Abstract
Parkinson's disease (PD) is the second most common neurodegenerative disorder behind Alzheimer's disease. There are currently no therapies proven to halt or slow the progressive neuronal cell loss in PD. A better understanding of the molecular and cellular causes of PD is needed to develop disease-modifying therapies. PD is an age-dependent disease that causes the progressive death of dopamine-producing neurons in the brain. Loss of substantia nigra dopaminergic neurons results in locomotor symptoms such as slowness of movement, tremor, rigidity and postural instability. Abnormalities in other neurotransmitters, such as serotonin, may also be involved in both the motor and non-motor symptoms of PD. Most cases of PD are sporadic but many families show a Mendelian pattern of inherited parkinsonism and causative mutations have been identified in genes such as Parkin, DJ-1, PINK1, alpha-synuclein and Leucine Rich Repeat Kinase 2 (LRRK2). Although the definitive causes of idiopathic PD remain uncertain, the activity of the antioxidant enzyme glutathione peroxidase 1 (Gpx1) is reduced in PD brains and has been shown to be a key determinant of vulnerability to dopaminergic neuron loss in PD animal models. Furthermore, Gpx1 activity decreases with age in human substantia nigra but not rodent substantia nigra. Therefore, we crossed mice deficient for both Parkin and DJ-1 with mice deficient for Gpx1 to test the hypothesis that loss-of-function mutations in Parkin and DJ-1 cause PD by increasing vulnerability to Gpx1 deficiency. Surprisingly, mice lacking Parkin, DJ-1 and Gpx1 have increased striatal dopamine levels in the absence of nigral cell loss compared to wild-type, Gpx1−/−, and Parkin−/−DJ-1−/− mutant mice. Additionally, Parkin−/−DJ-1−/− mice exhibit improved rotarod performance and have increased serotonin in the striatum and hippocampus. Stereological analysis indicated that the increased serotonin levels were not due to increased serotonergic projections. The results of our behavioral, neurochemical and immunohistochemical analyses reveal that PD-linked mutations in Parkin and DJ-1 cause dysregulation of neurotransmitter systems beyond the nigrostriatal dopaminergic circuit and that loss-of-function mutations in Parkin and DJ-1 lead to adaptive changes in dopamine and serotonin especially in the context of Gpx1 deficiency.
Introduction
Parkinson's disease (PD) is the most common neurodegenerative movement disorder and afflicts millions of people worldwide. The severity of the primary clinical symptoms, which include bradykinesia, resting tremor, rigidity, and postural instability, increases over the course of many years. Postmortem examinations reveal a profound and selective loss of dopaminergic neurons in the substantia nigra that project to the caudate and putamen of the dorsal striatum. The loss of dopaminergic innervation of the striatum underlies the primary clinical symptoms, which can be ameliorated with dopaminergic medications. Although the definitive cause of nigral dopamine neuron loss remains unknown, aging is the greatest risk factor for PD, consistent with the increased prevalence of PD in elderly populations. The capacity of cells to clear reactive oxygen species and repair oxidative damage to proteins, lipids and nucleic acids diminishes with age (Liddell et al., 2010), suggesting a potential role for cumulative oxidative stress in PD pathogenesis.
The majority of PD cases are idiopathic with no clear family history of parkinsonian symptoms. However, genetic linkage studies of families with Mendelian patterns of inherited parkinsonism have identified causal mutations in several genes (Dawson et al., 2010, Horowitz and Greenamyre, 2010, Lopez and Sidransky, 2010, Corti et al., 2011, Hattori, 2012, Varcin et al., 2012). Among these are the loss-of-function mutations in the Parkin and DJ-1 genes that were the first to be causally linked to recessive parkinsonism (Kitada et al., 1998, Bonifati et al., 2003). Both Parkin and DJ-1 are widely expressed throughout the brain and other tissues (Shimura et al., 1999, Stichel et al., 2000, Kuhn et al., 2004, Shang et al., 2004, Xie et al., 2009) but it is not obvious why loss of Parkin or DJ-1 function causes selective neurodegeneration and clinical PD symptoms. Presumably, dopaminergic neurons within the nigrostriatal pathway are more susceptible than other cells to both parkinsonian genetic mutations and to factors that cause idiopathic PD, which remain uncertain. Parkin functions as an E3 ubiquitin ligase (Shimura et al., 2000) and promotes autophagy of dysfunctional mitochondria (Narendra et al., 2008). This suggests an important role for Parkin in preventing the accumulation of damaged mitochondria, which are major cellular sources of free radicals and oxidative stress. The exact cellular function of DJ-1 remains uncertain, but it has been reported to be an atypical peroxiredoxin-like peroxidase (Andres-Mateos et al., 2007) and may be a sensor of oxidative stress (Choi et al., 2006).
Overexpression of either protein is neuroprotective in vitro and in vivo (Lo Bianco et al., 2004, Zhou and Freed, 2005, Vercammen et al., 2006, Ulusoy and Kirik, 2008, Hayashi et al., 2009, Junn et al., 2009, Bian et al., 2012). In particular, DJ-1 is protective against various oxidative stresses (Yokota et al., 2003, Taira et al., 2004, Kim et al., 2005, Menzies et al., 2005, Meulener et al., 2005, Moore et al., 2005, Yang et al., 2005, Zhang et al., 2005, Andres-Mateos et al., 2007, Junn et al., 2009) and both proteins localize to mitochondria in cells undergoing oxidative stress (Horowitz and Greenamyre, 2010, Kawajiri et al., 2010, Shulman et al., 2011, Thomas et al., 2011). Cysteine 106 of DJ-1 is unusually sensitive to oxidation and is required both for the neuroprotective effects of DJ-1 and for localization of DJ-1 to mitochondria in response to oxidative stresses (Canet-Aviles et al., 2004, Kim et al., 2005, Lev et al., 2008, Junn et al., 2009, Cookson, 2010, Mullett and Hinkle, 2011). Mutations in the Parkin gene lead to impaired mitochondrial respiratory chain function and to increased markers of oxidative stress in humans and genetic animal models (Muftuoglu et al., 2004, Palacino et al., 2004, Rodriguez-Navarro et al., 2007, Vinish et al., 2011, Vincent et al., 2012, Hauser and Hastings, 2013, Vincow et al., 2013). Together, these data suggest that the cellular mechanism by which loss-of-function mutations in Parkin and DJ-1 cause parkinsonism involves diminished protection against oxidative stress.
Despite the high penetrance of loss-of-function Parkin and DJ-1 mutations in humans (Bonifati, 2007), similar mutations in mice do not produce the characteristic loss of nigral dopaminergic neurons that is the most prominent postmortem neuropathological feature of PD (Goldberg et al., 2003, Itier et al., 2003, Palacino et al., 2004, Von Coelln et al., 2004, Fleming et al., 2005, Goldberg et al., 2005, Kim et al., 2005, Perez et al., 2005, Perez and Palmiter, 2005, Fleming and Chesselet, 2006, Sato et al., 2006, Andres-Mateos et al., 2007, Manning-Bog et al., 2007, Yang et al., 2007, Zhu et al., 2007, Chandran et al., 2008, Frank-Cannon et al., 2008, Kitada et al., 2009, Pham et al., 2010, Rousseaux et al., 2012). Thus, within the two-year lifespan of mice, additional stress may be required for Parkin and DJ-1 knockout mice to reproduce the neurodegeneration that occurs in humans. It has been demonstrated that mice deficient for DJ-1 are more susceptible to nigral cell loss induced by mitochondrial toxins and oxidative stressors such as paraquat, rotenone, and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (Kim et al., 2005, Manning-Bog et al., 2007, Paterna et al., 2007) and Parkin knockout mice are more susceptible to nigral cell loss induced by chronic exposure to lipopolysaccharide (Frank-Cannon et al., 2008). Compensatory changes in enzymes that protect against oxidative stress could explain the lack of nigrostriatal degeneration in Parkin and DJ-1 knockout mice in the absence of additional stresses (Andres-Mateos et al., 2007, Rodriguez-Navarro et al., 2007). Specifically, DJ-1 knockout mice have an age-dependent increase in both the levels and activity of glutathione peroxidase (Gpx1), but not catalase, the two major enzymes that remove hydrogen peroxide from cells (Andres-Mateos et al., 2007). Parkin knockout mice also have an age-dependent increase in Gpx1 activity as well as decreased levels of reduced glutathione in the midbrain of aged knockout mice (Rodriguez-Navarro et al., 2007). Moreover, Gpx1 knockout mice are more susceptible to MPTP and overexpression of Gpx1 can protect against 6-hydroxydopamine-induced nigral cell loss (Bensadoun et al., 1998, Klivenyi et al., 2000, Zhang et al., 2000, Ridet et al., 2006). These studies suggest that the level of Gpx1 activity is a key determinant of vulnerability to nigral neuron loss in PD animal models and that compensatory increases in Gpx1 activity might explain the absence of nigral cell loss in Parkin and DJ-1 knockout mice. Even in the absence of mutations, Gpx1 activity decreases with age in human substantia nigra (Venkateshappa et al., 2012) but not in rodent substantia nigra (Benzi et al., 1989), which may explain the increased vulnerability to nigral cell loss in humans compared to rodents bearing PD-linked mutations.
To better understand the role of Parkin and DJ-1 loss-of-function mutations in PD pathogenesis and to potentially generate a mouse model that better recapitulates the age-dependent neurochemical, neuropathological and behavioral characteristics of PD, we combined Parkin and DJ-1 loss-of-function mutations and tested them in the context of Gpx1 deficiency on a C57BL/6 mouse genetic background. Mice deficient in all three genes (Parkin−/−DJ-1−/−Gpx1−/−) were viable but did not exhibit age-dependent loss of nigral neurons, decreased striatal dopamine, or motor impairments consistent with PD. Contrary to our expectations, Parkin−/−DJ-1−/−Gpx1−/− mice had increased striatal dopamine levels while Parkin−/−DJ-1−/− mice did not have increased striatal dopamine levels, but showed increased serotonin levels in both the striatum and the hippocampus. Mice with increased serotonin also showed improved rotarod behavior performance and were less “distracted” when performing the rotarod test. These data reveal roles for Parkin and DJ-1 in regulating serotonin levels and potentially compensatory increases in striatal dopamine levels in the absence of Gpx1, Parkin and DJ-1. These surprising behavioral and neurochemical phenotypes expand the apparent functions of Parkin and DJ-1 and suggest that the pathogenic mechanisms of Parkin and DJ-1 mutations may involve dysregulation of dopaminergic and serotonergic neurotransmission.
