 hours, meaning there was only local irritation due to test occlusion.
Results to the test are measured by morphologic criteria recommended by the International Contact Dermatitis Research Group (ICDRG). These criteria are also adopted in Brazil and are shown in chart 3.16
CHART 3.
Possible responses to the patch test, according to the International Contact Dermatitis Research Group (ICDRG)
| ?+ | Doubtful |
| + | Mild reaction, possible erythema, infiltration and papules |
| + + | Strong reaction, erythema, infiltration, papules and vesicles |
| + + + | Very strong reaction, intense erythema, infiltra- tion and coalescing vesicles |
| IR | Irritant reaction, of various types |
| NT | Not tested |
Interpretation of results
This is the most difficult and challenging part for those working in the field of dermatology. The skill, expertise and curiosity of the professional are crucial for a reliable result.
False-positive results occur in the absence of a true allergic reaction. They may be related to different causes and are described below.
Causes of false-positive results:
1. Presence of impurities in the test preparation
2. The vehicle is irritating:
Positive results caused by irritation
To differentiate positive results caused by irritation from those caused by real allergens is one of the first challenges. The use of a standard battery aims at solving this problem. However, errors in the application technique can lead to reactions caused by irritation. These reactions usually appear as slightly pleated skin (tissue paper), mild erythema, follicular papules and pustules, petechiae, pustules, blisters and necrosis.
Attempts to study this aspect of patch testing have been made using electron microscopy, monoclonal antibodies, and recently, reflectance confocal microscopy, without good results.10,17
3. Lack of proper dilution of the antigen in the vehicle
4. Reaction to the adhesive used
5. Effect of local pressure exerted by solid materials or underwear
6. Excited skin syndrome (angry back): described by Mitchell in 1975, it corresponds to the presence of two or more positive results, some of which are not reproduced when the patient is retested. Mitchell initially described this reaction as "Angry Back", and later Maibach called it "excited skin syndrome" since this reaction can occur at any site of application of the tests. Several factors contribute to the development of this reaction, such as:
a. Influence of a reaction caused by a substance adjacent to the site of application
b. Current or recent dermatosis at the test site
c. Dermatosis in areas far from the test site
d. Substances prone to cross-reactivity or co-sensitization tested close to each other.18
False-negative results may also occur and, therefore, a negative result does not completely exclude the possibility of CD. Standard series include only statistically relevant substances, but the possibility of a rare, exotic or new sensitizing agent cannot be excluded.10,19 Some causes of false-negative results are described below.
1. Inadequate penetration of the antigen
a. Substance is not released from the vehicle or is retained on the paper filter
b. Insufficient occlusion
c. Short contact of the antigen with the skin (detachment of the adhesives)
d. Test applied to a non-recommended site
2. Reading done in an inappropriate time
3. Site of application previously treated with corticosteroids or exposed to UV radiation.
4. Patient underwent systemic treatment with corticosteroids and / or immunosuppressant drugs.
Oral corticosteroid therapy does not completely contraindicate the test for patients on chronic, lowdose use of these drugs; thus, strongly positive results are reliable; dubious tests should be repeated. The use of antihistamines does not prevent patch testing.10
5. The allergen is not in its active form or is degraded
6. Compound allergy: a term used to describe the condition in which the patient is tested using a finished product, generally cosmetics or topical drugs, obtaining a positive response; however, when the components of the products are applied individually, tests are negative. The special conditions of these components offered jointly cause CD.
7. Tests were wet or lost
8. The substance tested is photosensitizing and photopatch testing was not done
9. The conditions of the site with dermatitis, such as sweating, heat, friction or pressure, were not reproduced during the test. For instance, investigation of CD caused by footwear may be hampered because the same moist environment caused by wearing the shoes cannot be reproduced. In this case, test occlusion must be increased by applying plastic film over the chambers.
Upon suspicion of false positive or false negative results, the patient should be retested with least 30 days between tests.
Intensity of patch tests
Currently, the strength of the response to the test should be considered to characterize actual sensitivity. Thus, positive (++) and (+ + +) tests indicate sensitization by the substance tested. Moreover, low intensity (+) responses cannot be reproduced at other times, making it difficult to establish their relevance.
