Abstract
Hormone-induced changes in gene expression initiate periodic molts and metamorphosis during insect development. Successful execution of these developmental steps depends upon successive phases of rising and falling 20-hydroxyecdysone (20E) levels, leading to a cascade of nuclear receptor-driven transcriptional activity that enables stage- and tissue-specific responses to the steroid. Among the cellular processes associated with declining steroids is acquisition of secretory competence in endocrine Inka cells, the source of ecdysis triggering hormones (ETH). We show here that Inka cell secretory competence is conferred by the orphan nuclear receptor βFTZ-F1. Selective RNA silencing of βftz-f1 in Inka cells prevents ETH release, causing developmental arrest at all stages. Affected larvae display buttoned-up, the ETH-null phenotype characterized by double mouthparts, absence of ecdysis behaviors, and failure to shed the old cuticle. During the mid-prepupal period, individuals fail to translocate the air bubble, execute head eversion and elongate incipient wings and legs. Those that escape to the adult stage are defective in wing expansion and cuticle sclerotization. Failure to release ETH in βftz-f1 silenced animals is indicated by persistent ETH immunoreactivity in Inka cells. Arrested larvae are rescued by precisely-timed ETH injection or Inka cell-targeted βFTZ-F1 expression. Moreover, premature βftz-f1 expression in these cells also results in developmental arrest. The Inka cell therefore functions as a “gateway cell”, whose secretion of ETH serves as a key downstream physiological output enabling stage-specific responses to 20E that are required to advance through critical developmental steps. This secretory function depends on transient and precisely timed βFTZ-F1 expression late in the molt as steroids decline.
Keywords: Drosophila, ecdysis, ecdysis triggering hormone (ETH), Inka cells, βFTZ-F1, RNA-silencing, secretory competence
Introduction
Hormones regulate developmental sequences and reproductive state through induction of stage- and tissue-specific responses. The response of a cell at any given time depends upon patterns of gene expression that confer “competence”. In insects, levels of 20-hydroxyecdysone (20E) and juvenile hormones fluctuate throughout ontogeny to determine stage-specific developmental events during embryogenesis, larval stages, and metamorphosis to the adult. Stage-dependent responses to these generalized hormonal signals are specified by nuclear receptor cascades originally recognized as temporal sequences of chromosomal puffing patterns corresponding to transcriptional activity (Ashburner et al., 1974; Becker, 1959; Yamada et al., 2000).
A key molecule in the 20E-induced signaling cascade is the “competence factor” βFTZ-F1, an orphan nuclear receptor whose transient expression during the hours preceding each ecdysis is induced as steroids decline to minimal levels. Early accounts showed that βFTZ-F1 confers cellular competence for mid-prepupal responses to 20E (Broadus et al., 1999; Yamada et al., 2000). βFTZ-F1 mutants exhibit a series of deficits, including failure to translocate the air bubble, perform head eversion and elongate incipient legs and wings, all hallmarks of the pre-pupal to pupal transition that occurs during pupal ecdysis. These mutants also fail to advance through larval stages: they progress to the pharate stage, characterized by formation of new cuticle, spiracles, mouthparts, and trachea, but die through failure to shed the corresponding old structures via ecdysis (Yamada et al., 2000). These observations raise the question: What cellular elements are involved in these βFTZ-F1-dependent responses to 20E?
In moths, transition from high to low steroid levels during the latter part of the molt is essential for ecdysis, which can be delayed by prolongation of high steroid levels (Curtis et al., 1984; Truman et al., 1983; Zitnan et al., 1999). Maintenance of high steroid levels also inhibits acquisition of secretory competence in Inka cells, the source of ecdysis triggering hormones (ETH) (Kingan and Adams, 2000; Zitnan et al., 1999). Dependence of both βFTZ-F1 expression and Inka cell secretory competence on declining steroid levels suggests these two events may be causally related.
We therefore tested whether βFTZ-F1 confers secretory competence in Inka cells through use of the GeneSwitch/UAS system (Osterwalder et al., 2001) for conditional regulation of gene expression specifically in these cells. Our findings demonstrate that Inka cell-selective silencing of βftz-f1 blocks ETH secretion, leading to failure of larval ecdyses, the prepupal-pupal transition, and adult eclosion. The timing of βFTZ-F1 expression in Inka cells is critically important, since its premature expression causes similar ecdysis defects. Finally, precisely timed ETH injection or targeted expression of βFTZ-F1 in Inka cells rescues βftz-f1 null mutants. These findings indicate that Inka cells play a crucial “gateway” role by enabling physiological outcomes initiated by the 20E-induced nuclear receptor cascade at onset of each molt.
Materials and Methods
Fly Stocks
The βFTZ-F1 null mutant (yw; βftz-f1ex7hs-βFTZ-F1/TM3, y+- designated henceforth as βftz-f1ex7hs-βFTZ-F1) fly line was kindly provided by Dr. Hitoshi Ueda. Additional fly lines used in this study are described below. All Drosophila stocks were maintained on a standard cornmeal-molasses diet at 25°C in a 12/12 hour light/dark cycle.
Staging of animals
Larval stages were distinguished by morphology of the anterior spiracle (Ashburner, 1989). Larvae with double mouth hooks were selected and kept at 25°C until appearance of double vertical plates (dVP). Prepupae were staged by hours after puparium formation.
Construction of ETH-GeneSwitch, UAS-βFTZ-F1, and UAS-βFTZ-F1 dsRNA fly lines
To drive conditional, Inka cell-specific expression of transgenes, we constructed a driver fly line carrying the eth promoter upstream of the RU486-dependent GAL4-progesterone receptor fusion protein (GeneSwitch). The eth promoter consists of the 362 bp sequence from -367 to -5 immediately upstream of the eth open reading frame (ethup; Park et al., 2002). It was PCR amplified with primers carrying MluI and NotI restriction motifs for 5′ and 3′ ends, respectively; the forward primer was 5′-ACGCGTATACTTTGTATATTTATTTATT-3′ and the reverse primer was 5′-GCGGCCGCACCTGACTCCTGCTCCACAAT-3′. The amplified fragment was inserted into the Drosophila transformation vector pP{UAS-GeneSwitch} (Osterwalder et al., 2001) (kindly supplied by Dr. Thomas Osterwalder, Yale University) by replacing adjacent 5×UAS and hsp70 sequences with the ethup sequence using MluI and NotI restriction sites. This driver line was designated “ETHGS” (ETH-GeneSwitch). Plasmid constructs used for fly transgenesis were sent to Drosophila Genetic Service Center in Duke University for germ-line transformation. Inverse PCR determined the transgene insertion to be in the 2nd chromosome.
For creation of UAS-βFTZ-F1 fly lines, the entire ORF of Drosophila βftz-f1 (2450 bp) was PCR amplified from total RNA extracted from tracheal tissue. To facilitate cloning, EcoRI and KpnI adapter sequences were added to the PCR primers, whose sequences were as follows: the forward primer was 5′-CATGAATTCATGTTATTAGAAATGGATCAGC-3′, and the reverse primer was 5′-TGAGGTACCTCATAAATGATTAAGTATTCCG-3. Amplified cDNA encoding βftz-f1 was introduced into the pUAST vector and confirmed by nucleotide sequencing. Following germ-line transformation, the insertion was determined to be on the 2nd chromosome.