Materials and Methods
Animals
Parkin knockout mice and DJ-1 knockout mice were generated as previously described (Goldberg et al., 2003, Goldberg et al., 2005) and backcrossed to strain C57BL/6J for 10 generations, then intercrossed for two generations to obtain homozygous double knockout mice (Parkin−/−DJ-1−/−) and wild-type controls. Gpx1 knockout mice on a C57BL/6 background were obtained from Dr. Holly Van Remmen at The University of Texas Health Science Center at San Antonio. Gpx1 knockout mice were crossed with Parkin−/−DJ-1−/− double knockout mice for two generations to produce Parkin−/−DJ-1−/−Gpx1+/− mice, which were intercrossed to produce homozygous triple knockout mice (Parkin−/−DJ-1−/−Gpx1−/−) and Parkin−/−DJ-1−/− mice. When possible, paired littermates were used as controls. Experimental procedures involving the use of animals or animal tissue were performed in accordance with the NIH Guidelines for Animal Care and Use and approved by the Institutional Animal Care and Use Committee at The University of Texas Southwestern Medical Center. Animals were housed in a climate-controlled facility with ventilated cages and standard commercial lab diet. Behavioral tests were performed between 10 AM and 4 PM during the 6 AM to 6 PM light cycle.
Behavioral tests
Locomotor
To measure spontaneous locomotor activity, mice were placed individually in a clean cage within an infrared photobeam activity monitor (San Diego Instruments) and were allowed to move freely in the dark for 2 hours. The number of beam breaks was recorded in 5-minute bins as a measure of locomotor activity.
Rotarod
To measure motor function, mice were placed on an accelerating rotarod (IITC Life Science Inc) and the speed of rotation was increased from 5 to 45 revolutions per minute (RPM) over 5 minutes. The latency to fall from the rotarod was recorded. Data were collected for 4 trials per day for 2 days.
Fixed-speed rotarod and distraction measurements
To delineate the role of motor and non-motor factors (“distraction”) on rotarod performance, fully trained mice were tested on a rotarod with fixed rotation rates of 5, 10, 15, or 20 RPM for a maximum of 5 minutes. For each speed, the latency to fall from the rotarod was recorded for 4 trials per day for 2 days. Mice were videotaped during the last two trials and the first 30 seconds of each video were analyzed with a stopwatch for time not focused on the task, which was measured as time in which the mice were not facing forward. All mice included in the video analysis stayed on the rotarod for the full 30 seconds.
Odor test
To measure olfactory function, mice were placed in a clean cage containing a dry cotton swab within reach, and given 15 minutes to acclimate to their surroundings. The dry cotton swab was replaced by a cotton swab dipped in water for 3 minutes, followed by a cotton swab dipped in vanilla and finally a cotton swab dipped in urine collected from unfamiliar mice. Mice were videotaped during all three trials and the total time spent investigating each cotton swab was recorded using a stopwatch.
Stereology of Dopaminergic Neurons
Stereology was performed according to previously described methods (Frank-Cannon et al., 2008). Brains were removed and placed in 10% neutral buffered formalin at 4° overnight, processed for paraffin embedding and sectioned in the coronal plane at 20-micron thickness. Every fifth slide was stained for unbiased stereology. Slides were deparaffinized, rehydrated in graded ethanol solutions and blocked with 5% normal goat serum for 1 hour prior to incubation in primary antibody (anti-tyrosine hydroxylase AB152, Chemicon) diluted 1:1000 at 4°C overnight. Sections were washed, incubated with biotinylated goat anti-rabbit secondary antibody, horseradish peroxidase conjugated avidin (ABC Elite, Vector) and were developed in DAB solution with NiCl enhancement prior to dehydration and coverslipping. A microscope with a motorized stage and Stereoinvestigator software was used to count the tyrosine hydroxylase-positive neurons in the substantia nigra of each section, with a counting frame of 50 microns by 50 microns and a grid size of 100 microns by 100 microns. The total number of bilateral substantia nigra dopamine neurons was estimated by counting neurons from one hemisphere.
Stereology of Hippocampal Serotonergic Fibers
Mice were perfused with 50mL of 1× phosphate buffered saline (PBS) followed by 4% paraformaldehyde in 1×PBS. Following perfusion, the brain was removed and placed in 4% paraformaldehyde solution overnight, followed by 10% and 30% sucrose overnight. Brains were frozen in OCT and sectioned sagitally at 14-micron thickness on a cryostat. Every 40th section was stained and used for unbiased stereology. Tissue was permeabilized with 0.3% Triton-X 100 prior to blocking with 10% normal donkey serum. Slides were then incubated in primary antibody (goat anti-serotonin transporter HTT-G0-af970, Frontier Labs) diluted1:250 at 4°C overnight. Slides were washed in 1% fish skin gelatin prior to incubation in secondary antibody (donkey anti-goat Alexafluor 488, Jackson Immunoresearch) diluted 1:200. Slides were stained with DAPI prior to coverslipping. Stereology was performed to measure immunoreactive fibers using the Spaceballs probe in Stereoinvestigator software, with a 200 × 200 micron grid and a hemisphere with a 10-micron radius.
Measurement of tissue neurotransmitter levels
Mice were euthanized and the striatum was quickly dissected on an ice-cold glass dish, weighed, frozen on dry ice and stored at −80 prior to analysis. Samples were combined with 50-fold (weight:volume) ice-cold 0.1 N perchloric acid containing 0.2 mM sodium metabisulfite. The tissue was disrupted by brief sonication and centrifuged at 4°C for 20 minutes at 15,000 x g to pellet proteins and cell debris. 200 uL of the supernatant was transferred to a clean tube, and 20 uL was injected onto an HPLC with a C18 column and eluted with isocratic MDTM mobile phase (ESA) at a rate of 0.6 mL/min. Monoamines were detected with a model 5014B electrochemical cell (ESA) set to a potential of +220 mV. Peak areas were normalized to tissue weight and compared to external standards for quantification.
Statistics
Data analysis was performed using SigmaPlot software. One way ANOVA was used to account for multiple genotypes. Where appropriate, repeated measures two-way ANOVA was used to account for multiple trials. Where p<0.05, Tukey's post hoc analysis was used to further analyze differences between groups. Planned comparisons were performed using Student's t-test.
Results
Normal number of nigral dopaminergic neurons in Parkin−/−DJ-1−/−Gpx1−/− mice
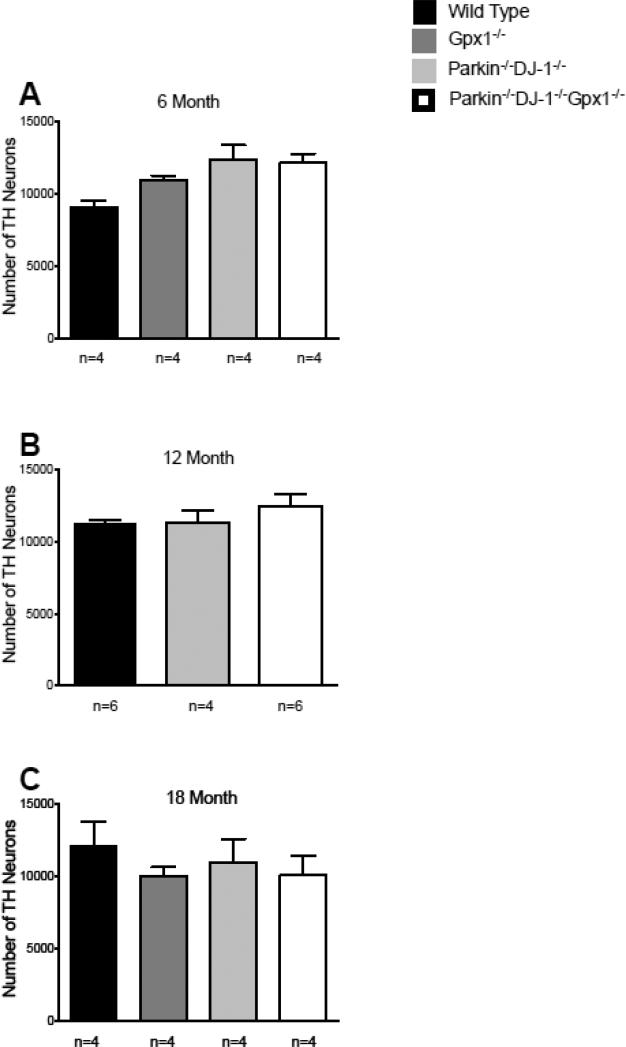
Triple knockout mice bearing combined loss-of-function mutations in the PD-linked genes Parkin and DJ-1, as well as the antioxidant Gpx1 gene, were born at the expected Mendelian ratio and had no apparent differences in viability or longevity compared to wild-type mice. Because age-dependent loss of dopaminergic neurons in the substantia nigra is the primary pathological characteristic of PD and the cause of the motor symptoms observed in patients, we investigated whether Parkin−/−DJ-1−/−Gpx1−/−mice exhibit progressive loss of nigral neurons. Coronal brain sections were stained using an antibody specific for tyrosine hydroxylase (TH), a marker of dopamine-containing neurons, and rigorous stereological methods were used to estimate the number of dopaminergic neurons in the substantia nigra of mice at ages 6, 12 and 18 months. We observed statistically similar numbers of TH-immunoreactive neurons in each genotype at age 6 months (Figure 1A), 12 months (Figure 1B) and 18 months (Figure 1C). These data indicate that the number of dopaminergic neurons is not significantly altered in Parkin−/−DJ-1−/−Gpx1−/− mice.
Figure 1.
Parkin−/−DJ-1−/− and Parkin−/−DJ-1−/−Gpx1−/− mice have normal substantia nigra cell numbers. Bars show the mean ± SEM bilateral number of tyrosine hydroxylase (TH)-positive neurons in the substantia nigra estimated by unbiased stereology. Separate cohorts of mice were analyzed at ages 6 (A), 12 (B) and 18 (C) months, n=4-6 mice per genotype at each age. The groups were not significantly different from each other (one-way ANOVA).
