Abstract
Multiple myeloma (MM) remains an incurable illness affecting nearly 20,000 individuals in the United States per year. High-dose melphalan (HDM) with autologous hematopoietic stem cell support (ASCT) is one of the mainstays of therapy for younger patients, but little advancement has been made with regards to conditioning regimens. We opted to combine 153Samarium ethylenediaminetetramethylenephosphonate (153Sm-EDTMP), a radiopharmaceutical approved for the palliation of pain caused by metastatic bone lesions, with HDM and ASCT in a phase II study. Individualized doses of 153Sm were based on dosimetry and were calculated to deliver 40 Gy to the bone marrow. The therapeutic dose of 153Sm-EDTMP was followed by HDM and ASCT. Forty-six patients with newly diagnosed or relapsed disease were treated. Study patients were compared to 102 contemporaneously patients treated with HDM and ASCT. Fifty-nine percent of study patients achieved a very good partial response or better. With a median follow-up of 7.1 years, the median overall survival and progression free survival from study registration was 6.2 years (95%CI 4.6–7.5 years) and 1.5 years (1.1–2.2 years), respectively, which compared favorably to contemporaneously treated non-study patients. Addition of high-dose 153Sm EDTMP to melphalan conditioning appears to be safe, well tolerated and worthy of further study in the context of novel agents and in the phase III setting.
Keywords: multiple myeloma, radiation, samarium, Quadramet®, bone marrow, stem cell transplant
Introduction
Multiple myeloma (MM) is a chemotherapy-responsive, but incurable disease. Prior to the advent of proteosome inhibitors and immune modulatory drugs, standard chemotherapy provided response rates of only 50–70% with a median survival of 30–40 months.(1, 2) The observation that dose intensification of melphalan resulted in higher response rates, even in patients who were refractory to standard dose melphalan (3) summoned the era of high dose chemotherapy with stem cell transplantation (SCT).(4, 5) Prior to incorporation of novel therapies into induction regimens, application of either single or tandem autologous SCT have provided complete response rates ranging from 19 to 44%, but all patients eventually relapsed. (4–7) The lack of survival benefit with transplants utilizing CD 34 selected autologous stem cells(8) and the high incidence of relapse after allogeneic SCT (9, 10) reflect the inadequacy of the current conditioning regimens for eradicating the neoplastic clone in vivo.
153Samarium ethylenediaminetetramethylenephosphonate (153Sm-EDTMP; Quadramet®) is a radiopharmaceutical approved for the palliation of pain caused by metastatic bone lesions. The compound consists of 153Sm, a beta emitter of medium energy with a physical half-life of 46.3 hours, and EDTMP, a diphosphonate compound, that avidly concentrates in bone. It is chemically and biologically stable and is rapidly cleared from non-osseous tissues (alpha t1/2 14 minutes; beta t1/2 11.5 hours). 153Sm-EDTMP has a beta emission range of 0.5 to 1.01 mm and emits a 103 keV gamma photon allowing for gamma camera imaging studies of the distribution of the radionuclide.(11–14) Use of low dose 153Sm-EDTMP in humans has demonstrated an excellent therapeutic ratio in carcinoma metastatic to bone (14–18) with myelosuppression as the major dose limiting toxicity. We aspired to exploit this “toxicity” in the context of a hematologic malignancy in the SCT setting as has been done in murine models. (19) We sought to capitalize on three observations: 1) myeloma cells are radiosensitive (20, 21); 2) melphalan 200 mg/m2 is as—if not more—effective as melphalan 140 mg/m2 with external beam total body irradiation;(22) and 3) 153Sm-EDTMP provides irradiation to the marrow since it localizes to bone and bony trabeculae.(18, 23, 24)
In our phase I study, we found that the standard myeloablative dose of melphalan could be administered safely with escalating doses of 153Sm-EDTMP. (25) Twelve patients were treated with escalating doses of 153Sm-EDTMP (N=3/group; 6, 12, 19.8, and 30 mCi/kg) and a fixed dose of melphalan (200 mg/m2). No dose limiting toxicity was seen. To better standardize the marrow compartment radiation dose, the study was modified such that an additional 6 patients were treated at a targeted absorbed radiation dose to the red marrow of 40 Gy based on a trace labeled infusion one week prior to the therapy. No dose limiting toxicity was seen, and overall response rate was 94% including 7 very good partial responses and 5 complete responses. Addition of 153Sm EDTMP to melphalan conditioning appeared to be safe, well tolerated, and worthy of further study.