Duarte et al., in a recently published study, showed that high intensity (+ + and + + +) tests were fully reproduced when patients were retested within a year after the first test.20 Positive (+ +) tests were reproduced in 86% of cases. Low intensity (+) tests did not show the same reliability. These data demonstrated that tests with a (+) result should be re-evaluated for their relevance.
Relevance of patch tests
The relevance of patch testing is defined as the ratio between the response obtained in the reading and the patient's contact with the causative agent of the dermatosis. A particular test may also be relevant in relation to a previous contact, that is, a positive result refers to a contact that is unrelated to the current dermatosis.
Three types of relevance are considered:
• Possible: positive result for a substance associated with the use of the material by the patient
• Probable: positive result for a substance and the material used by the patient
• Strong: reexposure to the material containing the sensitizing substance causes recurrence of CD.
Complications
Some complications are reported in the literature, but they are generally not serious and are presented in chart 4.
CHART 4.
Major complications arising from the application of patch tests
| Depigmentation |
| Hyperpigmentation, especially after sun exposure |
| Scars, keloids |
| Secondary infection by bacteria or viruses |
Ancillary testing
These are used to simulate everyday situations of product application to the skin, such as creams or topical medication. They reinforce a positive result or confirm a negative one.
Open test
The product is applied pure or dissolved in water or another solvent (e.g. ethanol, acetone, ether) freely spreading on the skin. They are recommended as a first step when unknown or little studied substances need to be tested.
Semi-open test
Method designed to evaluate products with irritating properties due to the presence of solvents or emulsifiers (e.g. detergents, shampoos, dyes, resins, varnishes, glues, waxes, freezing fluids, pharmaceuticals and cosmetics).10 A small amount of the product is applied using a cotton swab, to a 2x2 cm area. After it is completely dried, the area is covered with adhesive tape for two days. The site is reevaluated after 48 and 96 hours.
c) Repeated open application test (ROAT)
ROAT was described by Hannuksela and Sato in 1986. It has the function of refining positive, negative or doubtful responses, obtained in the closed test.21
Commercial products (cosmetics or drugs), in which the presence of a sensitizing substance is suspected, are applied twice a day for 7 days to the anterior portion of the arm, antecubital fossa or scapular region. The positive response (eczema) develops between 2-4 days, indicating that the product actually contains the sensitizing substance.
d) Photopatch test
This method is used in the diagnosis of skin eruptions in which ultraviolet radiation (UV) is an adjuvant in the onset of the dermatosis. It is the so-called photoallergic CD.
In this test, the application of the substances is duplicated on the back of the patient. After 48 hours, they are removed and one side is covered with a material that is opaque to UV radiation. The uncovered side is exposed to UVA. Different emission equipment can be used, but they are usually the same as those used in phototherapy treatments. The recommended dose is 5 to 15 J / cm2, varying according to skin type and hapten tested.
A new reading is done 48 hours after irradiation.
Several research centers worldwide have developed specific series for photopatch testing, something that has not occurred in Brazil.
Other uses of patch tests
Pharmacodermias
Patch tests are indicated for reactions that show a late hypersensitivity mechanism, such as maculopapular rash, erythroderma, eczematous rash, erythema multiforme, fixed drug eruption, AGEP (Acute Generalized Exanthematous Pustulosis) and DRESS.
The frequency of positive results for reactions to drugs varies from 7.5 to 54%, according to different studies and according to the patients selected, type of rash and drug involved.22
The test should be performed six weeks after the end of the event, adopting the same methodology as that used for CD tests. However, the results should be interpreted with caution, as negative results do not exclude culpability of the drug. There are several reasons for negative results; for example, inadequate bioavailability of the material tested, poor accuracy of medical history and when the allergen is a metabolite of the drug tested.
Patch tests in special situations
Patch tests in patients using immunomodulatory drugs
Immunosuppressive therapies have become more frequent over the years. Currently, drugs such as azathioprine, cyclosporine, tacrolimus, methotrexate, mycophenolate mofetil and TNF - α inhibitors are available to doctors, and many others are in experimental phases. Older drugs coexist with newer ones and are used in isolation or associated with other drugs.