Increased striatal dopamine in Parkin−/−DJ-1−/−Gpx1−/− mice
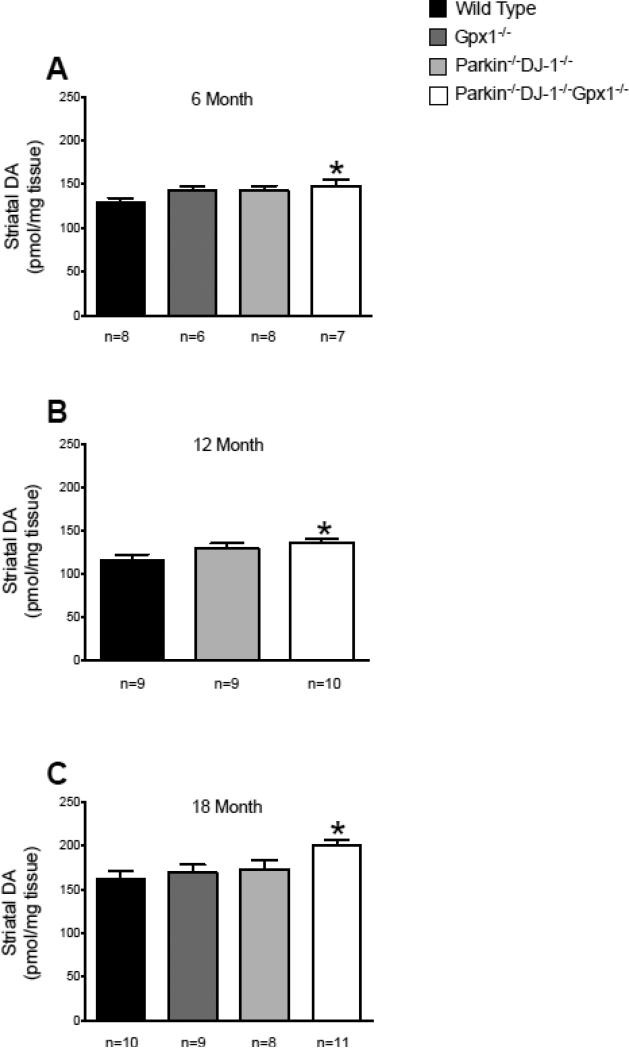
Nigral dopaminergic neurons project to the dorsal striatum (caudate and putamen) and compensatory changes in dopamine levels and dopamine turnover at presynaptic terminals in the striatum have been hypothesized to occur during presymptomatic stages of PD (Bernheimer et al., 1973). We therefore investigated whether Parkin−/−DJ-1−/−Gpx1−/− mice have altered levels of striatal dopamine even with normal nigral neuron numbers. We used HPLC with electrochemical detection to measure the levels of dopamine and its metabolites DOPAC, HVA and 3-MT in the striatum of mice at ages 6, 12 and 18 months. Parkin−/−DJ-1−/− mice and mice with a single Gpx1 deficiency showed no change in striatal dopamine levels compared to wild type mice (Figure 2). Surprisingly, dopamine levels were significantly elevated in Parkin−/−DJ-1−/−Gpx1−/− mice at 18 months (p<0.05, one-way ANOVA). At earlier ages, striatal dopamine levels are not significantly different by ANOVA, however, t-test shows significant differences between wild-type and Parkin−/−DJ-1−/−Gpx1−/− mice at age 12 months (p= 0.0019) and 6 months (p=0.0475) (Figure 2). We found no significant differences in dopamine turnover, calculated as the ratio of dopamine metabolites to dopamine (data not shown). These results suggest that Parkin−/−DJ-1−/−Gpx1−/− mice have compensatory changes in striatal dopamine levels.
Figure 2.
Striatal dopamine is increased in Parkin−/−DJ-1−/−Gpx1−/− mice. Levels of striatal dopamine (DA) measured by HPLC with electrochemical detection. Separate cohorts of mice were analyzed at ages 6 (A), 12 (B) and 18 (C) months, n=6-11 mice per genotype at each age. Bars show the mean ± SEM of the level of dopamine measured from microdissected striatum. Asterisks indicate significant differences compared to wild-type mice at the same age (*p<0.05, t-test). Parkin−/−DJ-1−/−Gpx1−/− mice have increased DA levels compared to wild type (**p<0.01, one-way ANOVA, Tukey's post-hoc) at age 18 months but not at 6 or 12 months.
Altered serotonin in multiple brain regions of Parkin−/−DJ-1−/− mice
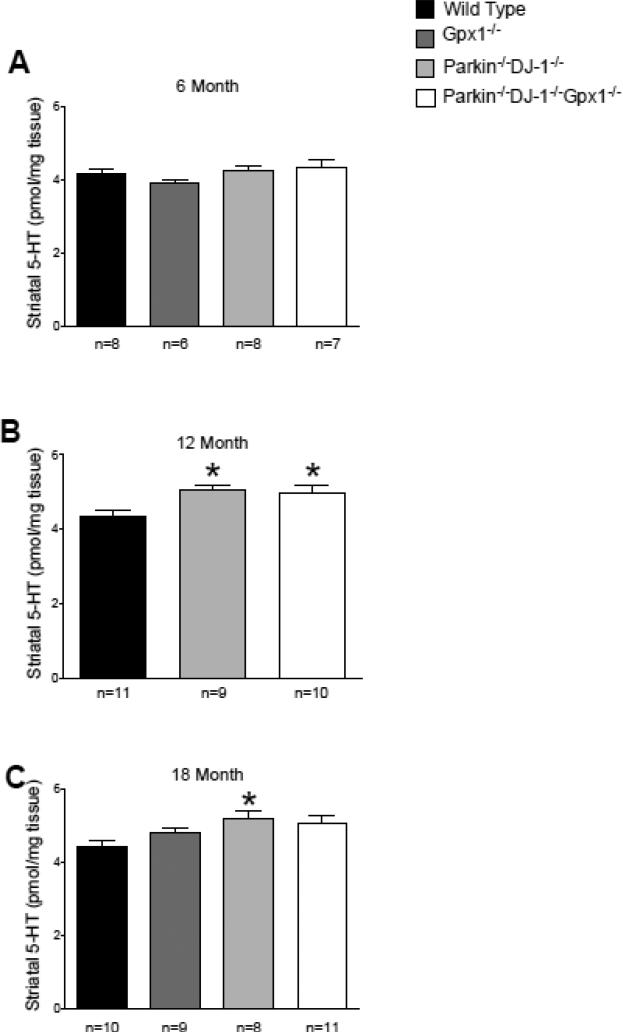
In addition to showing an increase in striatal dopamine, our HPLC analysis revealed that serotonin levels are significantly increased in the striatum of Parkin−/−DJ-1−/− mice and Parkin−/−DJ-1−/−Gpx1−/− mice at age 12 months (p<0.01, one-way ANOVA) and in the striatum of Parkin−/−DJ-1−/− mice at 18 months (p<0.05, one-way ANOVA) (Figure 3). No consistent differences were observed in serotonin turnover or in the levels of 5-HIAA, the primary metabolite of serotonin (data not shown).
Figure 3.
Striatal serotonin is increased in Parkin−/−DJ-1−/− and Parkin−/−DJ-1−/−Gpx1−/− mice. Levels of striatal serotonin (5-HT) measured by HPLC with electrochemical detection. Separate cohorts of mice were analyzed at ages 6 (A), 12 (B) and 18 (C) months, n=6-11 mice per genotype at each age. Bars show the mean ± SEM of the level of serotonin measured from microdissected striatum. *p<0.05, one-way ANOVA compared to wild-type mice at the same age.
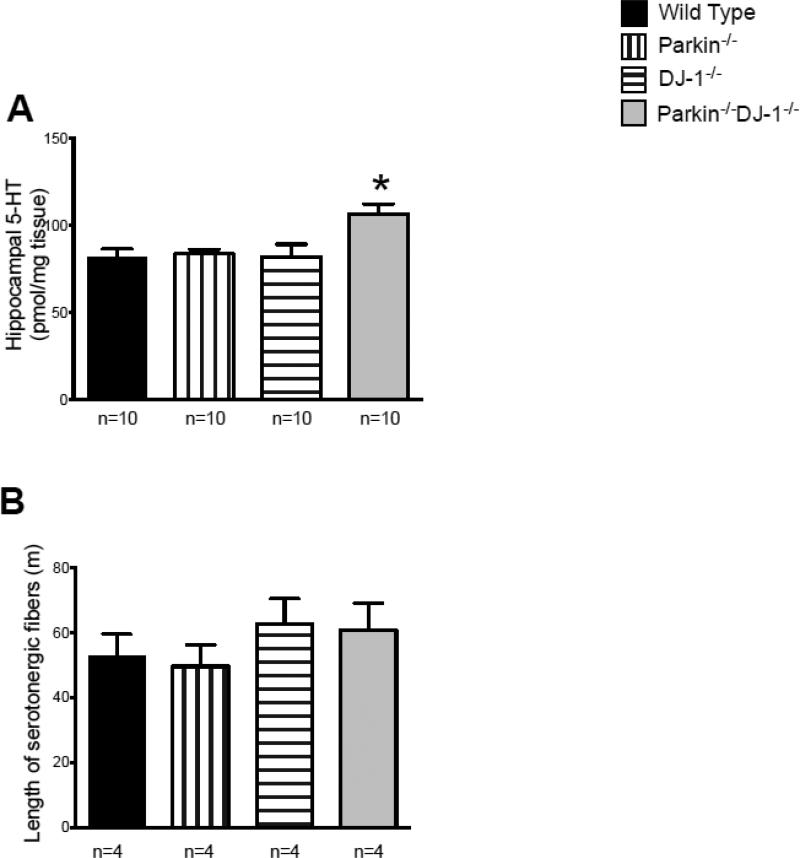
We found no genotype-dependent differences in dopamine levels in other brain regions, such as frontal cortex and hippocampus (data not shown). However, serotonin was significantly elevated in the hippocampus of Parkin−/−DJ-1−/− mice compared to wild type (p<0.05, one-way ANOVA) (Figure 4A). Deletion of both Parkin and DJ-1 is evidently required for the increased hippocampal serotonin because single knockout mice were not different from wild type (Figure 4A).
Figure 4.
Serotonin levels are increased in the hippocampus of 15- month Parkin−/−DJ-1−/− mice. (A) Levels of serotonin (5-HT) measured by HPLC with electrochemical detection. Bars show the mean ± SEM (n=10 per genotype). *p<0.05, one-way ANOVA compared to wild-type mice at the same age. (B) Length of serotonergic fibers in the hippocampus measured by unbiased stereology (n=4 mice per genotype).
One possible cause of the observed increase in hippocampal serotonin levels is an increase in serotonergic projections to the hippocampus. Therefore, we quantified serotonin-releasing fibers, defined as axons immunoreactive for the serotonin transporter (SERT) in the hippocampus, by stereological analysis. The estimated summed length of all serotonergic fibers in the hippocampus was calculated. Although there was a trend towards increased SERT-positive fibers in the hippocampus of DJ-1−/− mice and Parkin−/−DJ-1−/− mice compared to both wild type and Parkin−/− mice, this difference was not statistically significant (Figure 4B). Together, these data indicate the significant effects of Parkin and DJ-1 deficiency on the regulation of non-catecholamine neurotransmitters in the brain.
Improved rotarod performance of Parkin−/−DJ-1−/− mice
Because Parkinson's disease causes deficits in motor function, we investigated whether mice with combined PD-linked mutations have altered performance in established behavioral tests of motor function. Furthermore, because age is the greatest risk factor for PD, separate cohorts of mice were tested at ages 6, 12, and 18 months to assess whether motor abilities changed with age.
