Abstract
Castleman Disease (CD) is a rare, poorly understood lymphoproliferative disease. The spectrum of symptoms and course of disease are broad, but there is no large study describing the natural history of this disease. Basic clinic and laboratory data from the records of 113 patients with CD evaluated at the Mayo Clinic and University of Nebraska were abstracted. The impact of these variables on overall survival (OS) from time of diagnosis was evaluated. Sixty patients had multicentric disease. Of the patients with multicentric CD, 32% had criteria sufficient for a diagnosis of POEMS syndrome. For all patients, 2, 5, and 10-year OS was 92%, 76%, 59%. Most of the factors identified as risk factors for death on univariate analysis co-segregated with diagnostic criteria for POEMS syndrome, which supported the concept of 4 categories of CD, which are (along with their 5-year OS): 1) unicentric CD (91%); 2) multicentric CD associated with the osteosclerotic variant of POEMS syndrome (90%); 3); multicentric CD without POEMS syndrome (65%); and 4) multicentric CD with POEMS syndrome without osteosclerotic lesions (27%). We have demonstrated that CD represents a spectrum of disease that can be differentiated by simple prognostic factors that provide a framework for further study.
Keywords: Castleman's Disease, myeloma, lymphoma
Introduction
In 1954, Dr. Castleman first reported on 2 patients possessing localized mediastinal lymph node enlargement characterized by redundancy of lymphoid follicles with germinal-center involution and marked capillary proliferation with endothelial hyperplasia in both follicular and interfollicular regions.(1) This condition, which is now called Castleman's Disease (CD), was soon found to be associated with hypochromic anemia, hypergammaglobulinemia, and bone marrow plasmacytosis—features which typically resolved after surgical resection of a solitary mass involved with CD.(2, 3) By the late 1960s and the early 1970s, the histological descriptors in current in use were described (4, 5) and refined:(5) hyaline vascular variant, plasma cell variant, and mixed variant. Patients with the plasma cell variant were noted to be more prone to have B-symptoms as well as anemia and hypergammaglobulinemia.(4)
The first case of multicentric CD was reported in 1978.(6) Salient differences between hyaline vascular and plasma cell variants and their respective associations with unicentric (unifocal or localized) and multicentric (multifocal or generalized) presentations were subsequently recognized.(7, 8) Patients with unicentric disease had fewer associated symptoms in contrast to those with the multicentric disease who had a more complicated course.
In 1985 Lachant et al reported on 2 patients with the acquired immunodeficiency syndrome (AIDS) who developed multicentric CD followed by Kaposi's sarcoma.(9) More cases followed. In 1995, Soulier reported on the incidence of human herpes virus-8 (HHV-8 a.k.a. Kaposi sarcoma virus) in 31 patients multicentric CD.(10) All 14 of the human immunodeficiency virus (HIV) positive patients tested had HHV-8 in their affected lymph nodes; in contrast, 7 of 17 HIV-negative multicentric CD patients had HHV-8 present. Other investigators have confirmed that nearly all HIV-positive CD cases contain HHV-8 and that nearly half of HIV-negative multicentric CD are HHV-8 positive.(11-15)
Overproduction of circulating cytokines has been implicated in the pathogenesis and symptomatology of CD(16) and its sister syndrome POEMS syndrome (peripheral neuropathy, organomegaly, endocrinopathy, monoclonal protein, skin changes).(16-18) Interleukin-6 (IL-6) is related to the pathogenesis of CD for many patients as shown by the following 3 observations: removal of CD mass causes an abrupt drop in IL-6 levels and resolution of symptoms;(19) treatment with IL-6 receptor antibody relieves the symptoms and signs;(20, 21) overexpression of IL-6 in mice produces a phenotype similar to the multicentric CD phenotype.(22)
At present there is no state of the art for prognostication and management of CD. This retrospective analysis of 113 cases from 2 institutions evaluated the clinical, laboratory, and pathological parameters to define distinct prognostic subsets.
Methods
Patients
Patients were identified through pathology and lymphoma databases at the Mayo Clinic (n=87) and University of Nebraska (n=26). Eligibility for inclusion was contingent on a diagnosis of CD or angiofollicular lymph node hyperplasia and tissue available for central review. Efforts were made to contact patients for whom last follow-up was more than 1 year. The Social Security Death Index was used to document death in patients lost to follow-up. Clinical and laboratory data were abstracted and reviewed by ML, TMH, JC, KR, and AD at the Mayo Clinic and by JOA at the University of Nebraska.