For RNA silencing of βftz-f1, we created a transgenic fly line carrying a symmetrically transcribed, UAS-inducible double-stranded βftz-f1 sequence (Giordano et al., 2002). The construct was PCR amplified using the following primers: the forward primer was 5′-GTTCGAGCGGATAGAATGCGTGGTG-3′ and the reverse primer was 5′ AGTATTCCGTGTCACGTTCTCCCGAC - 3′. The fragment was cloned into pGEMT-easy, digested with EcoRI and inserted into the SympUAST vector. The resulting lines used in experiments described here carried transgenes on chromosome 3.
Use of RU486-induced GeneSwitch for RNA silencing and rescue
RU486 (Sigma) was dissolved in absolute ethanol at 10 mg/ml concentration and kept at -20°C until use. Working dilutions of RU486 were prepared in ethanol and mixed with larval diet to achieve a final concentration of 100-200 μg/ml RU486 and 4% ethanol. Fly lines were raised on a standard diet to the desired age (2nd or 3rd instar) and transferred to diet containing RU486 (100-200 μg/ml). Larvae maintained on RU486 diet were scored for developmental defects under a dissection microscope. Trachea containing Inka cells were extirpated and processed for in situ hybridization with a βFTZ-F1 probe followed by immunohistochemistry with antibodies to βFTZ-F1 or ETH (see below).
To rescue the βftz-f1ex7hs-βFTZ-F1 null mutant phenotype, we used either heat treatment or GeneSwitch-mediated expression of βFTZ-F1 specifically in Inka cells using progeny of the following parental cross: yw; ETHGS/ETHGS; βftz-f1ex7hs-βFTZ-F1/TM3, y+×UAS-FTZ-F1/UAS-FTZ-F1; βftz-f1ex7hs-βFTZ-F1/TM3, y+. Larval progeny homozygous for βftz-f1ex7 were recognized by the y- marker, selected every 2 hours for heat-shock treatment (33°C for 60 minutes) during the time period 13-16 hours after egg laying (AEL), and maintained at 25°C. Surviving 1st instar larvae were collected during 42-46 hours AEL and exposed either to a second heat treatment (33°C for 60 min) or to RU486-containing diet. The number of 1st and 2nd instar larvae was determined during the time interval 54-59 hours AEL.
Viability and developmental defects in flies with silenced and induced βFTZ-F1
Freshly ecdysed 2nd instar larvae produced from ETHGS × UAS-FTZi or ETHGS × UAS-βFTZ-F1 crosses were collected and transferred to RU486-containing diet (100-200 μg/ml). Animals were scored as developmentally arrested or surviving at 5-12 hour intervals; double mouth hook (dMH) larvae or puparia were selected for observation of ecdysis and survival. Mortality or developmental defects of flies were calculated as the percentage of arrested pharate 3rd instar larvae, pharate pupae or eclosed adults with short wings.
ETH injections into βFTZ-F1 silenced larvae
Pharate 3rd instar larvae at the dVP stage were immobilized on double sticky tape and injected with 1-10 fmol of Drosophila melanogaster ETH1 (DrmETH1) dissolved in 10 nl of distilled water using a glass capillary attached to a “Nanoject” nanoliter injector (Drummond Scientific). Surviving larvae were transferred to a small agar plate and observed for ecdysis behavior. In parallel experiments, control larvae were injected with the same volume of distilled water.
In situ hybridization and immunohistochemistry
For in situ hybridization and antibody staining, the tissues were dissected in phosphate-buffered saline (PBS) (130 mM NaCl, 7 mM Na2HPO4, 3 mM NaH2PO4, pH 7.4) and fixed in 4% paraformaldehyde (in PBS) for ∼1 hour at room temperature or overnight at 4°C. In situ hybridization and immunohistochemical staining were performed as previously described (Kim et al., 2006b). For detection of βFTZ-F1 expression in Inka cells, digoxygenin-labeled DNA probe was prepared by incorporation of Dig-11-dUTP (Roche Applied Science, Basel) into a single-stranded antisense DNA. Primers used for production of Drm βFTZ-F1-specific probe were as follows: βFTZ-F1 sense primer 5 -GGACACCACCTCCTCACACT-3′ and antisense primer 5′-GGAATTGGTTCCTCCTCCTC-3′. Negative control included in situ hybridization with a probe produced by the sense primer.
For immunohistochemical staining tracheae with attached Inka cells were incubated with primary antisera for 2 days at 4°C at a 1:2,000 dilution of DrmETH1 antiserum (Park et al., 2002) or 1:1,000 for DrmβFTZ-F1 antiserum, kindly supplied by Dr. Hitoshi Ueda (Yamada et al., 2000). To reveal specific binding of the primary antibodies, we used Alexa Fluor 488-labeled goat anti-rabbit IgG and Alexa Fluor 555-labeled goat anti-mouse IgG (Invitrogen). In situ hybridization and immunohistochemical staining were observed under confocal microscopes (Zeiss 510, or Leica TCS SPE-II).
Results
ethup-GeneSwitch Induces Conditional, Inka Cell-specific Expression of Transgenes
To analyze regulatory functions for βFTZ-F1 in secretory competence of Inka cells and ecdysis behaviors, we created a transgenic fly line capable of driving UAS-transgene expression under precise spatial and temporal control. We showed previously that the eth promoter sequence located immediately upstream of the eth open reading frame (ethup) drives Inka cell-specific gene expression (Park et al., 2002). We inserted this promoter sequence upstream of GeneSwitch (Osterwalder et al., 2001) and properties of the resulting ETH-GeneSwitch (ETHGS) line were checked first by crossing it to a UAS-EGFP line. No EGFP expression was observed unless progeny were fed RU486, which induced expression specifically in Inka cells within 2 hours; no EGFP fluorescence was observed in other tissues or organs (data not shown).
We performed an additional experiment, using the ETHGS driver line to induce βFTZ-F1 expression. Normally, βFTZ-F1 expression occurs during a narrow time window preceding ecdysis; i.e. in late 1st instart larvae (∼45-48 hours AEL), late 2nd instar larvae (∼69-72 hours AEL), and late 3rd instar larvae (∼8-12 hours APF) preceding pupal ecdysis (Yamada et al., 2000). We induced premature expression of βFTZ-F1 by crossing the ETHGS line with a UAS-βftz-f1 line; F1 progeny in 2nd or 3rd larval stages were fed RU486 and subjected to a double staining procedure: in situ hybridization was performed using a βftz-f1 digoxygenin-labeled DNA probe, followed by immunostaining with a DrmETH antibody. Inka-cell specific expression of the βFTZ-F1 transcript became detectable 1-2 hours after larvae were transferred to diet containing RU486 (Fig. 1). The in situ hybridization reaction product depicted in Fig. 1A corresponds to the location of the Inka cell, as evidenced by the occurrence of ETH-like immunoreactivity (ETH-IR) in the same location (Fig. 1B,C arrow). These results confirm that the ETHGS driver line induces transgene expression under precise spatial and temporal control.