Methods
Patient selection
Patients with multiple myeloma referred to the Mayo Clinic (Rochester, MN) for high dose chemotherapy with peripheral blood stem cell transplantation (PBSCT) were potentially eligible for this protocol. Between May 2001 and May 2003, 46 such patients were entered on a phase II study evaluating 153Sm EDTMP, followed by melphalan 200 mg/m2 with autologous stem cell support. Eligibility criteria included: Durie and Salmon criteria Stage II or III at diagnosis of MM; age greater than 18 years; Eastern Cooperative Oncology Group performance status less than or equal to 2; direct bilirubin less than or equal to 2.0 mg/dL; alkaline phosphatase less than or equal to 2.5 times the institutional normal; serum creatinine less than or equal to 3.0 mg/dL; cardiac left ventricular ejection fraction greater than or equal to 45%; a lung diffusing capacity of oxygen of greater than or equal to 50%; a forced vital capacity of greater than or equal to 50%; and a forced expiratory volume of greater than or equal to50%. Patients were also required to have greater than or equal to 2 × 106 CD34 cells/kg available for infusion after high dose therapy. No bisphosphonate drugs were allowed within 2 weeks prior to treatment. Patients signed a protocol specific consent form (MC9981) approved by the Institutional Review Board of the Mayo Clinic.
Study Design
This Phase II study was an extension of our previously reported Phase I trial. According to design, the 6 patients treated at the MTD on the Phase I trial were included in the Phase II analysis.(25) A total of 46 patients were treated at a uniform dose level—a targeted absorbed radiation dose to the red marrow of 40 Gy based on a trace labeled infusion one week prior to the therapy.
With the exception of engraftment, toxicity was defined according to National Cancer Institute Common Toxicity Criteria, version 2.0 toxicity criteria, including its bone marrow toxicity criteria. The Eastern Cooperative Oncology Group Autologous Bone Marrow Toxicity Criteria were used to define engraftment toxicity. Engraftment of granulocytes and platelets was defined as maintenance of respective counts of 0.5 × 109/L and 20 × 109/L for 3 consecutive days without transfusion support. The first day of the 3 consecutive days was considered the day of engraftment. For the purposes of toxicity reporting, each patient's treatment course was divided in two: the 153Sm-EDTMP period and the HDM period. The 153Sm-EDTMP period included the day of the 153Sm-EDTMP infusion up until the day of the HDM infusion, and the HDM period included the period of time from the HDM infusion until the time of their dismissal from Transplant Center.
Treatment
For the biodistribution study, 153Sm-EDTMP (30 mCi tracer dose) was injected intravenously. Details of biostribution calculations are as previously described.(25) Patients subsequently had two quantitative whole body gamma camera images obtained: shortly following injection (10 minute scan) and at 24 hours (30 minute scan). The therapeutic dose of 153Sm-EDTMP was infused intravenously over 30 minutes into well hydrated patients at the Mayo Clinic General Clinical Research Center in a lead-lined room. Patients were monitored for a goal urine output of at least 300 cc/2 hours and for initial dosimetry studies. Blood samples for drug clearance were taken at 0, 0.5, 1, 4, 48, and 72 to 120 hours. Gamma camera imaging was done at 0, 4, 24, 48, and 72–120 hours post 153Sm-EDTMP infusion. Patients were dismissed from the General Clinical Research Center after 48 hours. At 9 days post 153Sm-EDTMP infusion and prior to the high dose melphalan (HDM) and PBSCT, the patients had the residual whole body 153Sm measured as previously described (25) to assure that patients had less than 3.6 mCi at the time of PBSC infusion. The target red marrow dose of 40 Gy was achieved by administering doses ranging from 12 mCi/kg to 27 mCi/kg.
Melphalan 200 mg/m2 was administered IV over 1 hour on day -1 and PBSC were infused on day 0. Patients received GM-CSF 500 mcg subcutaneously beginning 6 days after the PBSC infusion. The GM-CSF was continued until the absolute neutrophil count (ANC) was greater than 500/uL on 2 consecutive days. Patients received antibiotic prophylaxis including fluconazole, a quinolone, penicillin, and valcyclovir and were treated on an outpatient basis as tolerated. Central parenteral nutrition, narcotics, IV antibiotics, transfusions, and hospitalization were used when clinically necessary.
Response Definitions
Response was as defined by Blade et al(26) with the addition of the very good partial response (VGPR) category which was defined as greater than or equal to 90% reduction of myeloma protein from serum, urine M-spike to be less than or equal to100 mg/24 hours, and less than or equal to 5% bone marrow plasma cells in the absence of bony progression. Responses were calculated from diagnosis among newly diagnosed patients and immediately prior to transplantation for relapsed or refractory patients.
Control group
The group of patients used for comparison consisted of 102 consecutive patients with MM who underwent autologous stem cell transplant at Mayo Clinic during the same interval (May 2001 and May 2003) as those who were enrolled in the trial. Data pertaining to the transplant patients were captured prospectively into a database, which is continuously updated. Complete follow-up was available for all the patients. All patients had provided written informed consent for use of their medical records.