Clinical characteristics abstracted and analyzed included age and presence or absence of: B-symptoms, dyspnea, organomegaly, papilledema, neuropathy, renal disease, sclerotic bone lesions, criteria for co-existing POEMS syndrome,(18, 23) and autoimmune phenomenon (for example, autoimmune hemolytic anemia or thrombocytopenia). B-symptoms were defined as weight-loss was defined as a 10% reduction in weight in prior 6-months, fevers, or night sweats. Physical findings like peripheral neuropathy, skin changes, splenomegaly, and palpable masses were tabulated according to the treating physician's documentation. A patient was considered to have a peripheral neuropathy if any of the following conditions were met: 1) a diagnosis of peripheral neuropathy was in the chart; or 2) there was a baseline report of significant lower extremity paresthesia, dysesthesia, or paresis. No mention of a symptom or sign was classified as absence of that finding. Extravascular overload included any mention of peripheral edema, ascites, pleural effusions, or pericardial effusion. If a test was not done, the finding was slated as not present. For example if bone radiographs or body CTs were not performed in a given patient, that individual was coded as not having bone lesions and non-palpable lymphadenopathy. Patients were classified as having unicentric (one site of lymphadenopathy) or multicentric (more than one lymph node region with lymph nodes greater than 1 cm). Clinical response to treatment was according to standard lymphoma response criteria according to treating physicians' documentation. Evaluated laboratory tests included complete blood count, creatinine, serum albumin, alkaline phosphatase, AST, sedimentation rate, and C-reactive protein. Since HIV testing was available for only 25 patients (1 positive), reviewers paid careful attention to any documented risk factors (high risk sexual practices) and other symptomology of HIV/AIDS (AIDS defining illnesses). No patient who had not undergone testing had a history or subsequent course consistent with AIDS. Patients were classified as POEMS by the abstracters according to published criteria.(23) Briefly, a clonal plasma cell disorder, a peripheral neuropathy, and at least one other major feature—Castleman's disease, osteosclerotic bone lesions, or elevated vascular endothelial growth factor—and at least one minor feature—endocrinopathy, organomegaly, classic skin changes, papilledema, extravascular overload, thrombocytosis, or erythrocytosis were required to make a diagnosis of POEMS syndrome.
Samples
Patients' diagnostic lymph nodes were biopsied between March 30, 1948 and June 18, 2002. The distribution of cases by time included: pre-1970, 4; 1970 to 1979, 10; 1980-1989, 29; 1990-1999, 59; and 2000-2002, 11. Lymph node specimens were reviewed by expert hematopathologists from the Mayo Clinic and University of Nebraska as per the methods of Castleman and Keller.(5) Pathological specimens were classified as hyaline vascular, plasma cell variant, or mixed variant.
Statistical Methodology
Differences between groups based on pathologic variants, extent of disease, peripheral neuropathy, osteosclerotic lesions, co-exiting POEMS syndrome, and documentation of HIV status were determined by the Fischer's Exact test for dichotomous variables and by the Kruskal-Wallis test for continuous variables. The impact of variables on overall survival (OS) from time of diagnosis was evaluated using univariate analyses, where their significance was determined based on the logrank statistic. Variables analyzed in relation to OS included: centricity of disease (unicentric vs. multicentric), pathologic variant, co-existing POEMS syndrome, age at diagnosis, serum albumin, abnormal platelet count, organomegaly, peripheral neuropathy, sclerotic bone lesions, and dyspnea. Further survival analyses were performed on patient subgroups based on known distinct natural histories of disease: patients with and without osteosclerotic lesions;(24, 25) patients with and without neuropathy; patients with and without POEMS syndrome; and patients with unicentric disease versus multicentric disease. Co-dependencies between variables prompted a convenient 4-group classification system, which is used to present patient characteristics. All statistical analyses were done using SAS or JMP software packages (SAS, Carey, North Carolina).
Results
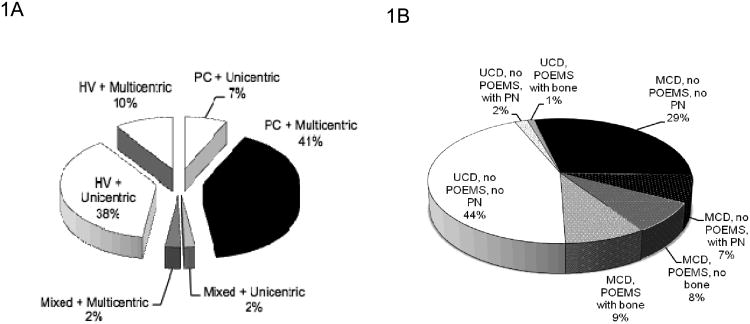
Of the 113 patients, 48% were male. The median age of the cohort was 43 years (range 4.2 to 78). Forty-seven percent were male. Overall, 60 (53%) patients had multicentric CD (Figure 1A). The breakdown by histology was: plasma cell variant, 54; hyaline vascular, 54; and mixed histology, 5. Patients with hyaline vascular disease were more likely to have unicentric disease; whereas, patients with plasma cell variant more commonly had multicentric disease. Three patients were under the age of 10, all of whom had unicentric hyaline vascular disease. Testing for a monoclonal protein was done in only 54 patients. Of these 29 had a monoclonal protein documented—3 in the unicentric group and 26 in the multicentric group. All but four of these were lambda restricted.
Figure 1. Interactions between histology, disease extent, and clinical features.
A. Relationship of histology and disease extent.
B. Relationship between disease extent, POEMS syndrome, osteosclerotic bone lesions, and peripheral neuropathy. All patients with osteosclerotic bone lesions had POEMS syndrome, and by definition all patients with POEMS had peripheral neuropathy, but one third of patients who had peripheral neuropathy did not have POEMS syndrome.
MCD, multicentric Castelman's disease; UCD, unicentric Castleman's disease.