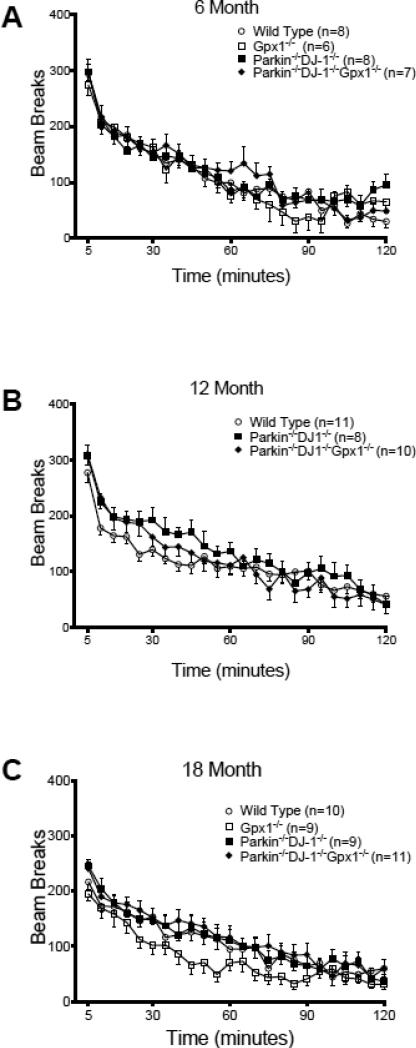
We examined spontaneous locomotor behavior by placing mice individually in automated activity monitors and measuring spontaneous locomotor activity for two hours. As expected, locomotor activity decreases during the two-hour test as the animals acclimate to a new environment. At all three ages tested, the activity of mutant mice was indistinguishable from wild type mice (Figure 5).
Figure 5.
Parkin−/−DJ-1−/− and Parkin−/−DJ-1−/−Gpx1−/− mice have normal locomotor activity in a novel environment. Spontaneous locomotor activity was measured for separate cohorts of mice at age 6 (A), 12 (B) and 18 months (C), n=6-8 mice per genotype, 8-11 mice per genotype and 9-11 mice per genotype, respectively. For all ages, the mice acclimated to the novel environment of the testing chamber over time, but there are no significant differences between genotypes (one-way ANOVA). Symbols represent the mean ± SEM number of infrared beam breaks in each 5-minute period of the 2-hour test.
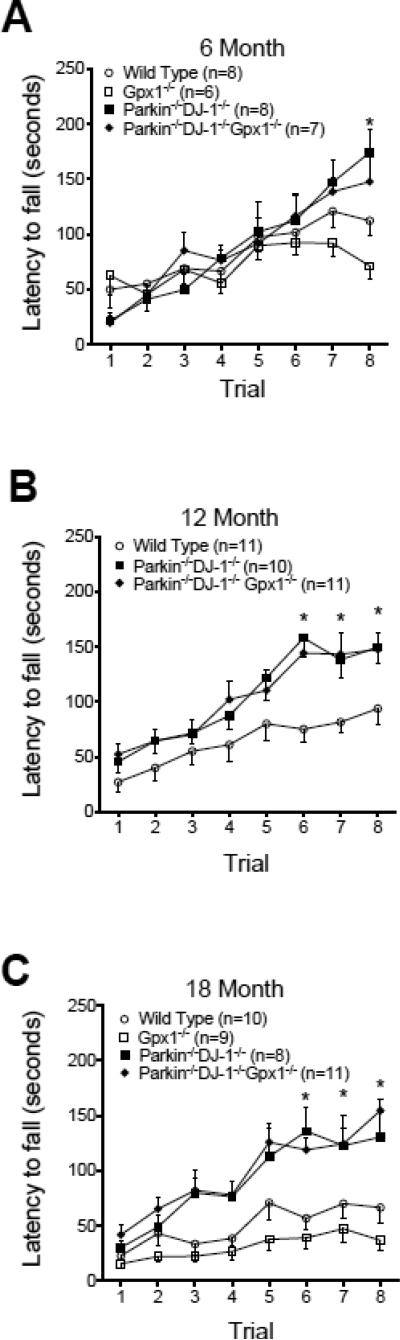
In addition to the locomotor test, we analyzed the behavior of mice on the rotarod test, which measures the ability of mice to stay on top of a rotating horizontal rod as the speed of rotation accelerates from 5 to 45 RPM over 5 minutes. The rotarod test has been used for many years to detect rodent neurological deficits affecting motor coordination and balance (Dunham and Miya, 1957). Separate cohorts of mice were tested at ages 6, 12 and 18 months and the latency to fall off the rotarod was analyzed by two-way repeated measures ANOVA with trial as the repeated measure. All genotypes showed increasing latency over the 8 trials, as expected. Contrary to our expectations, Parkin−/−DJ-1−/− mice and Parkin−/−DJ-1−/−Gpx1−/− mice were able to stay on the rotarod significantly longer than wild-type mice. At age 6 months, there was a trend towards increased latency to fall in Parkin−/−DJ-1−/− and Parkin−/−DJ-1−/−Gpx1−/− mice but this difference was not statistically significant (Figure 6A). However, at ages 12 and 18 months, Parkin−/−DJ-1−/− and Parkin−/−DJ-1−/−Gpx1−/− mice showed increased latency to fall compared to wild type in trials 6, 7 and 8 (p<0.05 at 12 months and p<0.001 at 18 months, two-way repeated measures ANOVA) (Figure 6B, C). There was no significant difference between wild-type and Gpx1−/− mice at any age nor was there an additive effect of Gpx1−/− to Parkin−/−DJ-1−/−. We did not observe differences in anxiety levels measured by open field or elevated plus maze, indicating that altered anxiety cannot explain the rotarod behavior differences (data not shown). These data suggest that Parkin−/−DJ-1−/− and Parkin−/−DJ-1−/−Gpx1−/− mice show age-dependent improvement in rotarod performance compared to Gpx1−/− and wild type mice.
Figure 6.
Parkin−/−DJ-1−/− and Parkin−/−DJ-1−/−Gpx1−/− mice have improved rotarod performance. The latency to fall off an accelerating rotating rod was measured for separate cohorts of mice at age 6 (A), 12 (B) and 18 months (C), n=6-8 mice per genotype, 10-11 mice per genotype and 9-11 mice per genotype, respectively. Symbols represent the mean ± SEM time (seconds) before falling off the rod for each of 8 trials. While all genotypes learned the task over multiple trials, Parkin−/−DJ-1−/− and Parkin−/−DJ- 1−/−Gpx1−/− mice showed a significant increase in the latency to fall compared to wild type at ages 12 and 18 months (**p<0.01, two-way ANOVA, Tukey's post-hoc for both Parkin−/−DJ-1−/− and Parkin−/−DJ-1−/−Gpx1−/−), with genotype and trial as factors.
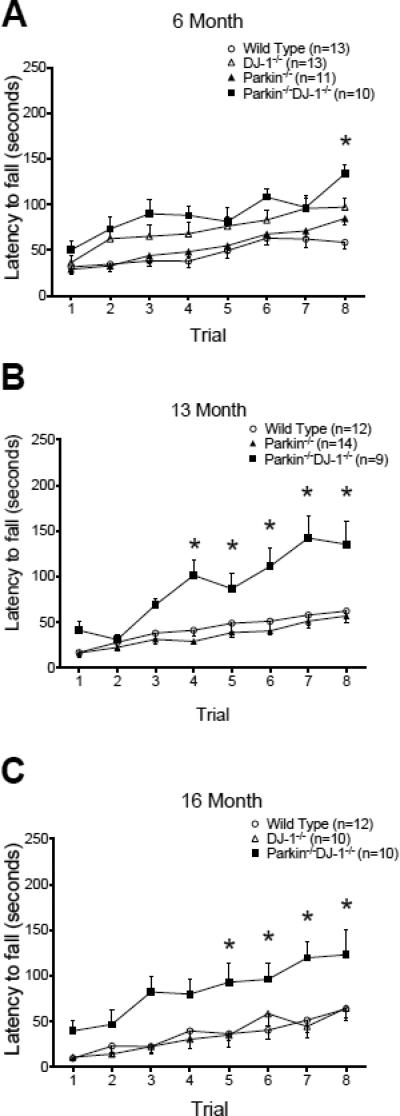
The surprising improvement in rotarod performance of Parkin−/−DJ-1−/− mice and Parkin−/−DJ-1−/−Gpx1−/− prompted us to re-examine the rotarod performance of Parkin−/− mice and DJ-1−/− mice, especially because they had been backcrossed from a hybrid to a pure C57BL/6 genetic background since our previous studies (Goldberg et al., 2003, Goldberg et al., 2005). We compared the rotarod performance of wild-type, Parkin−/−, DJ-1−/− and Parkin−/−DJ-1−/− mice in young (6 month) and aged (13 and 16 month) cohorts (Figure 7A-C). At ages 6, 13 and 16 months, Parkin−/−DJ-1−/− perform significantly better than wild type (p ≤ 0.001, two-way repeated measures ANOVA) (Figure 7B, C). Therefore, the improvement observed in Parkin−/−DJ-1−/− and Parkin−/−DJ-1−/−Gpx1−/− mice may be due to a synergistic effect of DJ-1 and Parkin deficiencies. The effect of Gpx1-deficiency on rotarod performance appears to be negligible because there is no difference in rotarod performance between Parkin−/-DJ-1−/− and Parkin−/−DJ-1−/−Gpx1−/− mice (Figure 6) and no difference in rotarod performance between wild-type mice and Gpx1−/− mice.
Figure 7.
Rotarod behavior of single and double knockout mice. The rotarod performance of three separate cohorts of mice is shown in (A), (B) and (C). 2-way ANOVA with genotype and trial as factors showed a significant effect of trial as all genotypes learned the task and a significant increase in the latency to fall for double knockout mice compared to wild-type mice at all ages (p<0.0001). Asterisks indicate trials with significant differences compared to wild-type mice according to post-hoc analysis. DJ-1−/− mice were significantly different from wild-type mice at age 6 months but not at age 16 months. Parkin−/− mice were not significantly different from wild-type mice at any age.