Statistical Analysis
A Fleming’s 2-stage design—one-stage with interim analysis—was employed to assess response. The primary endpoint was a best response of complete response (CR) or VGPR. The chosen design required 46 evaluable patients to test that the true CR/VGPR rate was at most 30% versus the alternative that it was at least 50%. The design had 90% power and a 0.10 level of significance to test this hypothesis. If there were at least 18 responses (out of the first 46 evaluable patients), the trial regimen was to be considered promising. All patients meeting the eligibility criteria who had signed a consent form and began treatment were considered evaluable for response. Duration of follow-up and survival were calculated from the date of transplant. Follow-up is through December 18, 2009. Progression free survival (PFS) was defined as the time from the date of transplant to the earlier of the dates of death from any cause or disease progression. Overall survival (OS) was defined as the time from the date of transplant until the date of death. Time to engraftment was defined as the number of days from transplant until engraftment. Fisher’s exact tests and Wilcoxon rank-sum tests were used to compare the baseline characteristics between the database and trial patients. The distributions of time to event data were estimated using the Kaplan-Meier method, and compared between the trial and the database using the stratified log rank test statistic.
RESULTS
Patient characteristics
The characteristics of patients treated with 153Sm-EDTMP and melphalan are listed in Table 1. Ten (22%) patients were treated for relapsed disease, 5 with chemoresistant relapse. Seven patients (15%) were transplanted for primary refractory myeloma. Ten of 46 patients had a high plasma cell labeling (>1.0%) index prior to transplant. Eighty-six percent of patients had myelomatous bone disease. One patient had a treatment related myelodysplastic syndrome and severe neutropenia before study entry.
Table 1.
Patient characteristics
| Characteristic | All Phase II, n=46 |
|---|---|
| Male gender, n (%) | 25 (54) |
| Age, median (range) | 58 (38 – 74) |
| Prior # of regimens, n (%) | |
| 1 / 2 / ≥ 3 | 36 / 7 / 4 (78 / 15 / 7) |
| Disease status, n (%) | |
| Primary responsive | 29 (63) |
| Primary refractory | 7 (15) |
| Relapsed | 10 (22) |
| Response status at entry, n (%) | 1 / 2 / 25 / 2 / 12 / 3 |
| CR / VGPR / PR / MR / NR / Prog | (2 / 4 / 54 / 4 / 26 / 7) |
| ECOG PS, n (%) | 11 / 27 / 7 |
| 0 / 1 / 2 | (24 /61 / 15) |
| BMPC >10%, n (%) | 10 (21.7) |
| Creatinine >1.5, n (%) | 3 (6.5) |
| PCLI >1%, n (%) | 10 (22.7) |
| Abnormal cytogenetics, n (%) | 8 (17.4) |
| Beta 2-M >2.7 mg/dL, n (%) | 23 (50) |
CR, complete response; VGPR, very good partial response; PR, partial response; MR, minimal response; NR, no response; and PD, progressive disease.
Adverse Events
Table 2 shows all the toxicity attributable to the single agent 153Sm-EDTMP alone. With the exception of myelosuppression, the toxicity of the 153Sm-EDTMP was minimal. The median time between administration of the 153Sm-EDTMP and of the HDM was 11 days, range 9 to 14 days. Times to an ANC of less than or equal to 0.5 × 10(9)/L and platelet count of 20 × 10(9)/L were day 3 and 4 post PBSCT. Sixty-seven percent of patients had nausea, 39% fatigue, and 20% vomiting. Seventeen percent of patients experienced grade 1 or 2 anorexia, headache, or bone pain. Thirty-five percent had asymptomatic transient hypocalcemia. Six patients had microscopic hematuria detected on scheduled urinalysis. Three patients had culture negative neutropenic fever and 4 patients had minor infections requiring oral antibiotics. Eight patients required red cell transfusion and one patient required a platelet transfusion prior to melphalan infusion.
Table 2.