There were differences in baseline features for patients with multicentric disease as compared to unicentric disease (Table 1). Patients with multicentric CD were more likely to be older, to have B-symptoms, palpable disease, peripheral neuropathy (Figure 1B), extravascular volume overload (i.e. edema, ascites or effusions), co-existing POEMS syndrome, documented bony sclerosis, anemia, leukocytosis, thrombocytosis, a high sedimentation rate, hypergammaglobulinemia, a low albumin, and an elevated creatinine. The neuropathy was more often sensory than motor. Because of the inter-relationships between POEMS syndrome and CD, the multicentric group was further parsed into 3-groups based on baseline characteristics. Sufficient features to diagnosis POEMS syndrome were present in 20 patients overall--19 patients with multicentric (34%) and 1 unicentric (2%) disease. Nine of these patients had only 3 major criteria for the diagnosis of POEMS syndrome with the 1st, 2nd, and 3rd being peripheral neuropathy, monoclonal protein, and CD, respectively, whereas 11 had sclerotic bone lesions as a 4th major criteria.(26) Within this group of 20, the only other difference in baseline characteristics between those with and without identified sclerotic bone lesions was that those with sclerotic bone lesions were more likely to have an endocrinopathy, consistent with their diagnosis of POEMS syndrome.
Table 1. Baseline characteristics*.
| UCD (n=53) | MCD with OSM POEMS (n=10) | MCD, no POEMS (n=41) | MCD, POEMS, No OSM (n=9) | P† | P‡ | P§ | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| % | Median (range) | % | Median (range) | % | Median (range) | % | |||||
| Age, years | 34 (4-74) | 38 (32-67) | 51 (16-78) | 49 (34-61) | <0.001 | NS | <0.001 | ||||
| Gender, M | 43 | 60 | 49 | 44 | NS | NS | NS | ||||
| PCV | 19 | 100 | 76 | 89 | <0.001 | NS | <0.001 | ||||
| B-symptoms | 11 | 60 | 49 | 22 | <0.001 | NS | <0.001 | ||||
| Dyspnea | 11 | 30 | 24 | 22 | 0.09 | NS | NS | ||||
| Organomegaly | 0 | 30 | 29 | 44 | <0.001 | NS | <0.001 | ||||
| Neuropathy | 6 | 100 | 20 | 100 | <0.001 | <0.001 | <0.001 | ||||
| Endocrine abn | 8 | 67 | 6 | 12 | NS | <0.001 | <0.001 | ||||
| Skin changes | 0 | 70 | 10 | 44 | <0.001 | <0.001 | <0.001 | ||||
| EVO | 9 | 60 | 34 | 56 | <0.001 | NS | <0.001 | ||||
| Papilledema | 0 | 30 | 0 | 12 | NS | 0.01 | <0.001 | ||||
| Sclerotic lesions | 2 | 100 | 0 | 0 | 0.01 | <0.001 | <0.001 | ||||
| Platelets (×109/L) | 264 (106-500) | 462 (202-929) | 315 (19-807) | 607 (160-1042) | 0.007 | 0.003 | <0.001 | ||||
| Albumin, g/dL | 4.2 (3.4-5.2) | 3.6 (3.2-4.1) | 3.4 (1.3-4.7) | 3.4 (2.5-4.1) | <0.001 | NS | <0.001 | ||||
| Hemoglobin, g/dL | 13 (8.1-16.8) | 13.8 (10.6-15.6) | 11.2 (6.9-16.9) | 14.6 (9.3-16.7) | 0.02 | 0.002 | <0.001 | ||||
| WBC (×109/L) | 6.0 (3.8-22) | 2.0 (4.0 -11.5) | 8.1 (2.5-65) | 8.0 (5.7-18.6) | 0.02 | NS | 0.08 | ||||
| ESR, mm/minute | 7 (1-139) | 20 (4-130) | 50 (1-147) | 31 (1-86) | <0.001 | 0.06 | <0.001 | ||||
| Creatinine, mg/dL | 0.9 (0.6-5.0) | 1.0 (0.8-1.3) | 1.2 (0.6-4.1) | 1.2 (0.8-2.2) | 0.01 | NS | 0.07 | ||||
| HIV tested | 11 | 40 | 34 | 22 | 0.007 | NS | 0.03 | ||||
| AIHA | 2 | 0 | 12 | 0 | NS | NS | NS | ||||
AIHA, autoimmune hemolytic anemia; ESR, erythrocyte sedimentation rate; EVO, extravascular volume overload; HIV, human immunodeficiency virus; MCD, multicentric Castleman's disease; PCV, plasma cell variant; UCD, unicentric Castleman's disease
Missing values for several parameters such that total number tested for each test is: hemoglobin, 103; WBC, 103; platelets, 93; ESR, 70; gammaglobulins, 66; albumin 82; creatinine, 58.