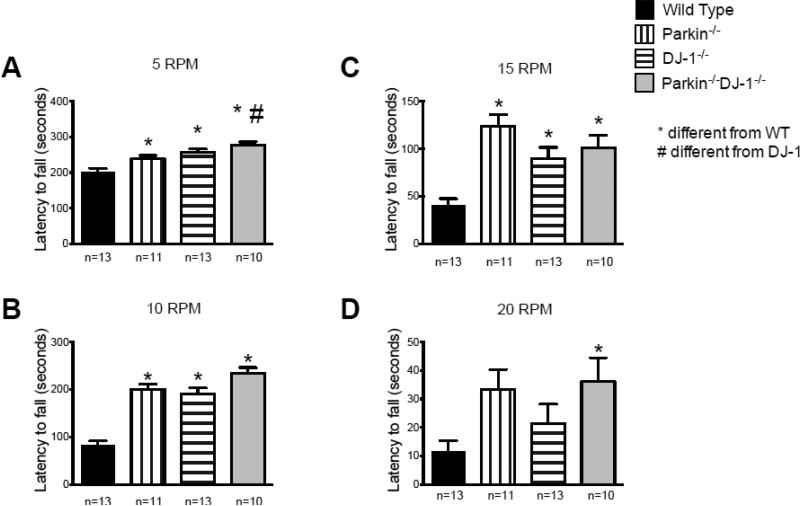
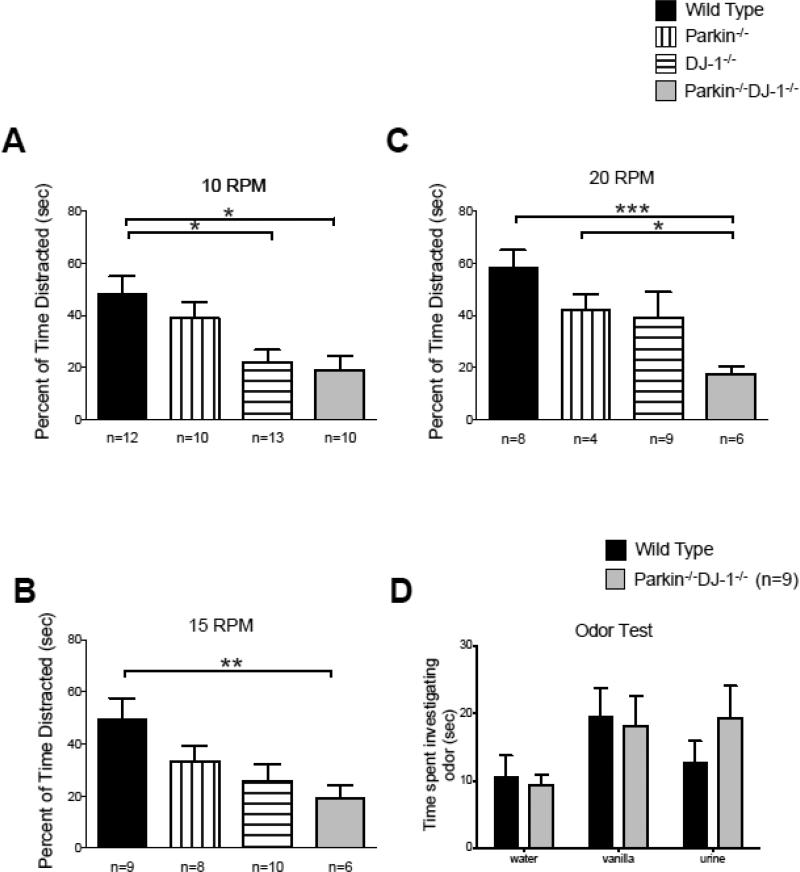
To investigate whether the increased rotarod latencies in Parkin−/−DJ-1−/− mice were due to improved motor skills or non-motor aspects of this test, we measured the latencies of fully trained 8-month-old wild-type and mutant mice to fall off the rotarod at fixed speeds of 5, 10, 15 and 20 RPM for 5 min (Figure 8). Wild-type mice fell off the rotarod significantly faster than Parkin−/−, DJ-1−/− and Parkin−/−DJ-1−/− mice at all speeds tested, including low speeds, such as 5 and 10 RPM, that did not challenge the motor abilities of the mice. We also analyzed video recordings of mice during the fixed-speed rotarod test. It was apparent that all mice could easily perform the task at slow speeds and that the mice that fell off did so upon turning around or exploring the left or right sides of the rod rather than facing forward. An investigator blind to genotype analyzed the videos with a stopwatch and measured the time each mouse was not facing forward, which was considered “distracted” from the task, during the first 30 seconds of the fixed-speed rotarod test. We observed that at 10, 15 and 20 RPM, Parkin−/−DJ-1−/− mice spent significantly less time “distracted” on the rotarod compared to wild type mice (p<0.05-0.001, one-way ANOVA) (Figure 9A-C). At 5 RPM, the same trend was observed, likely accounting for the longer latencies to fall compared to wild-type mice (Figure 8A).
Figure 8.
Improved motor and non-motor skills in Parkin−/−, DJ-1−/− and Parkin−/−DJ-1−/− mice. A cohort of mice at age 8 months was fully trained to perform the rotarod test and then tested at fixed rotarod speeds of 5 (A), 10 (B), 15 (C) and 20 rotations per minute (RPM) (D). Bars represent the mean ± SEM latency to fall off the rotating rod. The single and double knock-out mice showed increased latency to fall compared to wild type (*p<0.0001, one-way ANOVA, Tukey's post-hoc). Parkin−/−DJ-1−/− mice also showed increased latency to fall compared to DJ-1−/− at the lowest speed (A).
Figure 9.
Parkin−/−, DJ-1−/− and Parkin−/−DJ-1−/− mice display less “distraction” behavior compared to wild type mice. Video of the fixed-speed rotarod behavior (Figure 8) was analyzed to measure the percent of time each mouse was not facing straight forward on the rotarod apparatus as a surrogate measure of “distraction” (A-C). Bars show mean ± SEM percent time on the rotarod not facing forward. Parkin−/−DJ-1−/− mice spent more time facing forward at 10 (A), 15 (B), and 20 (C) rotations per minute (RPM) compared to wild type (*p<0.05, **p<0.01, and ***p<0.001, one-way ANOVA, Tukey's post-hoc). (D) Mice were tested for the amount of time spent investigating novel odors of vanilla and urine from unfamiliar mice. Bars represent mean ± SEM time spent investigating the odor during the 3-minute trial. Parkin−/−DJ-1−/− and wild type mice showed comparable olfactory function (one-way ANOVA).
Normal olfactory function in mutant mice
In order to rule out potential sensory deficits as a cause of altered rotarod behavior, we tested mice for olfactory function because one common preclinical symptom of PD is anosmia (Doty et al., 1988, Doty et al., 1992, Pellicano et al., 2007). We hypothesized that decreased olfactory function may cause Parkin−/−DJ-1−/− mice to explore less and consequently show longer latencies to fall off of the rotarod. However, we found no evidence of impaired olfactory function in mutant mice (Figure 9D). These data suggest that the improved rotarod performance of Parkin−/−DJ-1−/− mice is not attributable to a lack of olfactory distractions.
Discussion
In the years since mutations in Parkin and DJ-1 were identified as causes of recessive parkinsonism, we and others have analyzed Parkin−/− mice and DJ-1−/− mice to better understand the normal functions of Parkin and DJ-1 and to delineate the physiological mechanisms by which loss-of-function mutations in Parkin and DJ-1 cause familial PD. However, the absence of PD-relevant neuropathology in Parkin−/− mice and DJ-1−/− mice has impeded efforts to study the process of mutation-induced neurodegeneration and to test neuroprotective therapies in these knockout mice. We sought to overcome these obstacles by crossing Parkin−/− and DJ-1−/− mice together and with Gpx1−/− mice to test whether mutations in Parkin and DJ-1 cause PD by increasing vulnerability to Gpx1 deficiency, which normally occurs with age in human substantia nigra (Venkateshappa et al., 2012) but not in rodent substantia nigra (Benzi et al., 1989). The surprising absence of age-dependent nigral dopamine neuron loss in the substantia nigra of Parkin−/−DJ-1−/−Gpx1−/− mice (Figure 1) strongly suggests that the mechanisms by which loss-of-function mutations in Parkin and DJ-1 cause PD do not require Gpx1 deficiency. Our study also revealed several novel and surprising aspects of Parkin and DJ-1 function, including roles in the regulation of dopamine, serotonin and non-motor behaviors.
There are no reported postmortem examinations of cases of PD linked to DJ-1 mutations, but autopsies of Parkin-linked PD consistently show profound loss of nigral dopaminergic neurons. Evidently, deletion of both Parkin and DJ-1 is not sufficient to induce nigral cell loss in mice because we did not observe significant differences in the number of nigral dopaminergic neurons measured by unbiased stereology (Figure 1). Because Gpx1-deficiency has previously been shown to increase vulnerability to MPTP-induced nigral cell loss (Klivenyi et al., 2000, Zhang et al., 2000), we expected Parkin−/−DJ-1−/−Gpx1−/− mice to have an age-dependent decrease in nigral dopaminergic neurons, resulting in depletion of striatal dopamine. Instead, we observed a significant increase in the levels of striatal dopamine in Parkin−/−DJ-1−/−Gpx1−/− mice, but not in Gpx1−/− or Parkin−/−DJ-1−/− mice (Figure 2). This may be compensation for loss of dopaminergic terminals in the striatum in the absence of nigral neuron loss similar to the retrograde neurodegeneration thought to occur in PD patients (Bernheimer et al., 1973) and observed in mouse models following intrastriatal 6-hydroxydopamine injections or loss of mitofusin2 (Lee et al., 1996, Pham et al., 2012). Alternatively, all three genes may interact to affect dopamine production, trafficking, release, degradation, or pre-synaptic or post-synaptic signaling, resulting in a net increase in steady-state dopamine levels. In support of this, both Parkin and DJ-1 have been found to be located in the membranes of synaptic vesicles (Kubo et al., 2001, Usami et al., 2011) and altered dopamine release, reuptake and synaptic plasticity within the striatum has been observed in Parkin−/− mice and DJ-1−/− mice (Jiang et al., 2004, Goldberg et al., 2005, Kitada et al., 2009). It is possible that elevated striatal dopamine levels compensate for defects in dopaminergic signaling and that without this compensation there would be detectable locomotor behavior deficits if Parkin−/−DJ-1−/−Gpx1−/− mice. Alternatively, it is possible that the increased striatal dopamine causes behavioral phenotypes that are not detected by our tests of locomotor function.
Consistent with our results, triple mutant mice lacking Parkin, DJ-1 and PINK1 show no loss of nigral dopamine neurons but an increase in striatal dopamine levels at 24 months (Kitada et al., 2009). PINK1 acts in the same pathway as Parkin and is believed to recruit Parkin to impaired mitochondria (Narendra et al., 2008). Conceivably, increased mitochondrial free radical production in aged mice lacking Parkin, DJ-1 and PINK1 could mimic the effect of Gpx1-deficiency in our study and explain the similar increase in striatal dopamine. In contrast to other studies of Parkin−/− mice, there is one report of loss of dopaminergic neurons in aged Parkin−/− mice (Rodriguez-Navarro et al., 2007) and one report of nigral cell loss in 10-month-old Parkin conditional knockout mice (Shin et al., 2011), however, these results require independent replication with unbiased stereological sampling. Similarly, in contrast to other studies of DJ-1−/− mice, there is one report of nigral neuron loss in DJ-1−/− mice (Rousseaux et al., 2012), however, this was only observed in a subset of animals and may be due to sparse sampling methods. Our data are otherwise consistent with numerous reports of normal numbers of nigral dopamine neurons in Parkin−/− mice and DJ-1−/− mice (Goldberg et al., 2003, Itier et al., 2003, Palacino et al., 2004, Von Coelln et al., 2004, Fleming et al., 2005, Goldberg et al., 2005, Kim et al., 2005, Perez et al., 2005, Perez and Palmiter, 2005, Fleming and Chesselet, 2006, Sato et al., 2006, Andres-Mateos et al., 2007, Manning-Bog et al., 2007, Yang et al., 2007, Zhu et al., 2007, Chandran et al., 2008, Frank-Cannon et al., 2008, Kitada et al., 2009, Pham et al., 2010, Rousseaux et al., 2012).