Fig. 1. Inka cell-specific expression of βFTZ-F1 in ETHGS/UAS-βFTZ-F1 flies.
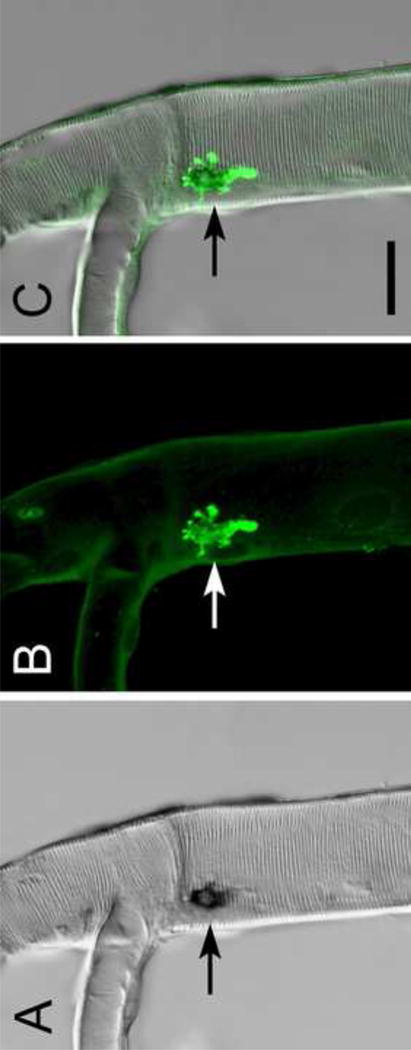
Targeted βFTZ-F1 expression in Inka cells (arrows) was induced by feeding RU486 to ETHGS/UAS-βFTZ-F1 larvae. (A) Tracheae were dissected from feeding 3rd instar larvae 2 hours later and processed for in situ hybridization with a βFTZ-F1 DNA probe. (B) Specific βFTZ-F1 expression in Inka cells was confirmed by subsequent immunostaining with an antiserum to DrmETH. (C) Merged A and B. Scale bar, 30 μm.
Selective Knockdown of βftz-f1 Transcripts in Inka Cells
We performed RNA-silencing of βftz-f1 in Inka cells by crossing the ETHGS driver line with the UAS-βftz-f1i line carrying a double-stranded RNA construct; progeny were fed RU486 during the 3rd instar. Presence of βFTZ-F1 protein in knock-down and control flies was analyzed by immunohistochemical staining. Inka cells of prepupal flies fed RU486 were devoid of βFTZ-F1-IR, while strong staining for the protein was detected in nuclei of Inka cells in control flies (Fig. 2). Neighboring tracheal epidermal cells exhibited positive βFTZ-F1 immunostaining in both sets of flies, demonstrating Inka cell specificity of βftz-f1 silencing.
Fig. 2. βFTZ-F1 expression is reduced selectively in Inka cells of ETHGS/UAS-βFTZ-F1i flies.

Third instar larvae were reared on diet containing RU486. Tracheae were dissected 8 hours after puparium formation and processed for immunostaining with antisera against ETH and βFTZ-F1. (A) Inka cell displaying ETH-IR in the cytoplasm (green; arrowheads), but not in the nucleus. (B) Lack of βFTZ-F1-IR in nuclei of Inka cells in RNA-silenced flies. (C) A and B merged. (A'-C') Inka cells of control animals reared on standard diet showed ETH-IR in the cytoplasm (A') and βFTZ-F1-IR in the nucleus (B'). (C') Merged A' and B'. Scale bar, 30 μm.
βFTZ-F1 Knockdown in Inka Cells Causes Lethal Ecdysis Deficiencies
We examined phenotypes in all stages (larva, pupa, adult) subjected to RNA silencing of βftz-f1, effected by feeding RU486 to ETHGS/UAS-βftz-f1i flies during larval development (2nd and 3rd instars). The developmental profile of RU486-fed larvae was indistinguishable from wild type controls until they reached the double vertical plate (dVP) stage. Targeted βFTZ-F1 knockdown in Inka cells produced lethal ecdysis deficiency in 47% of late 2nd instar larvae (Table 1). Larvae were arrested at the dVP stage, failed to shed the old cuticle and died 1-2 days later. Arrested larvae exhibited the typical “buttoned-up” phenotype (Fig. 3A), originally described in eth-null mutants (Park et al., 2002), consisting of dVP, double mouth hooks (Fig. 3B), double tubular tracheae with anterior and posterior spiracles, and a new layer of cuticle for the next instar. The high percentage of lethality (47%) in late 2nd instar larvae with fully developed structures appropriate for the next larval stage indicates that targeted βFTZ-F1 knock-down in Inka cells resulted in failure of the ecdysis sequence.
Table 1.
Lethality and developmental defects resulting from βFTZ-F1 silencing in Inka cells.
| Fly lines | n | Accumulated lethality | Short wings/normal | |
|---|---|---|---|---|
|
| ||||
| Pharate 3rd instar |
Pharate pupae |
Adults | ||
| RU486 + | ||||
| ETHGS × UAS-βFTZi | 68 | 32 (47) | 34 (97) | 2/0 |
| ETHGS × UAS-reaper-hid | 53 | 52 (98) | 1 (100) | 0/0 |
| ETHGS × UAS-EGFP | 60 | 0 | 0 | 0/60 |
| RU 486 - | ||||
| ETHGS × UAS-βFTZi | 50 | 0 | 0 | 0/50 |
| ETHGS × UAS-reaper-hid | 45 | 1 | 0 | 0/44 |
| W1118 | 60 | 0 | 0 | 0/60 |
Numbers in parentheses correspond to percent mortality at each stage.
Fig. 3. Targeted βFTZ-F1 silencing selectively in Inka cells leads to ecdysis failure throughout development.

(A) Ecdysis failure and “buttoned-up” phenotype in late 2nd instar larva; ecdysis failure is indicated by partially shed old cuticle (arrows) and death. (B) Failure to shed old mouthparts indicated by double mouth hooks (arrows) and double vertical plates (arrowheads). (C) Surviving larvae show normal pupariation indistinguishable from controls. (D, E) Pupal ecdysis failure, indicated by incomplete translocation of the air-bubble (black arrows), lack of head eversion (arrowheads) and gaps between pupal abdomen and puparium (white arrows). (F) A freshly pupated wild-type (W1118) pupa with completed head eversion (white arrowhead). (G-I) Arrested development after head eversion. (G) Developmental defects, indicated by lack of pigmentation, failure to elongate wings and legs (arrowheads), and a gap between the abdomen and puparium (arrow). (H) Successful development and pigmentation of the head and thorax, while wings and legs are short (arrowheads) and the abdomen exhibits persistent early prepupal phenotype (arrow). (I) Successful development and pigmentation, but failure to elongate wings and legs (arrowheads). (J) Normal development and elongated appendages (arrowheads) in a wild-type (W1118) pharate adult. (K) A few emerged adults with silenced βFTZ-F1 in Inka cells fail to expand their wings (arrows) and contain dimples on the dorsal thorax (arrowheads). (L) Normally developed control adult (W1118).