Between UCD and MCD
Across 3 multicentric CD groups
Across all 4 groups
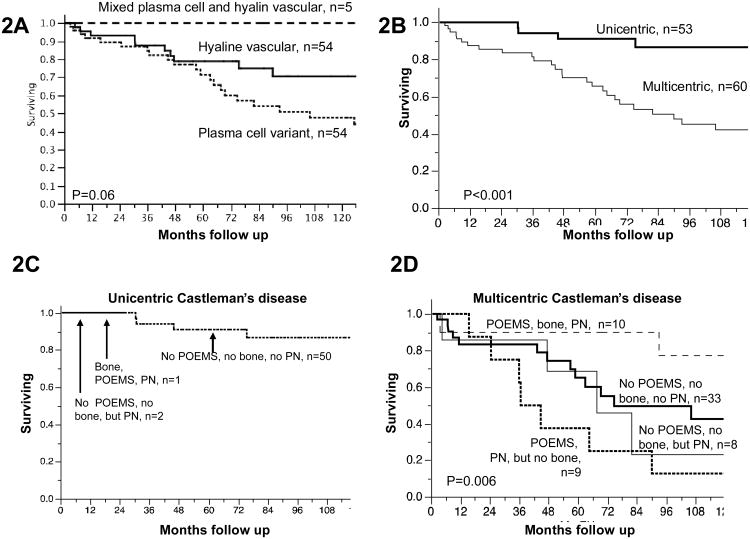
Thirty-seven patients have died. Median follow-up for surviving patients was 5.8 years. For all patients, the 2, 5, and 10-year survival rates were 92%, 76%, 59%. Baseline adverse risk factors on univariate analysis (Table 2) included age at diagnosis, dyspnea, peripheral neuropathy, organomegaly, plasma cell variant histology (Figure 2A), multicentric disease (Figure 2B), low serum albumin, and a platelet count that was either high or low. The presence of B-symptoms or extravascular volume overload was not prognostic. The 5-year overall survival rates for unicentric and multicentric were 91% and 65%, respectively.
Table 2. Univariate prognosticators for overall survival, Cox regression modeling.
| All (n=113) | Unicentric (n=53) | Multicentric (n=60) | ||||
|---|---|---|---|---|---|---|
| RR (95%CI) | p | RR (95%CI) | p | RR (95%CI) | p | |
| Age (by decade) | 1.7 (1.3-2.1) | <0.001 | 1.5 (0.9-2.5) | 0.1 | 1.6 (1.2-2.1) | <0.001 |
| Multicentric disease | 4.7 (2.1-12.8) | <0.001 | NA | NA | NA | NA |
| Plasma cell variant | 1.8 (0.9-3.8) | 0.08 | No events | NA | 1.1 (0.4-2.6) | 0.8 |
| Organomegaly | 3.3 (1.5-6.7) | 0.003 | NA | NA | 1.9 (0.9-4.0) | 0.1 |
| Peripheral neuropathy | 2.1 (1.1-4.1) | 0.03 | No events | NA | 0.9 (0.5-2.4)* | 0.7* |
| POEMS syndrome | 1.6 (0.8-3.2) | 0.2 | Too few pts | NA | 0.9 (0.4-1.9)† | 0.8† |
| Sclerotic bone lesions | 0.5 (0.1-1.4) | 0.2 | Too few pts | NA | 0.3 (0.1-0.8) | 0.01 |
| Abnormal platelet count‡ | 3.2 (1.6-6.7) | 0.001 | 4.0 (0.2-43) | 0.3 | 2.0 (0.9-4.5)§ | 0.07§ |
| Serum albumin (reciprocal) | 2.2 (1.4-3.5) | 0.001 | 4.0 (0.8-28) | 0.1 | 1.7 (0.9-2.9)# | 0.09# |
| Dyspnea | 2.6 (1.2-5.0) | 0.01 | 4.9 (0.8-29) | 0.08 | 1.7 (0.8-3.6) | 0.2 |
RR, risk ratio; 95%CI, 95% confidence interval; NA, not applicable; pts, patients
Excluding 10 patients with sclerotic bone lesions, peripheral neuropathy became borderline significant with a RR 2.1 (95%CI 1.0-4.6), p=0.05
Excluding 10 patients with sclerotic bone lesions, POEMS syndrome became significant with a RR 2.5 (95%CI 1.0-5.6), p=0.05
Only 75% of patients had platelet count available
Excluding 10 patients with sclerotic bone lesions, abnormal platelet count was more significant with a RR 1.5 (95%CI 1.1-5.6), p=0.03
Excluding 10 patients with sclerotic bone lesions, serum albumin was no longer significant with a RR 1.4 (95%CI 0.7-2.4), p=0.2
Figure 2. Kaplan meier estimates of overall survival.
Differences between groups were calculated by log-rank
A. By histology. 5 year OS for hyaline vascular, plasma cell variant, and mixed is 79%, 70%, and 100%.
B. By extent of disease. 5 year overall survival for unicentric and multicentric is 91% and 65% respectively.
C. By co-existing POEMS syndrome, osteosclerotic bone lesions, and peripheral neuropathy in patients with unicentric Castleman's disease. Limited follow-up for the 1 patient with POEMS syndrome associated with osteosclerotic bone lesions and the 2 patients without POEMS, but with peripheral neuropathy.
D. By co-existing POEMS syndrome, osteosclerotic bone lesions, and peripheral neuropathy in patients with multicentric Castleman's disease. 5 year overall survival for 10 patients with osteosclerotic variant of POEMS syndrome was 90%, for 9 patients with POEMS syndrome without osteosclerotic lesions was 27%, and for 41 patients without POEMS who either had (n=8) or did not (n=33) have peripheral neuropathy was 51% and 65%, respectively. The combined 5-year OS blending the last two groups was 65%.
For the 53 patients with unicentric disease, only dyspnea was of borderline significance for being an adverse risk factor (Table 2). The excellent overall survival enjoyed by these patients is seen in Figure 2C. Neither peripheral neuropathy (n=2) nor sclerotic bone lesions (n=1) appeared to have an impact on overall survival for patients with unicentric CD. In our original analyses, organomegaly was considered insufficient to classify a patient as having multicentric CD; however, these 4 patients with putative unicentric CD with organomegaly had a relative risk of death of 11.8 (95% confidence interval 2.3-54) as compared to their unicentric counterparts without organomegaly such that all analyses were rerun using the definition that organomegaly was sufficient to diagnosis multicentric disease. These are the results that are shown.