Adverse events (CTC 2.0) potentially attributable to 153Sm-EDTMP (pre-melphalan), n=46.*
| Adverse event | Grade | Any Gr 3–4 | Any Gr 1–4 | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| Neutropenia | 0 | 15 | 21 | 7 | 28 | 43 |
| Anemia | 5 | 20 | 5 | 0 | 5 | 30 |
| Thrombocytopenia | 6 | 6 | 1 | 0 | 1 | 13 |
| Febrile neutropenia | 0 | 0 | 3 | 0 | 3 | 3 |
| Infection | 0 | 2 | 0 | 0 | 0 | 2 |
| Nausea | 27 | 2 | 2 | 0 | 2 | 31 |
| Vomiting | 6 | 1 | 2 | 0 | 2 | 9 |
| Diarrhea | 5 | 1 | 0 | 0 | 0 | 6 |
| Hypocalcemia | 15 | 1 | 0 | 0 | 0 | 16 |
| Fatigue | 16 | 2 | 0 | 0 | 0 | 18 |
| Anorexia | 6 | 2 | 0 | 0 | 0 | 8 |
| Headache | 4 | 4 | 0 | 0 | 0 | 8 |
| Arthralgia | 1 | 2 | 0 | 0 | 0 | 3 |
| Pain-bone | 3 | 5 | 0 | 0 | 0 | 8 |
| Hematuria | 6 | 0 | 0 | 0 | 0 | 6 |
Includes adverse events (CTC 2.0) occurring in at least 3 patients and possibly, probably, definitely attributable to 153Sm-EDTMP
After the HDM, all but one patient had grade 3 or 4 myelosuppression (Table 3). Seventy percent had grade 3–4 nausea, vomiting, stomatitis, and/or diarrhea. Thirty-six patients had febrile neutropenia and/or documented infection, the majority of which were line associated bacteremias. Seven patients had microscopic hematuria <100 red blood cells per high power field during their transplant course. In all instances, the hematuria occurred during periods of platelet count less than 50 × 109/L. None had gross hematuria. With a median of 7.1 years of follow-up, none have developed microangiopathy or radiation nephritis.
Table 3.
Adverse events subsequent to high dose melphalan
| Body system | Grade 3 | Grade 4 | Any 3–5 |
|---|---|---|---|
| Bone marrow myelosuppression | 0 | 45 | 45 |
| Anemia | 30 | 1 | 31 |
| Neutropenia | 0 | 45 | 45 |
| Thrombocytopenia | 22 | 23 | 45 |
| Gastrointestinal | 13 | 18 | 31 |
| Allergy/Immunologic | 0 | 1 | 1 |
| Cardiovascular | 4 | 0 | 4 |
| Constitutional | 2 | 0 | 2 |
| Dermatologic | 0 | 0 | 0 |
| Hemorrhage | 13 | 0 | 13 |
| Hepatic | 1 | 0 | 1 |
| Infection/febrile neutropenia | 33 | 0* | 35* |
| Metabolic/laboratory | 17 | 0 | 17 |
| Neurology | 4 | 0 | 4 |
| Pain | 2 | 0 | 2 |
| Pulmonary | 2 | 1* | 3* |
| Renal | 1 | 0 | 1 |
Any grade 3–5 adverse events (CTC 2.0) subsequent to high dose melphalan regardless of attribution, maximum grade per organ system per patient
There was an additional patient who died of CMV pneumonitis.
One patient died related to CMV pneumonitis. His treatment with 153Sm-EDTMP and HDM was without incident. He engrafted his white cells on day 11 post-stem cell infusion, and had a nine day hospitalization from day 8 through day 17 for a culture negative neutropenic fever, mucositis, and dysuria. He was clinically improved on dismissal, but on day 18 developed fever, nausea, vomiting, and pulmonary infiltrate of the right upper lobe (on 5/9/02) for which he was re-hospitalized. CT scan showed consolidation right upper lobe and patchy infiltrates bilaterally. He became increasingly dyspneic and fever persisted. A BAL was performed on day 21, and both CMV and candida albicans grew from the washings. Antibiotic coverage was broadened to include liposomal amphotericin and ganciclovir, but pulmonary infiltrates and gas exchange worsened. There was concern that there was a component of periengraftment syndrome and high dose methyl prednisolone was administered. Patient and family refused intubation so BiPAP was initiated. Despite these efforts, the patient expired 27 days post stem cell infusion.
Hematopoietic Engraftment
The median time to ANC greater than or equal to 0.5 × 10(9)/L was 13 days (95%CI 12–14). The median time to unsupported platelet count of 50 × 10(9)/L was 30 days (95%CI 20–53). Two patients had a second stem cell aliquot infused at days 38 and 43 due to delayed platelet engraftment, and two patients never achieved a platelet count of 50 × 10(9)/L. These values were comparable to those of 102 patients treated at our Transplant Center during the same time period, who received melphalan 200 mg/m2 alone as their conditioning regimen. In this control group, the median time to a neutrophil count of 0.5 × 10(9) was 15 days (95%CI: 14–18) and a platelet count of 50 was 35 days (95%CI: 28–40). The percent of patients in the study group versus routine practice requiring re-infusion was also no different (p=0.46).
Response to therapy
Forty-five of the forty-six patients have been assessed for response. The one patient who died prior to day 100 was not assessed for response but has been classified as a non-responder. Post-transplant, fifteen patients (33%) had achieved a CR and another 12 (26%) had achieved a VGPR. Eighteen additional patients were in partial response after transplant.