Some patients who use them develop eczematous dermatitis and they are often not investigated due to the assumption that patch tests will be negative. These drugs inhibit the migration of Langerhans cells or prevent the activation, proliferation and maturation of T lymphocytes, which are key cells in the mechanism of allergic contact dermatitis.
In general, these drugs cannot be suspended for patch testing.
In 2008, Rosmarin et al. described the case of a patient using a drug with an anti-TNF - α action who developed allergic contact dermatitis in the hands. The authors patch tested the patient and found positive responses to various substances.23
Wee et al. retrospectively evaluated 38 patients who were patch tested and who were using different immunosuppressive drugs. Among these patients, 16 (44%) tested positive with intensity varying between (+) and (+ + +).24
The North American Contact Dermatitis Group (NACDG) recently published a compilation of the opinions of several members of the entity with respect to this issue. It was concluded that the impossibility of withdrawing the drug should not prevent patch testing, although false negative results may occur.
These facts create new perspectives for the study of the pathogenesis of ACD, as they show that although the best known immunological pathways of CD are inactive at this time, the reaction occurs and, therefore, the tests can be performed.25
Patch tests in children
Patch tests in children have always been the subject of controversy in the literature regarding their applicability, methodology and relevance. The clinical symptoms of CD in this population do not differ from those shown by adults. However, the most affected areas are the extremities and the most common allergens are metals, footwear, topical medication and cosmetics.
Many publications have shown that the frequency of sensitization among children is increasing, which makes patch testing increasingly important in this population.
Sensitization in children is described as early as neonates; however, patch tests should be based on a detailed medical history for their application. The size of the child's back must be considered, since it does not allow the placement of many allergens. The use of current chambers is well established in this age group.26
The concentration of the allergens to be applied is a controversial issue in the literature, but so far the same allergens are used in both adults and children. On the other hand, some studies have shown that there are differences in some substances, such as nickel sulfate. Mortz et al., in a recent publication, argued that patch testing with nickel sulfate should be done only when there is a very suggestive clinical history. Most positive reactions (62%) obtained in young chil-dren (12 and 18 months of age) were not reproduced when the same age group was later retested (3 and 6 years old), suggesting the potential irritating capacity of this allergen in young children.27
Future prospects
Much must be learned and studied about patch testing. All works published about this topic try to refine application techniques, reading of the results and the allergens employed.
A number of improvements are still needed to increase the quality of the tests. Among them are the improvement of the vehicles used to increase the bioavailability of the allergens; standardization of the reading criteria and relevance of weak reactions; study of the effect of factors such as weather variations, latitude, temperature and humidity on test reactivity, and improvement and diversification of the types of chambers to allow greater comfort for patients in their daily activities.
We still have a lot to do in Brazil, such as increase the number of centers that use patch tests as a diagnostic tool, conduct epidemiological studies of allergens in different parts of the country, improve or revise the current standard battery of tests, increase the number allergens available and strengthen the Brazilian Study Group of Contact Dermatitis, so that multicenter studies can be conducted.
Despite the fact that patch tests are considered category B by evidence-based medicine, the practice and experience of those who perform them in routine patient care show that this methodology is a powerful tool to define the diagnosis and etiology of allergic contact dermatitis. After becoming more knowledgeable in relation to proper indication, application technique and interpretation of the results, the attending physician feels gratified with the practical contribution of patch tests.