For the 60 patients with multicentric CD, age, sclerotic bone lesions, abnormal platelet count, and low serum albumin were all risk factors. Sclerotic bone lesions appeared to be protective with a risk ratio of death of 0.3 (95%CI 0.1-0.8). The question arose whether our multicentric CD population was over represented with patients with the osteosclerotic myeloma variant of POEMS syndrome, so the univariate analyses were repeated excluding the 10 patents with osteosclerotic lesions identified. The results were comparable with a couple of exceptions. For the 50 multicentric CD patients without sclerotic bone lesions, the presence of POEMS syndrome (non-osteosclerotic variant) was associated with a significant risk for death (Figure 4D)—relative risk of 2.5, 95%CI 1.0-5.6) p=0.05. Peripheral neuropathy was also of borderline risk on univariate, and low albumin was no longer significant. The interactions between POEMS syndrome, peripheral neuropathy and sclerotic bone lesions are shown in Figure 2D. The 5-year overall survival for 10 patients with osteosclerotic variant of POEMS syndrome was 90%, for the 9 patients with POEMS syndrome without osteosclerotic lesions was 27%, and for the 41 patients without POEMS who either had (n=8) or did not (n=33) have peripheral neuropathy was 51% and 65%, respectively. These outcomes prompted us to consider a 4-group classification system for describing out patients (Table 1). On multivariable analysis, this system retained significance (p<0.001) along with age at diagnosis (p<0.001).
Platelet count was another interesting variable among patients with multicentric CD. Both counts above (n=22) or below (n=8) the normal range predicted for poorer outcomes. The 5-year OS for normal, low, and high platelet counts were 69%, 50%, and 55%. There was a relationship between platelet count and the 3 multicentric CD groups with the highest platelet counts when POEMS syndrome was part of the presentation (Table 1).
HIV testing was done in only 25 patients, and all but 1 had a negative result. The only statistically significant differences between those patients tested and not tested were a higher likelihood in the tested group for multicentric disease (69% versus 44%, p=0.02), neuropathy (50% versus 20%, p=0.004), B-symptoms (50% versus 24%, p=0.02), and a higher white count (8.0 versus 6.2 cells/uL, p=0.01). Survival outcomes for those tested was not superior to those who were not tested (data not shown).
Other associated conditions among our CD cohort included: renal disease (n=6); thrombotic episodes (n=6); immune thrombocytopenic purpura (n=5); autoimmune hemolytic anemia (n=3); bronchiolitis obliterans (n=2); paraneoplastic pemphigus (n=1); and AA amyloidosis (n=1). Patients with multicentric disease were more likely to have these conditions—especially renal disease. Seven patients had malignant lymphoma. Five patients with multicentric CD subsequently developed lymphoma: diffuse large cell lymphoma (n=2), composite lymphoma, small lymphocytic lymphoma, and anaplastic plasmacytoma. Two other patients had Hodgkin's disease predating diagnosis of hyaline vascular CD.
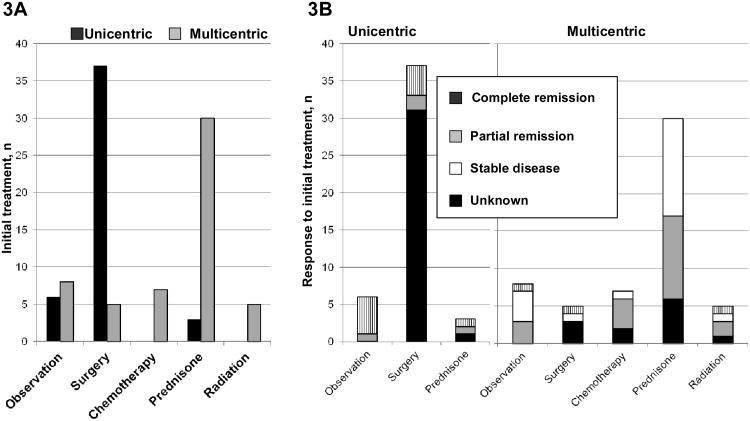
Treatment information was available for 103 patients (Figure 3), ten of whom had less than or equal to 2 months follow up. Fourteen percent (14 of 103) were initially observed. Eighty percent (37 of 46) of the unicentric CD patients were treated with surgery with 89% (33 of 37) achieving either a complete or a partial response. Both patients with unicentric CD had at least some response. Thirty multicentric CD patients were treated with prednisone as initial therapy, and of those 17 had clinical improvement. As first line treatment, only one multicentric CD patient received anthracycline-based therapy, and he responded. Low-dose alkylator-based therapy—cyclophosphamide or chlorambucil—was the initial cytotoxic therapy of choice with benefit in 5 of 6 multicentric CD patients. Two patients received interferon-based therapy, but there is no information on response. Of the 5 multicentric patients treated with radiation up front, response information was available for only 3, all of whom had response. Beyond first-line treatment, therapies included alkylator-based-chemotherapy (n=5), anthracycline-based-chemotherapy (n=2), interferon (n=2), rituximab (n=1).
Figure 3. Treatment decisions and outcomes.
A. Initial therapy based on unicentric or multicentric disease
Due to missing data, total unicentric and multicentric cases are 46 (7 missing) and 57 (3 missing).