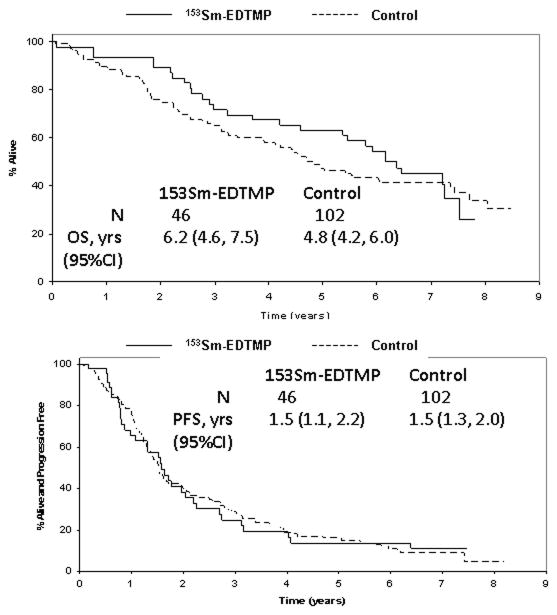
Overall survival and progression free survival
Median follow-up of surviving patients is 7.1 years (range 6.1 to 7.8). Thirty-two patients have progressed and 28 have died. Eight patients were censored for progression events when they enrolled onto maintenance trials (8 dendritic cell therapy and 1 2-methoxydiesterol). Median overall survival (OS) and progression free survival (PFS) from transplantation for all patients treated was 6.2 years (95%CI 4.6–7.5 years) and 1.5 years (1.1–2.2 years), respectively. OS from time of diagnosis was 7.1 years (95%CI 5.9–8.5).
One hundred and two patients with myeloma were treated contemporaneously off study with single agent high dose melphalan conditioning. The baseline characteristics of these two groups were no different (data not shown). There was no significant difference in CR rates, OS or PFS among the patients treated with or without 153Sm-EDTMP (Figure 1). The respective OS and PFS rates for the control group were 4.8 years (95%CI 4.2–6.0), and 1.5 years (1.3–2.0 years). The 6-year OS rates were 54% (95%CI 42–71%) versus 43% (95%CI 35–54%) for the study and control groups respectively.
Figure 1. Overall and progression free survival.
A. Overall survival for study (153Sm-EDTMP and high-dose melphalan) patients versus contemporaneously treated transplant patients
B. Progression free survival for study (153Sm-EDTMP and high-dose melphalan) patients versus contemporaneously treated transplant patients
DISCUSSION
HDM with autologous stem cell transplant has been an important addition to the treatment armamentarium for patients with MM.(27, 28) With regards to conditioning regimens, however, very little progress has been made. Only one randomized study evaluated the role of conditioning for MM, i.e. melphalan 200 mg/m2 versus melphalan 140 mg/m2 and total body irradiation.(22) That study showed that despite radiation’s utility against malignant plasma cells in vivo, the toxicity overrode the benefit. Others have tried other conditioning combinations like busulfan and melphalan, but so far these combinations do not appear to be superior to HDM alone. Incorporation of radiotherapeutics into transplantation conditioning regimens is an attractive approach which is being explored in MM, lymphoma, and leukemia. (29–34) For MM, skeletal targeted radiation is of special interest. When Giralt et al incorporated holmium-166 1, 4, 7, 10-tetraazcyclododecane-1, 4, 7, 10-tetramethylenephosphonate (166Ho-DOTMP) into a transplant conditioning regimen, initial results were promising with CR rates of 35%.(29) Unfortunately there was unexpected renal toxicity related to dosimetry issues, which initially lessened the enthusiasm for developing that isotope further. However, upon further analysis, it was demonstrated that for patients receiving an optimum dose—no more than 2400 mCi—there was a trend toward better EFS and OS compared to patients receiving the higher doses of 166Holumium-DOTMP or HDM alone.(30)
We have combined 153Sm-EDTMP with full dose melphalan in an attempt to optimize the therapeutic window for radiation and chemotherapy in patients with MM. Adverse events were manageable. Prior to HDM infusion the only AEs attributable to the radioisotope were mild, but manageable, nausea and vomiting in a minority of patients, microscopic hematuria in a minority, and prolonged myelosuppression that translated into a pre-transplant neutropenic fever rate of 4%. Engraftment of neutrophils was comparable to that seen in MM patients transplanted with standard HDM alone during the same time period.