At the end stage, PD patients have decreased levels of serotonin (Scatton et al., 1983) and midbrain serotonin transporter (SERT) (Politis et al., 2010a, Politis et al., 2010b, Roselli et al., 2010). However, decreased dopaminergic innervation may result in an initial compensatory increase in serotonergic innervation (Kish et al., 2008). Transgenic mice expressing mutated human alpha-synuclein linked to PD have increased SERT by western blot, suggesting increased serotonin levels (Graham and Sidhu, 2010, Yamakado et al., 2012). We show here that a combined loss of Parkin and DJ-1 results in altered serotonin levels.
Increases in neurotransmitter levels can result from increased neurotransmitter-releasing fibers. We hypothesized that the significant increase in hippocampal serotonin levels in Parkin−/−DJ-1−/− mice is due to an increase in hippocampal serotonergic fibers. Although we observed a trend towards increased hippocampal SERT-positive fibers in DJ-1−/− and Parkin−/−DJ-1−/− mice, they are not significantly different from wild-type and Parkin−/− mice. It is possible that older mice would show a greater difference in SERT-positive fiber staining. However, we believe this to be unlikely because significant differences in neurotransmitter levels are observed as early as 6 months by t-test.
The increased hippocampal serotonin in Parkin−/−DJ-1−/− mice, but not the increased striatal dopamine in Parkin−/−DJ-1−/−Gpx−/− mice, correlates with improved rotarod performance. Mice with serotonergic system disruption have decreased performance on the rotarod test (Holmes et al., 2002, Morelli et al., 2011) and changes in serotonin levels have been shown to alter cognitive abilities, such as attention and learning (Buhot, 1997). Therefore, it is possible that the altered rotarod behavior is related to the increased hippocampal serotonin.
Rotarod performance is routinely used to assess motor function in rodent models of neurodegenerative disease. Our data indicate that mice deficient for Parkin or DJ-1 do not have motor impairment but instead show improved performance on the rotarod task compared to controls (Figure 7). We determined that the increase in rotarod performance cannot be explained by an overall increase in activity because spontaneous locomotor behavior was unchanged in Parkin−/−DJ-1−/− mice compared to controls (Figure 5). Previous studies have shown that other factors can contribute to rotarod performance, such as body weight or sensory abilities (McFadyen et al., 2003). However, Parkin−/−DJ-1−/− mice do not exhibit changes in olfactory function, a common non-motor symptom of PD. Differences in weight are also unlikely to account for differences in rotarod behavior because wild type mice are, on average, larger than all other genotypes but perform no differently from Parkin−/− and DJ-1−/− mice despite the difference in weight.
We have rigorously tested the hypothesis that loss of Gpx1 is instrumental in the development of PD symptoms. While adaptive changes in brain antioxidants may indeed be neuroprotective, our results demonstrate that preventing these changes by genetic disruption of Gpx1 is not sufficient to induce nigral neuron loss in mice deficient for Parkin and DJ-1. In fact, our results indicate that Parkin−/−DJ-1−/− and Parkin−/−DJ-1−/−Gpx1−/− mice exhibit neurochemical and behavioral phenotypes that are contrary to the expected parkinsonian phenotype. Our study is the first to demonstrate that Parkin and DJ-1 mutations affect striatal serotonin levels and rotarod behavior in mice. Additionally, deficiency for all three genes causes a significant increase in striatal dopamine. We speculate that the increased striatal dopamine might be an early-stage manifestation of nigrostriatal dysfunction induced by these mutations in mice and suggest that this has important implications for studies of PD pathogenesis and for efforts to develop neuroprotective therapies.
Highlights.
We analyzed mice deficient for Parkin, DJ-1 and glutathione peroxidase
Parkin, DJ-1, Gpx1 triple mutant mice have increased striatal dopamine
Parkin, DJ-1 double mutant mice have increased striatal and hippocampal serotonin
Mice with increased serotonin also have improved rotarod behavior
The improved rotarod performance may be due to non-motor behavior changes
Acknowledgements
We thank Dr. Shari Birnbaum and the staff of the UTSW Rodent Behavior Core Facility for assistance with behavioral tests. Generous support from the following sources is gratefully acknowledged: American Parkinson Disease Association, NIH Training Grant 5T32GM00820322 from the National Institute of General Medical Sciences, the David M. Crowley Foundation and Parkinson's Benefactors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andres-Mateos E, Perier C, Zhang L, Blanchard-Fillion B, Greco TM, Thomas B, Ko HS, Sasaki M, Ischiropoulos H, Przedborski S, Dawson TM, Dawson VL. DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc Natl Acad Sci U S A. 2007;104:14807–14812. doi: 10.1073/pnas.0703219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensadoun JC, Mirochnitchenko O, Inouye M, Aebischer P, Zurn AD. Attenuation of 6-OHDA-induced neurotoxicity in glutathione peroxidase transgenic mice. Eur J Neurosci. 1998;10:3231–3236. doi: 10.1046/j.1460-9568.1998.00345.x. [DOI] [PubMed] [Google Scholar]
- Benzi G, Pastoris O, Marzatico F, Villa RF. Cerebral enzyme antioxidant system. Influence of aging and phosphatidylcholine. J Cereb Blood Flow Metab. 1989;9:373–380. doi: 10.1038/jcbfm.1989.56. [DOI] [PubMed] [Google Scholar]
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Bian M, Liu J, Hong X, Yu M, Huang Y, Sheng Z, Fei J, Huang F. Overexpression of parkin ameliorates dopaminergic neurodegeneration induced by 1- methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. PLoS One. 2012;7:e39953. doi: 10.1371/journal.pone.0039953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifati V. Genetics of parkinsonism. Parkinsonism Relat Disord. 2007;13(Suppl 3):S233–241. doi: 10.1016/S1353-8020(08)70008-7. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Buhot MC. Serotonin receptors in cognitive behaviors. Curr Opin Neurobiol. 1997;7:243–254. doi: 10.1016/s0959-4388(97)80013-x. [DOI] [PubMed] [Google Scholar]
- Canet-Aviles RM, Wilson MA, Miller DW, Ahmad R, McLendon C, Bandyopadhyay S, Baptista MJ, Ringe D, Petsko GA, Cookson MR. The Parkinson's disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci U S A. 2004;101:9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran JS, Lin X, Zapata A, Hoke A, Shimoji M, Moore SO, Galloway MP, Laird FM, Wong PC, Price DL, Bailey KR, Crawley JN, Shippenberg T, Cai H. Progressive behavioral deficits in DJ-1-deficient mice are associated with normal nigrostriatal function. Neurobiol Dis. 2008;29:505–514. doi: 10.1016/j.nbd.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Sullards MC, Olzmann JA, Rees HD, Weintraub ST, Bostwick DE, Gearing M, Levey AI, Chin LS, Li L. Oxidative Damage of DJ-1 Is Linked to Sporadic Parkinson and Alzheimer Diseases. J Biol Chem. 2006;281:10816–10824. doi: 10.1074/jbc.M509079200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson MR. DJ-1, PINK1, and their effects on mitochondrial pathways. Mov Disord. 2010;25(Suppl 1):S44–48. doi: 10.1002/mds.22713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti O, Lesage S, Brice A. What genetics tells us about the causes and mechanisms of Parkinson's disease. Physiol Rev. 2011;91:1161–1218. doi: 10.1152/physrev.00022.2010. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Ko HS, Dawson VL. Genetic animal models of Parkinson's disease. Neuron. 2010;66:646–661. doi: 10.1016/j.neuron.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL, Deems DA, Stellar S. Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology. 1988;38:1237–1244. doi: 10.1212/wnl.38.8.1237. [DOI] [PubMed] [Google Scholar]
- Doty RL, Stern MB, Pfeiffer C, Gollomp SM, Hurtig HI. Bilateral olfactory dysfunction in early stage treated and untreated idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1992;55:138–142. doi: 10.1136/jnnp.55.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham NW, Miya TS. A note on a simple apparatus for detecting neurological deficit in rats and mice. J Am Pharm Assoc Am Pharm Assoc (Baltim) 1957;46:208–209. doi: 10.1002/jps.3030460322. [DOI] [PubMed] [Google Scholar]
- Fleming SM, Chesselet MF. Behavioral phenotypes and pharmacology in genetic mouse models of Parkinsonism. Behav Pharmacol. 2006;17:383–391. doi: 10.1097/00008877-200609000-00004. [DOI] [PubMed] [Google Scholar]
- Fleming SM, Fernagut PO, Chesselet MF. Genetic mouse models of parkinsonism: strengths and limitations. NeuroRx. 2005;2:495–503. doi: 10.1602/neurorx.2.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank-Cannon TC, Tran T, Ruhn KA, Martinez TN, Hong J, Marvin M, Hartley M, Trevino I, O'Brien DE, Casey B, Goldberg MS, Tansey MG. Parkin deficiency increases vulnerability to inflammation-related nigral degeneration. J Neurosci. 2008;28:10825–10834. doi: 10.1523/JNEUROSCI.3001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MS, Fleming SM, Palacino JJ, Cepeda C, Lam HA, Bhatnagar A, Meloni EG, Wu N, Ackerson LC, Klapstein GJ, Gajendiran M, Roth BL, Chesselet MF, Maidment NT, Levine MS, Shen J. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J Biol Chem. 2003;278:43628–43635. doi: 10.1074/jbc.M308947200. [DOI] [PubMed] [Google Scholar]