B. Response to initial therapy based on extent of disease
First line chemotherapy regimens used were cyclophosphamide/prednisone (n=4), chlorambucil/prednisone (n=2), cyclophosphamide/vincristine/mitroxantrone/prednisone (n=1), and interferon/prednisone (n=2; data not shown)
Disscussion
In this report we illustrate baseline characteristics, treatment choices, and outcomes in this descriptive study spanning 6 decades of experience with a predominantly HIV negative Castleman's disease population from the Mayo Clinic and the University of Nebraska. The major limitations of this study are the incomplete testing and reporting inherent in a retrospective study and the period that cases were ascertained. In most of the 20th century, modern treatments like high-dose chemotherapy with peripheral blood stem cell transplantation, rituximab, and anit-interleukin-6 treatment strategies were not available for CD. Despite these limitations, we have made several important observations that should serve as a platform from which future prospective studies could be based.
The most intriguing observation from the current study is the difference in survival based on whether or not osteosclerotic bone lesions were present among those who satisfied criteria for POEMS syndrome. Those patients who had POEMS syndrome and osteosclerotic bone lesions had the best outcomes, and those who had POEMS syndrome without osteosclerotic bone lesions comprised the group with the worst outcomes. Patients with multicentric CD without POEMS syndrome had an overall survival rate between these two extremes. Although co-existing CD and POEMS syndrome have been reported since the 1970's,(6, 17, 25, 27-29) 18% of our group of 113 CD patients (and 32% of patients with multicentric disease) had criteria sufficient to diagnose co-existing POEMS syndrome. Peripheral neuropathy alone was not a risk factor for death unless it was associated with a non-osteosclerotic variant of POEMS syndrome. Others have also shown that when CD is associated with peripheral neuropathy, edema and impaired peripheral circulation are the most common systemic abnormalities observed,(8, 27, 29-34) which according to modern criteria, could represent undiagnosed POEMS syndrome. Within the context of POEMS syndrome, the absence of osteosclerotic lesions has been implicated as an adverse feature,(24) but the issue in CD had not been addressed. We and others have speculated previously that peripheral neuropathy is associated with a worse outcome,(35, 36) and the current data strongly support that hypothesis. Most of the factors identified as risk factors on univariate analysis, including multicentric disease, plasma cell variant, peripheral neuropathy, organomegaly, low albumin, and an abnormal platelet count, co-segregated with POEMS syndrome, which supports the concept of 4 categories of CD: 1) unicentric CD; 2) multicentric CD associated with the osteosclerotic variant of POEMS syndrome; 3) multicentric CD without POEMS syndrome; and 4) multicentric CD with POEMS syndrome without osteosclerotic lesions. This system supports the observation made by us and others that patients with unicentric disease generally have an excellent prognosis(37-43) and helps clarify which multicentric patients do well.(8, 37-39)
The observations that multicentric patients are older, more likely to have the plasma cell variant, and more likely to be symptomatic are not novel.(5, 7, 8, 35-40, 43, 44) For the 19 patients for whom there was discordance between disease extent and histology, the course of disease was dictated by extent rather than by histology, despite the fact that this was a population of patients who had limited imaging. In addition, the present study clarifies how CD patients with either hepatomegaly or splenomegaly should be classified. The presence of organomegaly, even without biopsy proof, is sufficient to classify a patient as having multicentric rather than unicentric disease, especially since CD histology has been reported in the spleen.(45) Shin et al demonstrated that age and the presence of splenomegaly were the most prognostic clinical variables in their series of 27 patients with multicentric CD.(46) Our composite model using the POEMS syndrome umbrella captures this feature.
CD is not infrequently associated with other conditions like lymphoma and other autoimmune diseases.(42, 44, 47) Seven of our patients had lymphoma sometime during their course. Immune thrombocytopenic purpura, autoimmune hemolytic anemia, renal disease, bronchiolitis obliterans, or thrombosis each affected fewer than 5% of patients in the present study.
This large cohort provides a retrospective overview of treatment options and outcomes, which may be of use for stratification in clinical trials using exciting antibodies directed against either interleukin-6 or receptor interleukin-6 like tocliuzumab or siltuximab.(20, 21, 48) In our series, surgery was the treatment of choice for unicentric CD with excellent outcomes in more than 90% of patients. Prednisone and/or chemotherapy were the most common interventions selected for patients with multicentric CD. As previously reported, low dose chemotherapy—predominantly non-anthracycline-based—resulted in higher response rates than did prednisone.(7, 36, 39, 40) Of interest, non-systemic therapies yielded good results in 5 of 6 multicentric CD patients so treated. There have been reports in the literature of a localized treatment approach resulting in distant response.(37, 49-51) None of the patients in this series had high-dose chemotherapy with autologous stem cell transplant given the timeframe of ascertainment of cases. Despite the omission of this modality, it should not be ignored as therapeutic strategy in this patient population.(26, 34, 52) High-dose chemotherapy with autologous stem cell transplant is considered by many to be the treatment of choice for patients with POEMS syndrome.