RU486-fed larvae that escaped to the 3rd instar developed normally and formed the puparium (Fig. 3C), but the vast majority failed to advance through the pre-pupal to pupal transition. Cumulative mortality reached 97% during or shortly after unsuccessful pupation (Table 1). Most of these animals (86%) were arrested during translocation of the air bubble to the anterior region, failed to complete head eversion, and exhibited an abnormal space between the posterior abdomen and puparium (Figs. 3D, E). In freshly ecdysed control pupae, the air bubble reached the anterior end of the puparium (Fig. 3F). Some RU486-treated pharate pupae (12%) completed air bubble translocation and head eversion, but showed a space at the posterior tip of the puparium and failed to expand legs and wings (Fig. 3G). These appendages reached only one third of the body length in the puparium (Figs. 3G-I), in contrast to those of control pupae, which extended appendages to two thirds of the body length (Fig. 3J). In certain instances, head and thorax continued to develop for 1-2 days, whereas the abdomen failed to show pigmentation and retained a morphology characteristic of the early pupal stage (Fig. 3H). Two flies eclosed successfully (Fig. 3I), but these individuals failed to expand the wings and exhibited dimples on the dorsal thorax and soft abdomens (Fig. 3K), indicative of incomplete cuticle sclerotization. No other abnormalities were observed in these flies. These observations indicate that βFTZ-F1 silencing in Inka cells leads to lethal defects at larval and pupal ecdysis and adult eclosion.
βFTZ-F1 Knockdown Blocks Release of ETH from Inka Cells
Resemblance of the βFTZ-F1 knockdown phenotype in larvae to the ETH-null mutant, buttoned-up (Park et al., 2002), led us to hypothesize failure of Inka cells to acquire secretory competence, resulting in ETH deficiency. To test this possibility, Inka cells of βftz-f1 knock-down larvae arrested at the dVP stage were subjected to immunohistochemical staining with ETH-specific antiserum. We observed strong ETH-IR in Inka cells of both control (ETHGS/UAS-EGFP) and βFTZ-F1 knock-down larvae at the dVP stage (Fig. 4A-D). ETH-IR disappeared during the 10 min following dVP in control flies, demonstrating ETH release from Inka cells (Figs. 4A'-C'). However ETH staining persisted for hours in Inka cells of dVP-arrested ETHGS/UAS-βftz-f1i larvae, indicating failure to release ETH (Fig. 3D'). This would explain ecdysis defects and lethal phenotypes observed in ETHGS/UAS-βftz-f1i flies. Accumulation of ETH-IR in Inka cells of βFTZ-F1 silenced larvae also implies that βFTZ-F1 is not required for ETH expression, but is essential for inducing competence to release this peptide hormone.
Fig. 4. Persistent ETH-IR in Inka cells of ETHGS/UAS-βftz-f1i flies.

ETHGS flies were crossed with UAS-EGFP line for Inka cell visualization at dVP larval stage (A-C) and just after ecdysis (A'-C'). A, A'; anti-GFP staining (green, Alexa 488). B, B'; The same tracheae were stained with ETH antiserum to detect ETH levels before and after ecdysis (red, Alexa 555); C, C; merged images. Note that ETH was depleted after ecdysis (B'). (D, D') In βFTZ-F1 knock-down flies, ETH staining showed no difference between Inka cells in dVP stage (D) and arrested larvae 3 hr after appearance of dVP (D'; red, Alexa 555). Scale bar, 25 μm.
ETH Injection Rescues Developmental Arrest in βFTZ-F1 Knockdown Larvae
If ETH deficiency is responsible for developmental arrest in ETHGS/UAS-βftz-f1i larvae, injection of synthetic peptide should be an effective rescue strategy. DrmETH1 was chosen because of its efficiency in rescue of ETH-null mutants (Park et al., 2002). First instar larvae (ETHGS/UAS-βftz-f1i) were transferred to a diet containing RU486 and injected when they reached the late 2nd instar stage. DrmETH1 (10 fmol) was injected 30 min after appearance of double vertical plates (dVP). The number of rescued animals was compared with that of water-injected control larvae. Most ETHGS/UAS-βftz-f1i larvae arrested at the dVP stage performed the ecdysis sequence following DrmETH1 injection (19 out of 20; Table 2). Considering that the percentage of lethality was 47% in βftz-f1 knock-down flies (see Table 1), injection of DrmETH1 rescued 89% of knock-down flies. This result supports the hypothesis that the failure of ecdysis in ETHGS/UAS-βftz-f1i is caused by suppression of ETH release.
Table 2.
Rescue of larvae with targeted βFTZ-F1 knockdown in Inka cells by DrmETH1 injection into late 2nd instar larvae.
| Fly lines | n | Ecdysis |
|---|---|---|
| ETHGS × UAS-βFTZi (100 fmol DrmETH1) | 20 | 19 |
| ETHGS × UAS-βFTZi (dist. water) | 20 | 9 |
Targeted Ablation of Inka Cells Phenocopies βFTZ-F1 silencing
We have shown that βftz-f1 silencing in Inka cells leads to failure of ETH release, resulting in a pattern of ecdysis defects similar to those observed previously in ETH-null mutants (Park et al., 2002). We sought to confirm that lack of ETH leads to ecdysis deficiencies in later developmental stages by comparing phenotypes of βFTZ-F1 silenced flies (ETHGS/UAS-βftz-f1i) with those of Inka cell-ablated flies (ETHGS/UAS-reaper-hid). Progeny of the latter cross, which were fed RU486 during the second instar, showed no DrmETH1-IR in the tracheal system, consistent with ablation of Inka cells (data not shown). At the time of ecdysis from 2nd to 3rd instar, these larvae showed phenotypes identical to those observed in the ETHGS/UAS-βftz-f1i. Affected larvae exhibited the buttoned-up phenotype and lethal defects at larval ecdysis (98%; Table 3). Likewise, all 3rd instar larvae fed RU486 showed severe defects at pupal ecdysis (Table 3), indicated by incomplete translocation of the air bubble, failure of head eversion and reduced extension of pupal wing pads and legs.
Table 3.
Lethality and developmental defects of flies with ablated Inka cells.
| Fly lines | n | Accumulated lethality | Short wings/normal | |
|---|---|---|---|---|
|
| ||||
| Pharate 3rd instar |
Pharate pupae |
Adults | ||
| RU486 + | ||||
| ETHGS × UAS-reaper-hid | 57 (2nd) | 56 (98) | 1 (100) | 0/0 |
| ETHGS × UAS-reaper-hid | 64 (3rd) | - | 64 (100) | 0/0 |
| RU 486 - | ||||
| ETHGS × UAS-reaper-hid | 42 (2nd) | 1 | 0 | 0/41 |
| ETHGS × UAS-reaper-hid | 38 (3rd) | - | 0 | 0/3 |
Numbers in parentheses correspond to percent mortality at each stage.