Our primary endpoints for this trial were met, i.e. manageable AEs and a combined CR/VGPR rate of 59%, which was an impressive rate during the time in which this protocol was conceived and conducted. These results may appear less remarkable in the era of novel therapies, a time in which more than 50% of patients proceed to transplant with rates of VGPR or better, but for its time, these response rates after steroid-based induction were notable. For example, in the IFM94 study, which compared a single ASCT versus a tandem ASCT strategy, CR/VGPR rates for one and two transplants were 42% and 50%, respectively, and the 7-year overall survival rates were 21% and 42% (p=0.01), respectively.(6) Moreover, our cohort contained 37% of patients who were transplanted with relapsed or refractory disease, a setting in which one would expect median OS and PFS of 20 and 11 months, respectively.(35) With a median follow-up of over 7 years, survival rates are comparable to what was seen in other patients transplanted with single agent high dose melphalan although it appears that there is a trend toward superior survival in the trial patients. In conclusion, the combination of 153Sm-EDTMP and HDM is active, tolerable, and warrants further study.
Acknowledgments
This trial was supported in part by grant M01-RR00585, CA15083 and the Mayo Clinic Hematology Malignancies Fund. The authors acknowledge the Research Nurses, Data Managers, nurses, physicians, and physicians assistants whose dedication and hard work made this study possible.
Footnotes
Financial disclosure statement: None of the authors have anything to disclose.
References
- 1.Rajkumar SV, Gertz MA, Kyle RA, Greipp PR. Current therapy for multiple myeloma. Mayo Clin Proc. 2002;77:813–822. doi: 10.4065/77.8.813. [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, Fonseca R, Rajkumar SV, Offord JR, Larson DR, Plevak ME, Therneau TM, Greipp PR. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 3.McElwain T, Powles R. High-dose intravenous melphalan for plasma-cell leukaemia and myeloma. Lancet. 1983;2:822–824. doi: 10.1016/s0140-6736(83)90739-0. [DOI] [PubMed] [Google Scholar]
- 4.Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, Casassus P, Maisonneuve H, Facon T, Ifrah N, Payen C, Bataille R. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome [see comments] New England Journal of Medicine. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 5.Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, Brown J, Drayson MT, Selby PJ Medical Research Council Adult Leukaemia Working P. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. New England Journal of Medicine. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 6.Attal M, Harousseau JL, Facon T, Guilhot F, Doyen C, Fuzibet JG, Monconduit M, Hulin C, Caillot D, Bouabdallah R, Voillat L, Sotto JJ, Grosbois B, Bataille R. Single versus double autologous stem-cell transplantation for multiple myeloma. The New England journal of medicine. 2003;349:2495–2502. doi: 10.1056/NEJMoa032290. [DOI] [PubMed] [Google Scholar]
- 7.Cavo M, Tosi P, Zamagni E, Cellini C, Tacchetti P, Patriarca F, Di Raimondo F, Volpe E, Ronconi S, Cangini D, Narni F, Carubelli A, Masini L, Catalano L, Fiacchini M, de Vivo A, Gozzetti A, Lazzaro A, Tura S, Baccarani M. Prospective, randomized study of single compared with double autologous stem-cell transplantation for multiple myeloma: Bologna 96 clinical study. J Clin Oncol. 2007;25:2434–2441. doi: 10.1200/JCO.2006.10.2509. [DOI] [PubMed] [Google Scholar]
- 8.Vescio R, Schiller G, Stewart AK, Ballester O, Noga S, Rugo H, Freytes C, Stadtmauer E, Tarantolo S, Sahebi F, Stiff P, Meharchard J, Schlossman R, Brown R, Tully H, Benyunes M, Jacobs C, Berenson R, DiPersio J, Anderson K, Berenson J. Multicenter phase III trial to evaluate CD34(+) selected versus unselected autologous peripheral blood progenitor cell transplantation in multiple myeloma. Blood. 1999;93:1858–1868. [PubMed] [Google Scholar]