In conclusion, when assessing a patient with CD, the most important parameter to consider aside from HIV status is extent of disease,(43) that is, unicentric versus multicentric disease. Although we did not have HIV status for most of our patients, those tested fared no better than those who were not, making it highly probable that our study set was not enriched for patients with HIV. Patients with multicentric CD should be questioned about the presence of peripheral neuropathy. If neuropathic symptoms, patients should be screened for a monoclonal protein with immunofixation of the serum their bones should be screened for osteosclerotic lesions. In the era of digital storage of CT images, bone windows are readily assessable for screening the spine and pelvis. Although we were not able to characterize the type of neuropathy these patients had based on the retrospective nature of this work, personal experience offers the insight that the peripheral neuropathy seen with CD is typically more subtle and more often sensory than motor. The prognostic role tests like interleukin-6, vascular endothelial growth factor, sedimentation rate, fibrinogen, human herpes virus 8, C-reactive protein, and serum immunoglobulin free light chain plays in CD cannot be assessed by the present study. Despite these omissions, this paper further clarifies the spectrum and the relationships between the heterogeneous entities ranging from unicentric CD to multicentric CD with and without the osteosclerotic variant of POEMS syndrome, and hopefully it will lay a framework both to treat patients and to understand the underlying pathogenic mechanisms.
Acknowledgments
AD is supported in part by grant CA91561 (A.D) from the National Cancer Institute, the Robert A. Kyle Hematologic Malignancies Fund, The JABBS Foundation, The Predolin Foundation.
Authorship and Conflict of Interest Statements
There is no conflict of interest. AD and TMH receive clinical trial funding from Ortho Biotech
| AD | JOA | MJL | SMG | JA | JKC | DMM | DDW | KR | AD | TMH | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | x | x | x | x | x | ||||||
| 2 | x | x | x | x | x | ||||||
| 3 | x | x | x | ||||||||
| 4 | x | x | x | ||||||||
| 5 | x | ||||||||||
| 6 | x | x | x | x | x | x | x | x | x | x | x |
1. Conceived design
2. Data abstraction
3. Data analysis
4. Pathologic review
5. Wrote manuscript
6. Edited manuscript
References
- 1.Castleman B, Iverson L, Menendez VP. Localized mediastinal lymph-node hyperplasia resembling thymoma. Cancer. 1956;9:822–830. doi: 10.1002/1097-0142(195607/08)9:4<822::aid-cncr2820090430>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Lee SL, Rosner F, Rivero I, Feldman F, Hurwitz A. Refractory Anemia with Abnormal Iron Metabolism: Its Remission after Resection of Hyperplastic Mediastinal Lymph Nodes. N Engl J Med. 1965 Apr 15;272:761–766. doi: 10.1056/NEJM196504152721502. [DOI] [PubMed] [Google Scholar]
- 3.Tung KS, McCormack LJ. Angiomatous lymphoid hamartoma. Report of five cases with a review of the literature. Cancer. 1967;20:525–536. doi: 10.1002/1097-0142(1967)20:4<525::aid-cncr2820200409>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 4.Flendrig JA, Schiillings PHM. Benign giant lymphoma: The clinical signs and symptoms and the morphological aspects.y. Folia Med. 1969;12:119–120. [Google Scholar]
- 5.Keller AR, Hocholzer L, Castleman B. Hyaline-vascular and plasma-cell types of giant lymph node hyperplasia of the mediastinum and other locations. Cancer. 1972;29:670–683. doi: 10.1002/1097-0142(197203)29:3<670::aid-cncr2820290321>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 6.Gaba AR, Stein RS, Sweet DL, Variakojis D. Multicentric giant lymph node hyperplasia. Am J Clin Pathol. 1978 Jan;69(1):86–90. doi: 10.1093/ajcp/69.1.86. [DOI] [PubMed] [Google Scholar]
- 7.Frizzera G, Banks PM, Massarelli G, Rosai J. A systemic lymphoproliferative disorder with morphologic features of Castleman's disease. Pathological findings in 15 patients. American Journal of Surgical Pathology. 1983 Apr;7(3):211–231. doi: 10.1097/00000478-198304000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Weisenburger DD, Nathwani BN, Winberg CD, Rappaport H. Multicentric angiofollicular lymph node hyperplasia: a clinicopathologic study of 16 cases. Human Pathology. 1985 Feb;16(2):162–172. doi: 10.1016/s0046-8177(85)80065-4. [DOI] [PubMed] [Google Scholar]
- 9.Lachant NA, Sun NC, Leong LA, Oseas RS, Prince HE. Multicentric angiofollicular lymph node hyperplasia (Castleman's disease) followed by Kaposi's sarcoma in two homosexual males with the acquired immunodeficiency syndrome (AIDS) American Journal of Clinical Pathology. 1985 Jan;83(1):27–33. doi: 10.1093/ajcp/83.1.27. [DOI] [PubMed] [Google Scholar]
- 10.Soulier J, Grollet L, Oksenhendler E, Miclea JM, Cacoub P, Baruchel A, et al. Molecular analysis of clonality in Castleman's disease. Blood. 1995 Aug 1;86(3):1131–1138. [PubMed] [Google Scholar]