- Goldberg MS, Pisani A, Haburcak M, Vortherms TA, Kitada T, Costa C, Tong Y, Martella G, Tscherter A, Martins A, Bernardi G, Roth BL, Pothos EN, Calabresi P, Shen J. Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial Parkinsonism-linked gene DJ-1. Neuron. 2005;45:489–496. doi: 10.1016/j.neuron.2005.01.041. [DOI] [PubMed] [Google Scholar]
- Graham DR, Sidhu A. Mice expressing the A53T mutant form of human alpha-synuclein exhibit hyperactivity and reduced anxiety-like behavior. J Neurosci Res. 2010;88:1777–1783. doi: 10.1002/jnr.22331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori N. Autosomal dominant parkinsonism: its etiologies and differential diagnoses. Parkinsonism Relat Disord 18 Suppl. 2012;1:S1–3. doi: 10.1016/S1353-8020(11)70003-7. [DOI] [PubMed] [Google Scholar]
- Hauser DN, Hastings TG. Mitochondrial dysfunction and oxidative stress in Parkinson's disease and monogenic parkinsonism. Neurobiol Dis. 2013;51:35–42. doi: 10.1016/j.nbd.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Ishimori C, Takahashi-Niki K, Taira T, Kim YC, Maita H, Maita C, Ariga H, Iguchi-Ariga SM. DJ-1 binds to mitochondrial complex I and maintains its activity. Biochem Biophys Res Commun. 2009;390:667–672. doi: 10.1016/j.bbrc.2009.10.025. [DOI] [PubMed] [Google Scholar]
- Holmes A, Murphy DL, Crawley JN. Reduced aggression in mice lacking the serotonin transporter. Psychopharmacology (Berl) 2002;161:160–167. doi: 10.1007/s00213-002-1024-3. [DOI] [PubMed] [Google Scholar]
- Horowitz MP, Greenamyre JT. Gene-environment interactions in Parkinson's disease: the importance of animal modeling. Clin Pharmacol Ther. 2010;88:467–474. doi: 10.1038/clpt.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itier JM, Ibanez P, Mena MA, Abbas N, Cohen-Salmon C, Bohme GA, Laville M, Pratt J, Corti O, Pradier L, Ret G, Joubert C, Periquet M, Araujo F, Negroni J, Casarejos MJ, Canals S, Solano R, Serrano A, Gallego E, Sanchez M, Denefle P, Benavides J, Tremp G, Rooney TA, Brice A, Garcia de Yebenes J. Parkin gene inactivation alters behaviour and dopamine neurotransmission in the mouse. Hum Mol Genet. 2003;12:2277–2291. doi: 10.1093/hmg/ddg239. [DOI] [PubMed] [Google Scholar]
- Jiang H, Jiang Q, Feng J. Parkin increases dopamine uptake by enhancing the cell surface expression of dopamine transporter. J Biol Chem. 2004;279:54380–54386. doi: 10.1074/jbc.M409282200. [DOI] [PubMed] [Google Scholar]
- Junn E, Jang WH, Zhao X, Jeong BS, Mouradian MM. Mitochondrial localization of DJ-1 leads to enhanced neuroprotection. J Neurosci Res. 2009;87:123–129. doi: 10.1002/jnr.21831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawajiri S, Saiki S, Sato S, Sato F, Hatano T, Eguchi H, Hattori N. PINK1 is recruited to mitochondria with parkin and associates with LC3 in mitophagy. FEBS Lett. 2010;584:1073–1079. doi: 10.1016/j.febslet.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Kim RH, Smith PD, Aleyasin H, Hayley S, Mount MP, Pownall S, Wakeham A, You-Ten AJ, Kalia SK, Horne P, Westaway D, Lozano AM, Anisman H, Park DS, Mak TW. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc Natl Acad Sci U S A. 2005;102:5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish SJ, Tong J, Hornykiewicz O, Rajput A, Chang LJ, Guttman M, Furukawa Y. Preferential loss of serotonin markers in caudate versus putamen in Parkinson's disease. Brain. 2008;131:120–131. doi: 10.1093/brain/awm239. [DOI] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- Kitada T, Tong Y, Gautier CA, Shen J. Absence of nigral degeneration in aged parkin/DJ-1/PINK1 triple knockout mice. J Neurochem. 2009;111:696–702. doi: 10.1111/j.1471-4159.2009.06350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klivenyi P, Andreassen OA, Ferrante RJ, Dedeoglu A, Mueller G, Lancelot E, Bogdanov M, Andersen JK, Jiang D, Beal MF. Mice deficient in cellular glutathione peroxidase show increased vulnerability to malonate, 3-nitropropionic acid, and 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. J Neurosci. 2000;20:1–7. doi: 10.1523/JNEUROSCI.20-01-00001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo SI, Kitami T, Noda S, Shimura H, Uchiyama Y, Asakawa S, Minoshima S, Shimizu N, Mizuno Y, Hattori N. Parkin is associated with cellular vesicles. J Neurochem. 2001;78:42–54. doi: 10.1046/j.1471-4159.2001.00364.x. [DOI] [PubMed] [Google Scholar]
- Kuhn K, Zhu XR, Lubbert H, Stichel CC. Parkin expression in the developing mouse. Brain Res Dev Brain Res. 2004;149:131–142. doi: 10.1016/j.devbrainres.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Lee CS, Sauer H, Bjorklund A. Dopaminergic neuronal degeneration and motor impairments following axon terminal lesion by instrastriatal 6-hydroxydopamine in the rat. Neuroscience. 1996;72:641–653. doi: 10.1016/0306-4522(95)00571-4. [DOI] [PubMed] [Google Scholar]
- Lev N, Ickowicz D, Melamed E, Offen D. Oxidative insults induce DJ-1 upregulation and redistribution: Implications for neuroprotection. Neurotoxicology. 2008;29:397–405. doi: 10.1016/j.neuro.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Liddell JR, Robinson SR, Dringen R, Bishop GM. Astrocytes retain their antioxidant capacity into advanced old age. Glia. 2010;58:1500–1509. doi: 10.1002/glia.21024. [DOI] [PubMed] [Google Scholar]
- Lo Bianco C, Schneider BL, Bauer M, Sajadi A, Brice A, Iwatsubo T, Aebischer P. Lentiviral vector delivery of parkin prevents dopaminergic degeneration in an alpha-synuclein rat model of Parkinson's disease. Proc Natl Acad Sci U S A. 2004;101:17510–17515. doi: 10.1073/pnas.0405313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez G, Sidransky E. Autosomal recessive mutations in the development of Parkinson's disease. Biomark Med. 2010;4:713–721. doi: 10.2217/bmm.10.96. [DOI] [PubMed] [Google Scholar]
- Manning-Bog AB, Caudle WM, Perez XA, Reaney SH, Paletzki R, Isla MZ, Chou VP, McCormack AL, Miller GW, Langston JW, Gerfen CR, Dimonte DA. Increased vulnerability of nigrostriatal terminals in DJ-1-deficient mice is mediated by the dopamine transporter. Neurobiol Dis. 2007;27:141–150. doi: 10.1016/j.nbd.2007.03.014. [DOI] [PubMed] [Google Scholar]
- McFadyen MP, Kusek G, Bolivar VJ, Flaherty L. Differences among eight inbred strains of mice in motor ability and motor learning on a rotorod. Genes Brain Behav. 2003;2:214–219. doi: 10.1034/j.1601-183x.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- Menzies FM, Yenisetti SC, Min KT. Roles of Drosophila DJ-1 in survival of dopaminergic neurons and oxidative stress. Curr Biol. 2005;15:1578–1582. doi: 10.1016/j.cub.2005.07.036. [DOI] [PubMed] [Google Scholar]
- Meulener M, Whitworth AJ, Armstrong-Gold CE, Rizzu P, Heutink P, Wes PD, Pallanck LJ, Bonini NM. Drosophila DJ-1 mutants are selectively sensitive to environmental toxins associated with Parkinson's disease. Curr Biol. 2005;15:1572–1577. doi: 10.1016/j.cub.2005.07.064. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Zhang L, Troncoso J, Lee MK, Hattori N, Mizuno Y, Dawson TM, Dawson VL. Association of DJ-1 and parkin mediated by pathogenic DJ-1 mutations and oxidative stress. Hum Mol Genet. 2005;14:71–84. doi: 10.1093/hmg/ddi007. [DOI] [PubMed] [Google Scholar]
- Morelli E, Moore H, Rebello TJ, Gray N, Steele K, Esposito E, Gingrich JA, Ansorge MS. Chronic 5-HT transporter blockade reduces DA signaling to elicit basal ganglia dysfunction. J Neurosci. 2011;31:15742–15750. doi: 10.1523/JNEUROSCI.2989-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muftuoglu M, Elibol B, Dalmizrak O, Ercan A, Kulaksiz G, Ogus H, Dalkara T, Ozer N. Mitochondrial complex I and IV activities in leukocytes from patients with parkin mutations. Mov Disord. 2004;19:544–548. doi: 10.1002/mds.10695. [DOI] [PubMed] [Google Scholar]
- Mullett SJ, Hinkle DA. DJ-1 deficiency in astrocytes selectively enhances mitochondrial Complex I inhibitor-induced neurotoxicity. J Neurochem. 2011;117:375–387. doi: 10.1111/j.1471-4159.2011.07175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacino JJ, Sagi D, Goldberg MS, Krauss S, Motz C, Wacker M, Klose J, Shen J. Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J Biol Chem. 2004;279:18614–18622. doi: 10.1074/jbc.M401135200. [DOI] [PubMed] [Google Scholar]
- Paterna JC, Leng A, Weber E, Feldon J, Bueler H. DJ-1 and Parkin modulate dopamine-dependent behavior and inhibit MPTP-induced nigral dopamine neuron loss in mice. Mol Ther. 2007;15:698–704. doi: 10.1038/sj.mt.6300067. [DOI] [PubMed] [Google Scholar]
- Pellicano C, Benincasa D, Pisani V, Buttarelli FR, Giovannelli M, Pontieri FE. Prodromal non-motor symptoms of Parkinson's disease. Neuropsychiatr Dis Treat. 2007;3:145–152. doi: 10.2147/nedt.2007.3.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez FA, Curtis WR, Palmiter RD. Parkin-deficient mice are not more sensitive to 6-hydroxydopamine or methamphetamine neurotoxicity. BMC Neurosci. 2005;6:71. doi: 10.1186/1471-2202-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez FA, Palmiter RD. Parkin-deficient mice are not a robust model of parkinsonism. Proc Natl Acad Sci U S A. 2005;102:2174–2179. doi: 10.1073/pnas.0409598102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham AH, Meng S, Chu QN, Chan DC. Loss of Mfn2 results in progressive, retrograde degeneration of dopaminergic neurons in the nigrostriatal circuit. Hum Mol Genet. 2012;21:4817–4826. doi: 10.1093/hmg/dds311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TT, Giesert F, Rothig A, Floss T, Kallnik M, Weindl K, Holter SM, Ahting U, Prokisch H, Becker L, Klopstock T, Hrabe de Angelis M, Beyer K, Gorner K, Kahle PJ, Vogt Weisenhorn DM, Wurst W. DJ-1-deficient mice show less TH-positive neurons in the ventral tegmental area and exhibit non-motoric behavioural impairments. Genes Brain Behav. 2010;9:305–317. doi: 10.1111/j.1601-183X.2009.00559.x. [DOI] [PubMed] [Google Scholar]