QUESTIONS
1 - It is correct to state the following about patch tests:
a) Any skin area can be used for testing
b) Any substance can be applied in its raw state, as long as the pH of the substance is known
c) Vehicles used in the battery should be chosen based on the final cosmetic characteristics of the product to be tested
d) Batteries to be tested should be based on population and laboratory studies
2 - Patch tests can be used in all of the cases below, except:
a) Patients with possible diagnosis of Allergic Contact Dermatitis
b) Patients with chronic dermatosis with probable secondary sensitization
c) Drug eruptions with a late hypersensitivity mechanism
d) Patients with acute dermatitis for rapid establishment of the etiology and withdrawal of contact
3 - It is correct to state the following about the application technique of patch tests:
a) The upper back is the best place for their application
b) The use of adhesive tape over the chambers is contraindicated due to the possibility of contact dermatitis caused by the tape
c) The minimum time for exposure to the allergens is 24 hours
d) The second reading done after 72-96 hours of the application of the test is unnecessary if the tests are already positive
4 - Among the causes of false positive results, all of the state ments below are correct, except:
a) The antigens were prepared with impurities
b) The vehicle used is irritating
c) Intense reaction to the adhesives used over the chambers
d) Positive results are always related to an allergic reaction
5 - It is correct to state the following about the Excited Skin Syndrome:
a) It is characterized by the presence of at least two positive reactions to substances without cross-reactivity or co-sensitization
b) It may occur as a result of a strongly positive test
c) After retest, all initial responses remain positive
d) It may be caused by the presence of acute dermatitis
6 - It is correct to state the following about false-negative tests:
a) Inadequate occlusion does not affect the response of patch tests
b) Use of topical corticosteroids at the test site does not interfere with the response because this route of administration does not alter the immune response
c) Test results of patients undergoing phototherapy are unreliable
d) The vehicle used in the preparation of allergens does not interfere with the response
7 - It is correct to state the following about patch tests:
a) Current chambers allow the patient to continue with all normal activities during the testing process
b) The use of special techniques, such as occlusion with plastic wrap, can favor the response in patients with contact dermatitis caused by footwear
c) After removal of the chambers, the erythema observed should be considered in the reading
d) The reading of the results is done in a random manner
8 - It is correct to state the following about the relevance of patch tests:
a) It is defined as the ratio between the response obtained in the reading of the results and the patient's contact with the causative agent of the dermatosis
b) It is possible when the test is positive for a substance and the material used by the patient
c) It is probable when reexposure to the material that contains the positive substance causes recurrence of the dermatitis
d) All tests should be considered relevant because the patient may not recall previous contacts with the agent
9 - Choose the correct alternative:
a) A patient with hand eczema, use of rubber gloves, positive patch test reactions to thiuram-mix (rubber vulcanization agent) is considered to show a response with possible relevance
b) A patient with hand eczema, history of use of rubber gloves, positive patch test reactions to thiuram-mix (rubber vulcanizing agent), and a positive test for a fragment of the glove is considered to show a response with clear relevance
c) The relevance of the tests should not be considered because a positive response to the test is what matters
d) Current relevance tests refer to the patch tests currently performed
10 - We can state the following about patch tests:
a) Complications arising from the test can be serious and, therefore, it should only be done in a hospital
b) Secondary infections caused by fungi at the test site are common, as the tests are applied to the dorsal area, where there is a large fungal population
c) Hyperpigmentation at the test site may occur after sun exposure
d) Keloid scars are common after intense responses to the tests
11 - It is incorrect to state the following about ancillary tests:
a) They are used to simulate everyday situations of product application on the skin
b) The open test may be used as the first test when substances are unknown
c) The semi-open test is used to evaluate products with irritating properties
d) Repeated open application tests can be done with any product such as detergents, oils, shampoos, dyes and resins
12 - Photopatch tests:
a) should be done in all patients with diagnosis of phototoxic contact dermatitis, such as the one caused by some fruit juice
b) are used in cases in which UV radiation acts as adjuvant in the onset of contact dermatitis
c) the most widely used radiation is UVB, which is the most erythemogenic
d) the reading time after application of UV light should be of a few hours, as it takes some time for radiation to cause erythema
13 - Among the patients below, which one should undergo patch testing again (retest):
a) A patient that tested positive for the four vulcanizing rubber agents present in the standard battery
b) A patient that tests positive (3+) for nickel sulfate and (2+) for cobalt chloride
c) A patient that tests negative when the reading is done only 48 hours after application
d) A patient whose patch test was negative in the 48 and 96-hour readings
14 - Patch testing in adverse drug reactions:
a) can be done for any type of drug eruption
b) the frequency of responses is usually high, making the test highly sensitive
c) negative results completely exempt the drug
d) if the rash is caused by a metabolite of the drug, test results will be negative
15 - Choose the correct alternative:
a) patch tests are contraindicated for patients treated with infliximab
b) low intensity (+) patch tests should be evaluated with caution
c) patients with psoriasis should never be patch tested
d) patch tests can be done with antigens chosen according to the experience of each physician who applies them
16 - Choose the correct alternative:
a) positive patch tests due to irritation are easily distinguishable from those caused by allergens
b) electron microscopy is a good method to differentiate skin irritant from allergy tests
c) tests with previous relevance are a cause of false positive results
d) products such as cosmetics and topic drugs can be used in repeated open application tests
17 - Choose the incorrect alternative:
a) the use of antihistamines does not prevent patch testing
b) the use of corticosteroids at high doses the week before the test changes the response to the test
c) the chronic use of systemic corticosteroids at low doses does not alter the response to the test
d) Oral antihistamines should be discontinued a week before the test not to affect the response
18 - It is correct to state the following about standardized series:
a) the vehicle of the antigen is always solid vaseline
b) the concentration of the antigen is critical for an appropriate response
c) they are identical in all countries
d) chamber adhesives are potentially sensitizing
19 - Choose the incorrect alternative:
a) the position of the antigens can interfere with the test response
b) Pregnant women should always be tested as they are easily sensitized
c) sun exposure interferes with the skin response in patch testing
d) the patient with excited skin syndrome should be retested after at least 30 days of the initial test
20 - Which of the following is incorrect for a patient with clinical symptoms of contact dermatitis whose patch tests were negative?