- 9.Gahrton G, Svensson H, Cavo M, Apperly J, Bacigalupo A, Bjorkstrand B, Blade J, Cornelissen J, de Laurenzi A, Facon T, Ljungman P, Michallet M, Niederwieser D, Powles R, Reiffers J, Russell NH, Samson D, Schaefer UW, Schattenberg A, Tura S, Verdonck LF, Vernant JP, Willemze R, Volin L, Marrow T The European Group for B. Progress in allogenic bone marrow and peripheral blood stem cell transplantation for multiple myeloma: a comparison between transplants performed 1983--93 and 1994--8 at European Group for Blood and Marrow Transplantation centres. Br J Haematol. 2001;113:209–216. doi: 10.1046/j.1365-2141.2001.02726.x. [DOI] [PubMed] [Google Scholar]
- 10.Bjorkstrand B. European Group for Blood and Marrow Transplantation Registry studies in multiple myeloma. Semin Hematol. 2001;38:219–225. doi: 10.1016/s0037-1963(01)90013-7. [DOI] [PubMed] [Google Scholar]
- 11.Goeckeler WF, Troutner DE, Volkert WA, Edwards B, Simon J, Wilson D. 153Sm radiotherapeutic bone agents. Int J Rad Appl Instrum B. 1986;13:479–482. doi: 10.1016/0883-2897(86)90028-0. [DOI] [PubMed] [Google Scholar]
- 12.Goeckeler WF, Stoneburner LK, Kasi LP, Fossella FV, Price DR, Fordyce WA. Analysis of urine samples from metastatic bone cancer patients administered 153Sm-EDTMP. Nucl Med Biol. 1993;20:657–661. doi: 10.1016/0969-8051(93)90036-t. [DOI] [PubMed] [Google Scholar]
- 13.Ketring AR. 153Sm-EDTMP and 186Re-HEDP as bone therapeutic radiopharmaceuticals. Int J Rad Appl Instrum B. 1987;14:223–232. doi: 10.1016/0883-2897(87)90046-8. [DOI] [PubMed] [Google Scholar]
- 14.Singh A, Holmes RA, Farhangi M, Volkert WA, Williams A, Stringham LM, Ketring AR. Human pharmacokinetics of samarium-153 EDTMP in metastatic cancer. J Nucl Med. 1989;30:1814–1818. [PubMed] [Google Scholar]
- 15.Turner JH, Claringbold PG, Hetherington EL, Sorby P, Martindale AA. A phase I study of samarium-153 ethylenediaminetetramethylene phosphonate therapy for disseminated skeletal metastases. J Clin Oncol. 1989;7:1926–1931. doi: 10.1200/JCO.1989.7.12.1926. [DOI] [PubMed] [Google Scholar]
- 16.Turner JH, Claringbold PG. A phase II study of treatment of painful multifocal skeletal metastases with single and repeated dose samarium-153 ethylenediaminetetramethylene phosphonate. Eur J Cancer. 1991;27:1084–1086. doi: 10.1016/0277-5379(91)90297-q. [DOI] [PubMed] [Google Scholar]
- 17.Collins C, Eary JF, Donaldson G, Vernon C, Bush NE, Petersdorf S, Livingston RB, Gordon EE, Chapman CR, Appelbaum FR. Samarium-153-EDTMP in bone metastases of hormone refractory prostate carcinoma: a phase I/II trial. J Nucl Med. 1993;34:1839–1844. [PubMed] [Google Scholar]
- 18.Serafini AN. Systemic metabolic radiotherapy with samarium-153 EDTMP for the treatment of painful bone metastasis. Q J Nucl Med. 2001;45:91–99. [PubMed] [Google Scholar]
- 19.Turner JH, Claringbold PG, Berger JD, Martindale AA, Glancy JR. 153Sm-EDTMP and melphalan chemoradiotherapy regimen for bone marrow ablation prior to marrow transplantation: an experimental model in the rat. Nucl Med Commun. 1992;13:321–329. doi: 10.1097/00006231-199205000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Mill WB. Radiation therapy in multiple myeloma. Radiology. 1975;115:175–178. doi: 10.1148/115.1.175. [DOI] [PubMed] [Google Scholar]
- 21.Bosch A, Frias Z. Radiotherapy in the treatment of multiple myeloma. Int J Radiat Oncol Biol Phys. 1988;15:1363–1369. doi: 10.1016/0360-3016(88)90232-5. [DOI] [PubMed] [Google Scholar]
- 22.Moreau P, Facon T, Attal M, Hulin C, Michallet M, Maloisel F, Sotto JJ, Guilhot F, Marit G, Doyen C, Jaubert J, Fuzibet JG, Francois S, Benboubker L, Monconduit M, Voillat L, Macro M, Berthou C, Dorvaux V, Pignon B, Rio B, Matthes T, Casassus P, Caillot D, Najman N, Grosbois B, Bataille R, Harousseau JL. Comparison of 200 mg/m(2) melphalan and 8 Gy total body irradiation plus 140 mg/m(2) melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myelome 9502 randomized trial. Blood. 2002;99:731–735. doi: 10.1182/blood.v99.3.731. [DOI] [PubMed] [Google Scholar]
- 23.Brenner W, Kampen WU, Kampen AM, Henze E. Skeletal uptake and soft-tissue retention of 186Re-HEDP and 153Sm-EDTMP in patients with metastatic bone disease. J Nucl Med. 2001;42:230–236. [PubMed] [Google Scholar]