- Politis M, Wu K, Loane C, Kiferle L, Molloy S, Brooks DJ, Piccini P. Staging of serotonergic dysfunction in Parkinson's disease: an in vivo 11C-DASB PET study. Neurobiol Dis. 2010a;40:216–221. doi: 10.1016/j.nbd.2010.05.028. [DOI] [PubMed] [Google Scholar]
- Politis M, Wu K, Loane C, Turkheimer FE, Molloy S, Brooks DJ, Piccini P. Depressive symptoms in PD correlate with higher 5-HTT binding in raphe and limbic structures. Neurology. 2010b;75:1920–1927. doi: 10.1212/WNL.0b013e3181feb2ab. [DOI] [PubMed] [Google Scholar]
- Ridet JL, Bensadoun JC, Deglon N, Aebischer P, Zurn AD. Lentivirus-mediated expression of glutathione peroxidase: neuroprotection in murine models of Parkinson's disease. Neurobiol Dis. 2006;21:29–34. doi: 10.1016/j.nbd.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Navarro JA, Casarejos MJ, Menendez J, Solano RM, Rodal I, Gomez A, Yebenes JG, Mena MA. Mortality, oxidative stress and tau accumulation during ageing in parkin null mice. J Neurochem. 2007;103:98–114. doi: 10.1111/j.1471-4159.2007.04762.x. [DOI] [PubMed] [Google Scholar]
- Roselli F, Pisciotta NM, Pennelli M, Aniello MS, Gigante A, De Caro MF, Ferrannini E, Tartaglione B, Niccoli-Asabella A, Defazio G, Livrea P, Rubini G. Midbrain SERT in degenerative parkinsonisms: a 123I-FP-CIT SPECT study. Mov Disord. 2010;25:1853–1859. doi: 10.1002/mds.23179. [DOI] [PubMed] [Google Scholar]
- Rousseaux MW, Marcogliese PC, Qu D, Hewitt SJ, Seang S, Kim RH, Slack RS, Schlossmacher MG, Lagace DC, Mak TW, Park DS. Progressive dopaminergic cell loss with unilateral-to-bilateral progression in a genetic model of Parkinson disease. Proc Natl Acad Sci U S A. 2012;109:15918–15923. doi: 10.1073/pnas.1205102109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Chiba T, Nishiyama S, Kakiuchi T, Tsukada H, Hatano T, Fukuda T, Yasoshima Y, Kai N, Kobayashi K, Mizuno Y, Tanaka K, Hattori N. Decline of striatal dopamine release in parkin-deficient mice shown by ex vivo autoradiography. J Neurosci Res. 2006;84:1350–1357. doi: 10.1002/jnr.21032. [DOI] [PubMed] [Google Scholar]
- Scatton B, Javoy-Agid F, Rouquier L, Dubois B, Agid Y. Reduction of cortical dopamine, noradrenaline, serotonin and their metabolites in Parkinson's disease. Brain Res. 1983;275:321–328. doi: 10.1016/0006-8993(83)90993-9. [DOI] [PubMed] [Google Scholar]
- Shang H, Lang D, Jean-Marc B, Kaelin-Lang A. Localization of DJ-1 mRNA in the mouse brain. Neurosci Lett. 2004;367:273–277. doi: 10.1016/j.neulet.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Shimura H, Hattori N, Kubo S, Mizuno Y, Asakawa S, Minoshima S, Shimizu N, Iwai K, Chiba T, Tanaka K, Suzuki T. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet. 2000;25:302–305. doi: 10.1038/77060. [DOI] [PubMed] [Google Scholar]
- Shimura H, Hattori N, Kubo S, Yoshikawa M, Kitada T, Matsumine H, Asakawa S, Minoshima S, Yamamura Y, Shimizu N, Mizuno Y. Immunohistochemical and subcellular localization of Parkin protein: absence of protein in autosomal recessive juvenile parkinsonism patients. Ann Neurol. 1999;45:668–672. doi: 10.1002/1531-8249(199905)45:5<668::aid-ana19>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Shin JH, Ko HS, Kang H, Lee Y, Lee YI, Pletinkova O, Troconso JC, Dawson VL, Dawson TM. PARIS (ZNF746) repression of PGC-1alpha contributes to neurodegeneration in Parkinson's disease. Cell. 2011;144:689–702. doi: 10.1016/j.cell.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman JM, De Jager PL, Feany MB. Parkinson's disease: genetics and pathogenesis. Annu Rev Pathol. 2011;6:193–222. doi: 10.1146/annurev-pathol-011110-130242. [DOI] [PubMed] [Google Scholar]
- Stichel CC, Augustin M, Kuhn K, Zhu XR, Engels P, Ullmer C, Lubbert H. Parkin expression in the adult mouse brain. Eur J Neurosci. 2000;12:4181–4194. [PubMed] [Google Scholar]
- Taira T, Saito Y, Niki T, Iguchi-Ariga SM, Takahashi K, Ariga H. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KJ, McCoy MK, Blackinton J, Beilina A, van der Brug M, Sandebring A, Miller D, Maric D, Cedazo-Minguez A, Cookson MR. DJ-1 acts in parallel to the PINK1/parkin pathway to control mitochondrial function and autophagy. Hum Mol Genet. 2011;20:40–50. doi: 10.1093/hmg/ddq430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulusoy A, Kirik D. Can overexpression of parkin provide a novel strategy for neuroprotection in Parkinson's disease? Exp Neurol. 2008;212:258–260. doi: 10.1016/j.expneurol.2008.04.026. [DOI] [PubMed] [Google Scholar]
- Usami Y, Hatano T, Imai S, Kubo S, Sato S, Saiki S, Fujioka Y, Ohba Y, Sato F, Funayama M, Eguchi H, Shiba K, Ariga H, Shen J, Hattori N. DJ-1 associates with synaptic membranes. Neurobiol Dis. 2011;43:651–662. doi: 10.1016/j.nbd.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Varcin M, Bentea E, Michotte Y, Sarre S. Oxidative stress in genetic mouse models of Parkinson's disease. Oxid Med Cell Longev. 2012;2012:624925. doi: 10.1155/2012/624925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkateshappa C, Harish G, Mythri RB, Mahadevan A, Bharath MM, Shankar SK. Increased oxidative damage and decreased antioxidant function in aging human substantia nigra compared to striatum: implications for Parkinson's disease. Neurochem Res. 2012;37:358–369. doi: 10.1007/s11064-011-0619-7. [DOI] [PubMed] [Google Scholar]
- Vercammen L, Van der Perren A, Vaudano E, Gijsbers R, Debyser Z, Van den Haute C, Baekelandt V. Parkin protects against neurotoxicity in the 6-hydroxydopamine rat model for Parkinson's disease. Mol Ther. 2006;14:716–723. doi: 10.1016/j.ymthe.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Vincent A, Briggs L, Chatwin GF, Emery E, Tomlins R, Oswald M, Middleton CA, Evans GJ, Sweeney ST, Elliott CJ. parkin-induced defects in neurophysiology and locomotion are generated by metabolic dysfunction and not oxidative stress. Hum Mol Genet. 2012;21:1760–1769. doi: 10.1093/hmg/ddr609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincow ES, Merrihew G, Thomas RE, Shulman NJ, Beyer RP, Maccoss MJ, Pallanck LJ. The PINK1-Parkin pathway promotes both mitophagy and selective respiratory chain turnover in vivo. Proc Natl Acad Sci U S A. 2013;110:6400–6405. doi: 10.1073/pnas.1221132110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinish M, Anand A, Prabhakar S. Altered oxidative stress levels in Indian Parkinson's disease patients with PARK2 mutations. Acta Biochim Pol. 2011;58:165–169. [PubMed] [Google Scholar]
- Von Coelln R, Thomas B, Savitt JM, Lim KL, Sasaki M, Hess EJ, Dawson VL, Dawson TM. Loss of locus coeruleus neurons and reduced startle in parkin null mice. Proc Natl Acad Sci U S A. 2004;101:10744–10749. doi: 10.1073/pnas.0401297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Zhuang X, Chen L. DJ-1 mRNA anatomical localization and cell type identification in the mouse brain. Neurosci Lett. 2009;465:214–219. doi: 10.1016/j.neulet.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakado H, Moriwaki Y, Yamasaki N, Miyakawa T, Kurisu J, Uemura K, Inoue H, Takahashi M, Takahashi R. alpha-Synuclein BAC transgenic mice as a model for Parkinson's disease manifested decreased anxiety-like behavior and hyperlocomotion. Neurosci Res. 2012;73:173–177. doi: 10.1016/j.neures.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Yang W, Chen L, Ding Y, Zhuang X, Kang UJ. Paraquat induces dopaminergic dysfunction and proteasome impairment in DJ-1-deficient mice. Hum Mol Genet. 2007;16:2900–2910. doi: 10.1093/hmg/ddm249. [DOI] [PubMed] [Google Scholar]
- Yang Y, Gehrke S, Haque ME, Imai Y, Kosek J, Yang L, Beal MF, Nishimura I, Wakamatsu K, Ito S, Takahashi R, Lu B. Inactivation of Drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/Akt signaling. Proc Natl Acad Sci U S A. 2005;102:13670–13675. doi: 10.1073/pnas.0504610102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T, Sugawara K, Ito K, Takahashi R, Ariga H, Mizusawa H. Down regulation of DJ-1 enhances cell death by oxidative stress, ER stress, and proteasome inhibition. Biochem Biophys Res Commun. 2003;312:1342–1348. doi: 10.1016/j.bbrc.2003.11.056. [DOI] [PubMed] [Google Scholar]
- Zhang J, Graham DG, Montine TJ, Ho YS. Enhanced N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity in mice deficient in CuZn-superoxide dismutase or glutathione peroxidase. J Neuropathol Exp Neurol. 2000;59:53–61. doi: 10.1093/jnen/59.1.53. [DOI] [PubMed] [Google Scholar]
- Zhang L, Shimoji M, Thomas B, Moore DJ, Yu SW, Marupudi NI, Torp R, Torgner IA, Ottersen OP, Dawson TM, Dawson VL. Mitochondrial localization of the Parkinson's disease related protein DJ-1: implications for pathogenesis. Hum Mol Genet. 2005;14:2063–2073. doi: 10.1093/hmg/ddi211. [DOI] [PubMed] [Google Scholar]
- Zhou W, Freed CR. DJ-1 up-regulates glutathione synthesis during oxidative stress and inhibits A53T alpha-synuclein toxicity. J Biol Chem. 2005;280:43150–43158. doi: 10.1074/jbc.M507124200. [DOI] [PubMed] [Google Scholar]
- Zhu XR, Maskri L, Herold C, Bader V, Stichel CC, Gunturkun O, Lubbert H. Non-motor behavioural impairments in parkin-deficient mice. Eur J Neurosci. 2007;26:1902–1911. doi: 10.1111/j.1460-9568.2007.05812.x. [DOI] [PubMed] [Google Scholar]