Proper Timing of βFTZ-F1 Expression in Inka cells is Essential for Larval Ecdysis, Pupation, and Adult Development
βFTZ-F1 expression is temporally restricted to a short period prior to each larval ecdysis and pupation, coinciding with decline of the ecdysteroid peak to a low level (Yamada et al., 2000). To assess the importance of stage-specific βFTZ-F1 expression in Inka cells, we analyzed the effect of its premature and prolonged expression on larval development and ecdysis in ETHGS/UAS-βFTZ-F1 flies. βFTZ-F1 expression in Inka cells was activated by raising newly eclosed 2nd instar larvae on diet containing RU486. Premature βFTZ-F1 expression in Inka cells did not affect feeding and larval growth, but resulted in severe ecdysis defects. Almost one-third of these larvae (average 30%) failed to execute ecdysis and exhibited the same morphological “buttoned-up” phenotype as observed in βFTZ-F1 -silenced animals shown in Table 1: double mouth hooks, double vertical plates, and new cuticle, spiracles and tracheae for the next larval instar (Table 4). Survivors continued to receive RU486 in the diet throughout the 3rd instar. All larvae in this group showed normal growth and puparium formation, but most (96%) failed to progress through pupal ecdysis and died (Table 4). Phenotypes included failure of air bubble translocation, head eversion and elongation of wings and legs. Three survivors eclosed to the adult stage, but failed to expand wings as observed in βFTZ-F1- silenced flies (Table 4). These results show that premature expression of βFTZ-F1 in Inka cells disrupts ecdysis with lethal consequences at all stages (larval, pupal, adult).
Table 4.
Lethality and developmental defects caused by premature expression of βFTZ-F1 in Inka cells.
| Fly lines | n | Accumulated lethality | Short wings/normal | |
|---|---|---|---|---|
|
| ||||
| Pharate 3rd instar |
Pharate pupae |
Adults | ||
| RU486 + | ||||
| ETHGS × UAS-βFTZ | 50 | 13 (26%) | 35 (95%) | 2/0 |
| ETHGS × UAS-βFTZ | 42 | 14 (33%) | 27 (97%) | 1/0 |
| ETHGS × UAS-EGFP | 54 | 1 | 2 | 0/51 |
| RU 486 - | ||||
| ETHGS × UAS-βFTZ | 47 | 0 | 0 | 0/47 |
| W1118 | 64 | 0 | 0 | 0/64 |
Targeted βFTZ-F1 Expression in Inka Cells Rescues βftz-f1 Deletion Mutants
Homozygous βftz-f1ex7 progeny are embryonic lethal, but can be rescued by temporally restricted heat treatment, resulting in ectopic expression of βFTZ-F1 and progression to the first instar (Yamada et al., 2000). These rescued larvae are arrested at ecdysis to the 2nd instar, exhibiting the buttoned-up phenotype unless rescued by a second heat treatment. While βFTZ-F1 is expressed widely in Drosophila tissues (Lam and Thummel, 2000), we have shown here that similar lethal phenotypes are caused by Inka cell-specific knockdown in ETHGS/UAS-βftz-f1i flies. This suggests that failure of βFTZ-F1 expression in Inka cells may be responsible for the most severe defects in βftz-f1 mutants. To test this hypothesis, we asked whether βftz-f1ex7 mutants could be rescued through temporally restricted, Inka cell-selective βFTZ-F1 expression using GeneSwitch.
We compared viability of βftz-f1ex7 null flies treated by heat shock (hs), which induces βFTZ-F1 expression in all tissues versus Inka cell-selective βFTZ-F1 expression via activation of GeneSwitch. To accomplish this, we collected homozygote progeny (yw; ETHGS/UAS-FTZ-F1; βftz-f1ex7hs-βFTZ-F1/βftz-f1ex7hs-βFTZ-F1) obtained from the following cross: yw; ETHGS/ETHGS; βftz-f1ex7hs-βFTZ-F1/TM3, y+ × yw; UAS-FTZ-F1/UAS-FTZ-F1; βftz-f1ex7hs-βFTZ-F1/TM3, y+, using the y- marker for selection. First instar larvae were rescued through the embryonic stage by heat treatment 13-16 hours after egg laying (AEL). Surviving 1st instar larvae were transferred during the time interval 42-46 hours AEL into a new dish containing food with RU486 (100-200 μg/ml) to elicit βFTZ-F1 expression only in Inka cells. In parallel experiments, larvae with the same genotype were placed into a dish containing standard diet and heat treated during 42-46 hours AEL, which resulted in organism-wide expression of βFTZ-F1. The number of 1st and 2nd instar larvae was counted during 54–60 hours AEL. We found that RU486 treatment rescued ∼77% to the 2nd instar, similar to 80% rescue of larvae by the ectopic expression of βFTZ-F1 following heat treatment (Table 5). These results show that restricted expression of βFTZ-F1 in Inka cells is sufficient for rescue of the βftz-f1ex7 null mutant.
Table 5.
Rescue of the βftz-f1ex7hs-βFTZ-F1 null mutant larvae by targeted βFTZ-F1 expression in Inka cells.
| Total number of larvae used after hs of embryos | Number and % of rescued larvae after heat or RU486 treatment | ||||
|---|---|---|---|---|---|
|
| |||||
| 1st instar | 1st instar | 2nd instar | rescued | ||
| ETHGS/UAS-βFTZ-F1; ftz-f1ex7hs-βFTZ-F1 | |||||
| hs | 59 | hs | 11 | 48 | 81% |
| hs | 67 | hs | 14 | 53 | 79% |
| hs | 56 | RU486 | 11 | 45 | 80% |
| hs | 59 | RU486 | 15 | 44 | 75% |
| hs | 90 | RU486 | 22 | 68 | 76% |
|
| |||||
| ftz-f1ex7hs-βFTZ-F1 | |||||
| hs | 87 | hs | 18 | 69 | 79% |
| hs | 75 | RU486 | 75 | 0 | 0% |
Embryos were rescued by heat shock (hs) during 13-16 hours AEL. Surviving 1st instar larvae were heat treated or fed RU486 treated during 42-46 hours AEL. The number of 1st or 2nd instar larvae was counted during 54-60 hours AEL.
As a control experiment, homozygous embryos from yw; βFTZ-F1ex7hs-βFTZ-F1/TM3, y+ parents were first rescued by heat shock during 13-16 hours AEL. Surviving 1st instar larvae were exposed again either to heat shock or RU486 during the developmental interval 42-46 hours AEL. The number of 1st or 2nd instar larvae was tabulated during 54-59 hours AEL. The proportion of larvae rescued by heat shock (79%) is similar to that obtained in the above described experiment, whereas those fed RU486 failed to undergo ecdysis and died as expected (Table 5).