a) we should disregard this diagnosis and think of other possibilities
b) irritant contact dermatitis may be considered
c) test adhesion was not adequate
d) the responsible antigen was not tested
Papers
Information for all members: The EMC-D questionnaire is now available at the homepage of the Brazilian Annals of Dermatology: www.anaisdedermatologia.org.br. The deadline for completing the questionnaire is 30 days from the date of online publication.
Footnotes
Work conducted at Irmandade da Santa Casa de Misericórdia de São Paulo - São Paulo (SP), Brazil.
Financial Support: None.
Conflict of Interest: None.
REFERENCES
- 1.Lachapelle JM. Patch testing: historical aspects. Ann Dermatol Venereol. 2009;136:575–577. doi: 10.1016/j.annder.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Lachapelle JM. Historical aspects. In: Johansen JD, Frosch PJ, Lepoittevin JP, editors. Contact Dermatitis. 5th ed. Verlag: Springer; 2011. pp. 1–8. [Google Scholar]
- 3.White JML. Patch testing: what allergists should know. Clin Exp Allergy. 2012;42:180–185. doi: 10.1111/j.1365-2222.2011.03862.x. [DOI] [PubMed] [Google Scholar]
- 4.Contactderm.org American Contact Dermatitis Society. History Genesis of the American Contact Dermatitis Society. [cited 2012 nov 22]. [homepage on the Internet] Available from: http://www.contactderm.org/i4a/pages/index.cfm?pageid=3277.
- 5.De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132:949–963. doi: 10.1038/jid.2011.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abbas AK, Lichtman AH. Imunologia Básica. 3 ed. Rio de Janeiro: Elsevier; 2009. Capítulo 11, Doenças de hipersensibilidade; pp. 210–215. [Google Scholar]
- 7.Motta AA, Aun MV, Kalil J, Giavina-Bianchi P. Dermatite de contato. Rev Bras Alerg Imunopatol. 2011;34:73–82. [Google Scholar]
- 8.Rustemeyer T, Hoogstraten IMW, Blomberg ME, Scheper RJ. Mechanisms of Allergic Contact Dermatitis. In: Rustemeyer T, Elsner P, John SM, Maibach HI, editors. Kanerva's Occupational Dermatology. Berlin Heidelberg: Springer-Verlag; 2012. pp. 113–146. [Google Scholar]
- 9.Okazaki F, Kanzaki H, Fujii K, Arata J, Akiba H, Tsujii K, et al. Initial recruitment of interferon-gamma-producing CD8+ effector cells, followed by infiltration of CD4+ cells in 2,4,6-trinitro-1-chlorobenzene (TNCB)-induced murine contact hypersensitivity reactions. J Dermatol. 2002;29:699–708. doi: 10.1111/j.1346-8138.2002.tb00206.x. [DOI] [PubMed] [Google Scholar]
- 10.Lindberg M, Matura M. Patch Testing. In: Johansen JD, Frosch PJ, Lepoittevin JP, editors. Contact Dermatitis. 5th. ed. Verlag: Springer; 2011. pp. 439–464. [Google Scholar]
- 11.Tennstedt D. Patch tests: indications or when testing should be performed? Ann Dermatol Venereol. 2009;136:579–583. doi: 10.1016/j.annder.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Contact Dermatitis Brazilian Studying Group Multicentric study for the development of a standard Brazilian patch test series. An Bras Dermatol. 2002;75:147–156. [Google Scholar]
- 13.Duarte I, Lazzarini R, Buense R. Interference of the position of substances in an epicutaneous patch test battery with the occurrence of false-positive results. Am J Contact Dermat. 2002;13:125–132. [PubMed] [Google Scholar]