- 24.Franzius C, Schuck A, Bielack SS. High-dose samarium-153 ethylene diamine tetramethylene phosphonate: low toxicity of skeletal irradiation in patients with osteosarcoma and bone metastases. J Clin Oncol. 2002;20:1953–1954. doi: 10.1200/JCO.2002.20.7.1953. [DOI] [PubMed] [Google Scholar]
- 25.Dispenzieri A, Wiseman GA, Lacy MQ, Litzow MR, Anderson PM, Gastineau DA, Tefferi A, Inwards DJ, Micallef INM, Ansell SM, Porrata L, Elliott MA, Lust JA, Greipp PR, Rajkumar SV, Fonseca R, Witzig TE, Erlichman C, Sloan JA, Gertz MA. A Phase I Study of 153Sm-EDTMP with Fixed High Dose Melphalan as a Peripheral Blood Stem Cell Conditioning Regimen in Patients with Multiple Myeloma. Leukemia. 2005;19:118–125. doi: 10.1038/sj.leu.2403575. [DOI] [PubMed] [Google Scholar]
- 26.Blade J, Samson D, Reece D, Apperley J, Bjorkstrand B, Gahrton G, Gertz M, Giralt S, Jagannath S, Vesole D. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. British Journal of Haematology. 1998;102:1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 27.Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Zeldenrust SR, Dingli D, Russell SJ, Lust JA, Greipp PR, Kyle RA, Gertz MA. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brenner H, Gondos A, Pulte D. Recent major improvement in long-term survival of younger patients with multiple myeloma. Blood. 2008;111:2521–2526. doi: 10.1182/blood-2007-08-104984. [DOI] [PubMed] [Google Scholar]
- 29.Giralt S, Bensinger W, Goodman M, Podoloff D, Eary J, Wendt R, Alexanian R, Weber D, Maloney D, Holmberg L, Rajandran J, Breitz H, Ghalie R, Champlin R. 166Ho-DOTMP plus melphalan followed by peripheral blood stem cell transplantation in patients with multiple myeloma: results of two phase 1/2 trials. Blood. 2003;102:2684–2691. doi: 10.1182/blood-2002-10-3250. [DOI] [PubMed] [Google Scholar]
- 30.Christoforidou AV, Saliba RM, Williams P, Qazilbash M, Roden L, Aleman A, Weber D, Mendoza F, Podoloff D, Wendt R, 3rd, Breitz H, Alexanian R, Champlin R, Giralt S. Results of a retrospective single institution analysis of targeted skeletal radiotherapy with (166)Holmium-DOTMP as conditioning regimen for autologous stem cell transplant for patients with multiple myeloma. Impact on transplant outcomes. Biol Blood Marrow Transplant. 2007;13:543–549. doi: 10.1016/j.bbmt.2006.12.448. [DOI] [PubMed] [Google Scholar]
- 31.Krishnan A, Nademanee A, Fung HC, Raubitschek AA, Molina A, Yamauchi D, Rodriguez R, Spielberger RT, Falk P, Palmer JM, Forman SJ. Phase II trial of a transplantation regimen of yttrium-90 ibritumomab tiuxetan and high-dose chemotherapy in patients with non-Hodgkin's lymphoma. J Clin Oncol. 2008;26:90–95. doi: 10.1200/JCO.2007.11.9248. [DOI] [PubMed] [Google Scholar]
- 32.Winter JN, Inwards DJ, Spies S, Wiseman G, Patton D, Erwin W, Rademaker AW, Weitner BB, Williams SF, Tallman MS, Micallef I, Mehta J, Singhal S, Evens AM, Zimmer M, Molina A, White CA, Gordon LI. Yttrium-90 ibritumomab tiuxetan doses calculated to deliver up to 15 Gy to critical organs may be safely combined with high-dose BEAM and autologous transplantation in relapsed or refractory B-cell non-Hodgkin's lymphoma. J Clin Oncol. 2009;27:1653–1659. doi: 10.1200/JCO.2008.19.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buchmann I, Bunjes D, Kotzerke J, Martin H, Glatting G, Seitz U, Rattat D, Buck A, Dohner H, Reske SN. Myeloablative radioimmunotherapy with Re-188-anti-CD66-antibody for conditioning of high-risk leukemia patients prior to stem cell transplantation: biodistribution, biokinetics and immediate toxicities. Cancer Biother Radiopharm. 2002;17:151–163. doi: 10.1089/108497802753773775. [DOI] [PubMed] [Google Scholar]
- 34.Burke JM, Jurcic JG, Scheinberg DA. Radioimmunotherapy for acute leukemia. Cancer Control. 2002;9:106–113. doi: 10.1177/107327480200900203. [DOI] [PubMed] [Google Scholar]
- 35.Gertz MA, Lacy MQ, Inwards DJ, Gastineau DA, Tefferi A, Chen MG, Witzig TE, Greipp PR, Litzow MR. Delayed stem cell transplantation for the management of relapsed or refractory multiple myeloma. Bone Marrow Transplant. 2000;26:45–50. doi: 10.1038/sj.bmt.1702445. [DOI] [PubMed] [Google Scholar]