Discussion
Lethal Phenotypes of βFTZ-F1 Deficiency are Induced by Blocking ETH Signaling
We have shown that GeneSwitch-mediated βftz-f1 silencing selectively in Inka cells prevents stage-specific responses to 20E in larvae, mid-prepupae, and adults that were observed previously for the βftz-f1ex7 null mutant (Yamada et al., 2000). Larval phenotypes include duplicate mouthparts, spiracles, and cuticular layers, all of which are ecdysis defects characteristic of ETH deficiency. Identical phenotypes result from Inka cell ablation (this work) and eth excision (Park et al., 2002). βftz-f1 knockdown in Inka cells is further characterized by persistent ETH-IR, suggesting failure to release the peptide. Rescue of these phenotypes by ETH injection lends credence to this view.
We also performed genetic rescue of the βftz-f1ex7 null mutant using two different methods. It was shown previously that the embryonic lethal genotype βftz-f1ex7hs-βFTZ-F1 could be rescued to the first instar by heat treatment. Although these survivors fail to advance to the 2nd instar, a second heat treatment rescues a majority (∼70%) of them (Yamada et al., 2000). By incorporating ETH-GeneSwitch and βFTZ-F1 into this fly line, we were able to compare rescue efficiencies of organism-wide βFTZ-F1 expression by heat treatment with Inka cell-specific βFTZ-F1 expression. Our results indicate that larval mortality at the end of the first instar can be rescued equally well by heat treatment (80%) and Inka cell specific expression of βftz-f1 (77%). These results provide further evidence that the major defects arising from the βftz-f1ex7 null mutant are ecdysis related and stem from ETH deficiency.
Inka cell-specific βftz-f1 silencing also phenocopies the βftz-f1ex7 null mutation at the mid-prepupal to pupal transition, which involves air bubble translocation (pre-ecdysis), head eversion (ecdysis) and elongation of incipient wings and legs (post-ecdysis). Despite normal muscle morphology, βftz-f1 mutants fail to perform abdominal contractions necessary to generate internal hydrostatic pressure for the prepupal-pupal transition. These observations can be explained by block of ETH release required for activation of motor circuitry driving abdominal musculature at the appropriate developmental time (Fortier et al., 2003). The entire ecdysis sequence is triggered by ETH via a downstream receptor-mediated signaling cascade of central peptidergic neurons that express ETH receptors (ETHR) (Kim et al., 2006b; 2006a; Park et al., 2002). ETHR neurons are organized as sequentially recruited peptidergic ensembles that release kinin, diuretic hormones, FMRFamides, eclosion hormone (EH), crustacean cardioactive peptide (CCAP), myoinhibitory peptide (MIP) and bursicon (Kim et al., 2006b; 2006a). These ensembles recruit successive steps in the ecdysis sequence; their disruption by cell-specific ablation causes defects similar to those observed in βftz-f1-null mutants and in flies in which βftz-f1 is silenced selectively in Inka cells (Clark, 2004; Kim et al., 2006b; Loveall and Deitcher, 2010; McNabb et al., 1997; J. H. Park, 2003; Peabody et al., 2008). For example, targeted ablation of the CCAP/MIPs/bursicon ensemble causes failure of head eversion and incomplete wing and leg extension, most likely due to insufficient blood pressure and cuticle extensibility normally conferred by co-release of these peptides (Kim et al., 2006b; J. H. Park, 2003). Further studies with pburs null mutants showed that this gene is mainly responsible for these phenotypes (Lahr et al., 2012). In the case of Inka cell specific βftz-f1 knock-down flies, most animals show the same phenotypes as described above and die during pupal ecdysis, while a small number of surviving adults fail to expand the wings. These defects are most likely explained by failure to recruit CCAP/MIP/bursicon neurons, normally activated by ETH (Kim et al., 2006b).
It is important to note that the phenotype associated with βftz-f1 loss of function mutants could be explained partially by defects in remodeling of motor neurons innervating larval muscles during metamorphosis. Proper pupal ecdysis requires activation of EcR-B1 expression in muscles controlled by the ftz-f1/Hr39 nuclear receptor pathway. Production of TGF-β/BMP ligands during muscle degeneration provides a signal that initiates retraction of motor neurons (Boulanger et al., 2012). However this cannot explain lethal phenotypes observed in ETHGS/UAS-βftz-f1i knockdown prepupae deficient in βFTZ-F1 protein only in Inka cells. Equally severe developmental defects clearly demonstrate the importance of Inka cell peptides in activation of neuronal networks controlling key processes in pupation.
Similarity of phenotypes resulting from deficiencies in βFTZ-F1 and ETH indicates they are signals in a common pathway initiated by 20E at the beginning of the molt and culminating with the ETH-driven behavioral cascade that terminates molting through completion of the ecdysis sequence.
Correct Timing of βFTZ-F1 Expression and ETH Release is Critical for Normal Development
Previous experiments with βftz-f1ex7hs-βftz-f1 null mutant flies demonstrated that developmental arrest could be rescued by heat-induced βFTZ-F1 expression, but only at developmental times corresponding to normal expression of βFTZ-F1; premature heat treatment was ineffective for rescue (Yamada et al., 2000). Likewise, our results show that, while Inka cell-specific βFTZ-F1 expression at the appropriate time rescues βftz-f1ex7 null mutants, premature expression of βFTZ-F1 in Inka cells leads to high mortality. Such mortality is likely due to premature ETH release. We have shown previously that premature ETH injection fails to rescue ETH-null mutants (Park et al., 2002). We also showed in the moth, Manduca sexta that premature injection of ETH into pharate larvae is lethal (Zitnanová et al., 2001). Taken together, these observations suggest that temporal pattern of βFTZ-F1 expression is critically important in determining the correct timing of ETH release.
βFTZ-F1 Confers Secretory Competence in Inka Cells
In Manduca sexta, release of ETH from Inka cells requires decline of 20E to low levels (≤ 0.1 μg/ml; 210 nM) and new gene expression in the hours preceding ecdysis (Kingan and Adams, 2000). Prior to this time, Inka cells have not yet acquired competence to release ETH in response to eclosion hormone. Among the genes newly expressed during this period is βftz-f1 (Hiruma and Riddiford, 2001).
In Drosophila, a similar pattern of steroid decline and βFTZ-F1 expression occurs at the end of each developmental stage prior to ecdysis, namely during the hours 45-47 hours AEL (first instar), 69-71 hours AEL (second instar), and 8-12 hours after pupariation (Yamada et al., 2000). The null mutant βftz-f1ex7hs-βFTZ-F1 is arrested at the first ecdysis, but can be rescued by heat treatment to the second instar. Rescued animals become arrested at the transition from 2nd to 3rd instar, but can be rescued again by heat treatment. Surviving animals fail to pass through the pre-pupal to pupal transition unless heat-treated again. These treatments induce βFTZ-F1 expression in all tissues that normally express this gene. Quite striking are the results presented here, showing that the βftz-f1ex7hs-βFTZ-F1 null mutant line is rescued by Inka cell-specific expression of βFTZ-F1. Although not all of the cellular phenotypes (e.g., salivary gland histolysis) associated the βFTZ-F1 knockout line may be rescued by Inka cell expression alone, it is nevertheless noteworthy that such restricted expression of βFTZ-F1 is sufficient to advance development to the next stage.