- 14.Silva EA, Bosco MRM, Mozer E. Study of the frequency of allergens in cosmetics components in patients with suspected allergic contact dermatitis. An Bras Dermatol. 2012;87:263–268. doi: 10.1590/s0365-05962012000200011. [DOI] [PubMed] [Google Scholar]
- 15.Lachapelle JM. Patch testing methods in different climatic conditions. Ann Dermatol Venereol. 2009;136:621–622. doi: 10.1016/j.annder.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Fregert S. Manual of Contact Dermatitis. 2nd ed. Munksgaard, Copenhagen: Year Book Medical Publishers, Inc; 1981. Chapter 10, Patch Testing; pp. 71–81. [Google Scholar]
- 17.Astner S, González E, Cheung AC, Rius-Díaz F, Doukas AG, William F, et al. Noninvasive evaluation of the kinetics of allergic and irritant contact dermatitis. J Invest Dermatol. 2005;124:351–359. doi: 10.1111/j.0022-202X.2004.23605.x. [DOI] [PubMed] [Google Scholar]
- 18.Duarte I. The excited skin syndrome: review. An Bras Dermatol. 1995;70:153–162. [Google Scholar]
- 19.Rietschel RL, Fowler JF., Jr . Fisher's Contact Dermatitis. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2001. Chapter 2, Pratical Aspects of patch testing; pp. 9–26. [Google Scholar]
- 20.Duarte I, Silva MF, Malvestiti AA, Machado BAR, Lazzarini R. Evaluation of the permanence of skin sensitization to allergens in patients with allergic contact dermatites. An Bras Dermatol. 2012;87:833–837. doi: 10.1590/S0365-05962012000600002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hannuksela M, Salo H. The repeated open application test (ROAT) Contact Dermatitis. 1986;14:221–227. doi: 10.1111/j.1600-0536.1986.tb01229.x. [DOI] [PubMed] [Google Scholar]
- 22.Gonçalo M, Bruynzeel DP. Patch Testing in Adverse Drug Reactions. In: Johansen JD, Frosch PJ, Lepoittevin JP, editors. Contact Dermatitis. 5th ed. Verlag: Springer; 2011. p. 481. [Google Scholar]
- 23.Rosmarin D, Bush M, Scheinman PL. Patch testing a patient with allergic contact hand dermatitis who is taking infliximab. J Am Acad Dermatol. 2008;59:145–147. doi: 10.1016/j.jaad.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Wee JS, White JM, McFadden JP, White IR. Patch testing in patients treated with systemic immunosupression and cytokine inhibitors. Contact Dermatitis. 2010;62:165–169. doi: 10.1111/j.1600-0536.2009.01695.x. [DOI] [PubMed] [Google Scholar]
- 25.Fowler JF, Jr, Maibach HI, Zirwas M, Taylor JS, Dekoven JG, Sasseville D, et al. Effects of immunomodulatory agents on patch testing: expert opinion 2012. Dermatitis. 2012;23:301–303. doi: 10.1097/DER.0b013e318275969f. [DOI] [PubMed] [Google Scholar]
- 26.Vigan M. Peculiarities of patch testing in children. Ann Dermatol Venereol. 2009;136:617–620. doi: 10.1016/j.annder.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Mortz CG, Kjaer HF, Eller E, Osterballe M, Norberg LA, Høst A, et al. Positive nickel patch tests in infants are of low clinical relevance and rarely reproducible. Pediatr Allergy Immunol. 2013;24:84–87. doi: 10.1111/pai.12027. [DOI] [PubMed] [Google Scholar]