We conclude that a major physiological outcome downstream of transient, precisely timed expression of βFTZ-F1 is ETH secretion by Inka cells. Based on our findings, βFTZ-F1 appears to be both necessary and sufficient to confer secretory competence in Inka cells at stage-specific times to advance development.
20E-induced Signaling Cascades in the Inka Cell
Although the precise sequence of molecular events leading to Inka cell secretory competence following βFTZ-F1 expression is unknown, previous findings shed some light on signal transduction in this endocrine cell. We propose a model combining evidence gained from studies on moths and flies (see graphical abstract). The surge of ecdysteroid levels that initiates changes in gene expression, including induction of the ETH gene via EcR and CRC (Gauthier and Hewes, 2006; Gauthier et al., 2012; Zitnan et al., 1999). High ecdysteroid levels induce expression of the gene eth (downward curved arrow) and inhibit expression of βftz-f1 (downward inhibitory bar); only after 20E levels fall to low levels (straight downward arrow) does βftz-f1 expression occur, allowing for acquisition of secretory competence. We have shown in this paper that silencing of βftz-f1 prevents ETH release, leading to ecdysis defects larvae, pre-pupae, and adults. Injection of ETH or targeted expression of βftz-f1 in Inka cells rescues these phenotypes.
Studies in Manduca sexta and Bombyx mori showed that Inka cells release ETH in response to corazonin and EH. Corazonin released from Ia1 neurons in the brain induces an initial, low level secretion of ETH, which acts back on the CNS to elicit the release of EH from VM neurons (Ewer et al., 1997; Kim et al., 2004; Kingan et al., 1997). EH induces elevation of cGMP in Inka cells, followed by massive release and complete depletion ETH. Pharmacological manipulations of Inka cells using various kinase and phosphatase inhibitors revealed Ca2+ and cGMP-mediated pathways are likely to be involved in EH-induced secretion of ETH in M. sexta (Kingan, 2001). Identification of membrane bound guanylyl cyclases as EH receptors confirmed a cGMP-mediated transduction pathway for this neuropeptide also in flies (Chang et al., 2009). Elevation of cGMP in Inka cells at ecdysis thus provides evidence in support of EH-induced ETH release in Drosophila (Clark, 2004). Further support for neuropeptide regulated release of ETH comes from detection of corazonin and EH receptor expression in Inka cells of moths and flies (Chang et al., 2009; Kim et al., 2004; Zitnan and Adams, 2013). However, EH alone appears not to be essential for ETH secretion in Drosophila, since EH knockout flies complete ecdysis even in the absence of this neuropeptide and cGMP elevation in Inka cells. In these flies ETH release may be induced by corazonin. Thus far, injections of corazonin or EH in Drosophila failed to induce premature ETH secretion and initiation of the ecdysis sequence (Clark, 2004). It is possible that this failure is related to a very brief window of neuropeptide receptor expression induced by βFTZ-F1. We speculate that brief appearance of receptors for corazonin and EH shortly before ecdysis initiation may be crucial for acquisition of Inka cell secretory competence mediated by βFTZ-F1.
Conclusions
Our findings provide precise physiological outcomes resulting from 20E-induced βFTZ-F1 expression in insect development and help to explain various lethal phenotypes associated with βFTZ-F1-null mutants in different developmental stages (Lam and Thummel, 2000; Yamada et al., 2000). Furthermore, they implicate the Inka cell as a key cellular “gateway” for stage-specific outcomes induced by 20E. Further investigation of the precise actions of βFTZ-F1 in Inka cell signal transduction will hopefully reveal molecular mechanisms underlying secretory competence in endocrine cells.
Highlights.
Silencing of βftz-f1 in Inka cells blocks ETH release and arrests development.
Developmental arrest rescued by ETH injection.
βftz-f1 null mutants rescued by Inka cell-specific expression of βftz-f1.
The Inka cell provides a “gateway” endocrine output of ecdysone signaling.
Acknowledgments
We thank Hitoshi Ueda for a generous gift of the βftz-f1ex7hs-βFTZ-F1 null mutant fly line and for the βFTZ-F1 antiserum. This work was supported by a grant from National Institutes of Health GM 067310 and Slovak grant agencies, Agentúra na podporu výskumu a vývoja (APVV-51-039105) and Vedecká grantová agentúra (VEGA 2/0132/09).
Abbreviations
- ETH
ecdysis triggering hormone
- ETH-IR
ecdysis triggering hormone-like immunoreactivity
- EH
eclosion hormone
- DrmETH
Drosophila melanogaster ETH
- ETHGS
ETH-GeneSwitch
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Ashburner M. A laboratory handbook. Cold Spring Harbor Laboratory Press; 1989. Drosophila. Cold Spring Harbor Laboratory Press. [Google Scholar]
- Ashburner M, Chihara C, Meltzer P, Richards G. Temporal control of puffing activity in polytene chromosomes. Cold Spring Harb Symp Quant Biol. 1974;38:655–662. doi: 10.1101/sqb.1974.038.01.070. [DOI] [PubMed] [Google Scholar]
- Becker HJ. Die Puffs der Speicheldrüsenchromosomen von Drosophila melanogaster. Chromosoma. 1959;10:654–678. doi: 10.1007/BF00396591. [DOI] [PubMed] [Google Scholar]
- Boulanger A, Farge M, Ramanoudjame C, Wharton K, Dura JM. Drosophila motor neuron retraction during metamorphosis is mediated by inputs from TGF-β/BMP signaling and orphan nuclear receptors. PLoS ONE. 2012;7:e40255. doi: 10.1371/journal.pone.0040255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadus J, McCabe JR, Endrizzi B, Thummel CS, Woodard CT. The Drosophila beta FTZ-F1 orphan nuclear receptor provides competence for stage-specific responses to the steroid hormone ecdysone. Mol Cell. 1999;3:143–149. doi: 10.1016/s1097-2765(00)80305-6. [DOI] [PubMed] [Google Scholar]
- Chang JC, Yang RB, Adams ME, Lu KH. Receptor guanylyl cyclases in Inka cells targeted by eclosion hormone. Proc Natl Acad Sci USA. 2009;106:13371–13376. doi: 10.1073/pnas.0812593106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AC. Neuroendocrine Control of Larval Ecdysis Behavior in Drosophila: Complex Regulation by Partially Redundant Neuropeptides. Journal of Neuroscience. 2004;24:4283–4292. doi: 10.1523/JNEUROSCI.4938-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AT, Hori M, Green JM, Wolfgang WJ, Hiruma K, Riddiford LM. Ecdysteroid regulation of the onset of cuticular melanization in allatectomized and Black mutant Manduca sexta larvae. Journal of Insect Physiology. 1984;30:597–606. [Google Scholar]
- Ewer J, Gammie SC, Truman JW. Control of insect ecdysis by a positive-feedback endocrine system: roles of eclosion hormone and ecdysis triggering hormone. J Exp Biol. 1997;200:869–881. doi: 10.1242/jeb.200.5.869. [DOI] [PubMed] [Google Scholar]
- Fortier TM, Vasa PP, Woodard CT. Orphan nuclear receptor betaFTZ-F1 is required for muscle-driven morphogenetic events at the prepupal-pupal transition in Drosophila melanogaster. Developmental Biology. 2003;257:153–165. doi: 10.1016/s0012-1606(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Gauthier SA, Hewes RS. Transcriptional regulation of neuropeptide and peptide hormone expression by the Drosophila dimmed and cryptocephal genes. J Exp Biol. 2006;209:1803–1815. doi: 10.1242/jeb.02202. [DOI] [PubMed] [Google Scholar]
- Gauthier SA, VanHaaften E, Cherbas L, Cherbas P, Hewes RS. Cryptocephal, the Drosophila melanogaster ATF4, is a specific coactivator for ecdysone receptor isoform B2. PLoS Genetics. 2012;8:e1002883. doi: 10.1371/journal.pgen.1002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano E, Rendina R, Peluso I, Furia M. RNAi triggered by symmetrically transcribed transgenes in Drosophila melanogaster. Genetics. 2002;160:637–648. doi: 10.1093/genetics/160.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiruma K, Riddiford LM. Regulation of Transcription Factors MHR4 and βFTZ-F1 by 20- Hydroxyecdysone during a Larval Molt in the Tobacco Hornworm, Manduca sexta. Developmental Biology. 2001;232:265–274. doi: 10.1006/dbio.2001.0165. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Spalovská-Valachová I, Cho KH, Zitnanová I, Park Y, Adams ME, Zitnan D. Corazonin receptor signaling in ecdysis initiation. Proc Natl Acad Sci USA. 2004;101:6704–6709. doi: 10.1073/pnas.0305291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Zitnan D, Cho KH, Schooley DA, Mizoguchi A, Adams ME. Central peptidergic ensembles associated with organization of an innate behavior. Proc Natl Acad Sci USA. 2006a;103:14211–14216. doi: 10.1073/pnas.0603459103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Zitnan D, Galizia CG, Cho KH, Adams ME. A command chemical triggers an innate behavior by sequential activation of multiple peptidergic ensembles. Current Biology. 2006b;16:1395–1407. doi: 10.1016/j.cub.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Kingan TG. Signal Transduction in Eclosion Hormone-induced Secretion of Ecdysis-triggering Hormone. Journal of Biological Chemistry. 2001;276:25136–25142. doi: 10.1074/jbc.M102421200. [DOI] [PubMed] [Google Scholar]
- Kingan TG, Adams ME. Ecdysteroids regulate secretory competence in Inka cells. J Exp Biol. 2000;203:3011–3018. doi: 10.1242/jeb.203.19.3011. [DOI] [PubMed] [Google Scholar]
- Kingan TG, Gray W, Zitnan D, Adams ME. Regulation of ecdysis-triggering hormone release by eclosion hormone. J Exp Biol. 1997;200:3245–3256. doi: 10.1242/jeb.200.24.3245. [DOI] [PubMed] [Google Scholar]
- Lahr EC, Dean D, Ewer J. Genetic analysis of ecdysis behavior in Drosophila reveals partially overlapping functions of two unrelated neuropeptides. Journal of Neuroscience. 2012;32:6819–6829. doi: 10.1523/JNEUROSCI.5301-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam G, Thummel CS. Inducible expression of double-stranded RNA directs specific genetic interference in Drosophila. Curr Biol. 2000;10:957–963. doi: 10.1016/s0960-9822(00)00631-x. [DOI] [PubMed] [Google Scholar]
- Loveall BJ, Deitcher DL. The essential role of bursicon during Drosophila development. BMC Dev Biol. 2010;10:92. doi: 10.1186/1471-213X-10-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNabb SL, Baker JD, Agapite J, Steller H, Riddiford LM, Truman JW. Disruption of a behavioral sequence by targeted death of peptidergic neurons in Drosophila. Neuron. 1997;19:813–823. doi: 10.1016/s0896-6273(00)80963-0. [DOI] [PubMed] [Google Scholar]
- Osterwalder T, Yoon KS, White BH, Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc Natl Acad Sci USA. 2001;98:12596–12601. doi: 10.1073/pnas.221303298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH. Targeted ablation of CCAP neuropeptide-containing neurons of Drosophila causes specific defects in execution and circadian timing of ecdysis behavior. Development. 2003;130:2645–2656. doi: 10.1242/dev.00503. [DOI] [PubMed] [Google Scholar]
- Park Y, Filippov V, Gill SS, Adams ME. Deletion of the ecdysis-triggering hormone gene leads to lethal ecdysis deficiency. Development. 2002;129:493–503. doi: 10.1242/dev.129.2.493. [DOI] [PubMed] [Google Scholar]
- Peabody NC, Diao F, Luan H, Wang H, Dewey EM, Honegger HW, White BH. Bursicon functions within the Drosophila CNS to modulate wing expansion behavior, hormone secretion, and cell death. Journal of Neuroscience. 2008;28:14379–14391. doi: 10.1523/JNEUROSCI.2842-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman JW, Rountree DB, Reiss SE, Schwartz LM. Ecdysteroids regulate the release and action of eclosion hormone in the tobacco hornworm, Manduca sexta (L.) Journal of Insect Physiology. 1983;29:895–900. [Google Scholar]
- Yamada M, Murata T, Hirose S, Lavorgna G, Suzuki E, Ueda H. Temporally restricted expression of transcription factor betaFTZ-F1: significance for embryogenesis, molting and metamorphosis in Drosophila melanogaster. Development. 2000;127:5083–5092. doi: 10.1242/dev.127.23.5083. [DOI] [PubMed] [Google Scholar]
- Zitnan D, Adams ME. Neuroendocrine regulation of insect ecdysis. In: Gilbert LI, editor. Insect Endocrinology. San Diego: 2013. pp. 253–309. [Google Scholar]
- Zitnan D, Ross LS, Zitnanová I, Hermesman JL, Gill SS, Adams ME. Steroid induction of a peptide hormone gene leads to orchestration of a defined behavioral sequence. Neuron. 1999;23:523–535. doi: 10.1016/s0896-6273(00)80805-3. [DOI] [PubMed] [Google Scholar]
- Zitnanová I, Adams ME, Zitnan D. Dual ecdysteroid action on the epitracheal glands and central nervous system preceding ecdysis of Manduca sexta. J Exp Biol. 2001;204:3483–3495. doi: 10.1242/jeb.204.20.3483. [DOI] [PubMed] [Google Scholar]