- 11.Chadburn A, Cesarman E, Nador RG, Liu YF, Knowles DM. Kaposi's sarcoma-associated herpesvirus sequences in benign lymphoid proliferations not associated with human immunodeficiency virus. Cancer. 1997 Aug 15;80(4):788–797. [PubMed] [Google Scholar]
- 12.Parravinci C, Corbellino M, Paulli M, Magrini U, Lazzarino M, Moore PS, et al. Expression of a virus-derived cytokine, KSHV vIL-6, in HIV-seronegative Castleman's disease. American Journal of Pathology. 1997 Dec;151(6):1517–1522. [PMC free article] [PubMed] [Google Scholar]
- 13.O'Leary J, Kennedy M, Howells D, Silva I, Uhlmann V, Luttich K, et al. Cellular localisation of HHV-8 in Castleman's disease: is there a link with lymph node vascularity? Molecular Pathology. 2000 Apr;53(2):69–76. doi: 10.1136/mp.53.2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suda T, Katano H, Delsol G, Kakiuchi C, Nakamura T, Shiota M, et al. HHV-8 infection status of AIDS-unrelated and AIDS-associated multicentric Castleman's disease. Pathology International. 2001 Sep;51(9):671–679. doi: 10.1046/j.1440-1827.2001.01266.x. [DOI] [PubMed] [Google Scholar]
- 15.Amin HM, Medeiros LJ, Manning JT, Jones D. Dissolution of the lymphoid follicle is a feature of the HHV8+ variant of plasma cell Castleman's disease. American Journal of Surgical Pathology. 2003 Jan;27(1):91–100. doi: 10.1097/00000478-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Rieu P, Noel LH, Droz D, Beaufils H, Gessain A, Hermine O, et al. Glomerular involvement in lymphoproliferative disorders with hyperproduction of cytokines (Castleman, POEMS) Advances in Nephrology From the Necker Hospital. 2000;30:305–331. [PubMed] [Google Scholar]
- 17.Bardwick PA, Zvaifler NJ, Gill GN, Newman D, Greenway GD, Resnick DL. Plasma cell dyscrasia with polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes: the POEMS syndrome. Report on two cases and a review of the literature. Medicine (Baltimore) 1980 Jul;59(4):311–322. doi: 10.1097/00005792-198007000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Dispenzieri A, Kyle RA, Lacy MQ, Rajkumar SV, Therneau TM, Larson DR, et al. POEMS syndrome: definitions and long-term outcome. Blood. 2003 Apr 1;101(7):2496–2506. doi: 10.1182/blood-2002-07-2299. [DOI] [PubMed] [Google Scholar]
- 19.Yoshizaki K, Matsuda T, Nishimoto N, Kuritani T, Taeho L, Aozasa K, et al. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman's disease. Blood. 1989 Sep;74(4):1360–1367. [PubMed] [Google Scholar]
- 20.Nishimoto N, Kanakura Y, Aozasa K, Johkoh T, Nakamura M, Nakano S, et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood. 2005 Oct 15;106(8):2627–2632. doi: 10.1182/blood-2004-12-4602. [DOI] [PubMed] [Google Scholar]
- 21.van Rhee F, Fayad L, Voorhees P, Furman R, Lonial S, Borghaei H, et al. Siltuximab, a novel anti-interleukin-6 monoclonal antibody, for Castleman's disease. J Clin Oncol. 2010 Aug 10;28(23):3701–3708. doi: 10.1200/JCO.2009.27.2377. [DOI] [PubMed] [Google Scholar]
- 22.Brandt SJ, Bodine DM, Dunbar CE, Nienhuis AW. Retroviral-mediated transfer of interleukin-6 into hematopoietic cells of mice results in a syndrome resembling Castleman's disease. Curr Top Microbiol Immunol. 1990;166:37–41. doi: 10.1007/978-3-642-75889-8_5. [DOI] [PubMed] [Google Scholar]
- 23.Dispenzieri A. POEMS syndrome: 2011 update on diagnosis, risk-stratification, and management. American Journal of Hematology. 2011 Jul;86(7):591–601. doi: 10.1002/ajh.22050. [DOI] [PubMed] [Google Scholar]
- 24.Soubrier MJ, Dubost JJ, Sauvezie BJ. POEMS syndrome: a study of 25 cases and a review of the literature. French Study Group on POEMS Syndrome. American Journal of Medicine. 1994 Dec;97(6):543–553. doi: 10.1016/0002-9343(94)90350-6. [DOI] [PubMed] [Google Scholar]
- 25.Nakanishi T, Sobue I, Toyokura Y, Nishitani H, Kuroiwa Y, Satoyoshi E, et al. The Crow-Fukase syndrome: a study of 102 cases in Japan. Neurology. 1984 Jun;34(6):712–720. doi: 10.1212/wnl.34.6.712. [DOI] [PubMed] [Google Scholar]
- 26.Dispenzieri A. POEMS syndrome. Blood reviews. 2007 Nov;21(6):285–299. doi: 10.1016/j.blre.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Yu GS, Carson JW. Giant lymph-node hyperplasia, plasma-cell type, of the mediastinum, with peripheral neuropathy. Am J Clin Pathol. 1976 Jul;66(1):46–53. doi: 10.1093/ajcp/66.1.46. [DOI] [PubMed] [Google Scholar]
- 28.Weisenburger DD, DeGowin RL, Gibson P, Armitage JO. Remission of giant lymph node hyperplasia with anemia after radiotherapy. Cancer. 1979 Aug;44(2):457–462. doi: 10.1002/1097-0142(197908)44:2<457::aid-cncr2820440212>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 29.Hineman VL, Phyliky RL, Banks PM. Angiofollicular lymph node hyperplasia and peripheral neuropathy: association with monoclonal gammopathy. Mayo Clin Proc. 1982 Jun;57(6):379–382. [PubMed] [Google Scholar]
- 30.Mallory A, Spink WW. Angiomatous lymphoid hamartoma in the retroperitoneum presenting with neurologic signs in the legs. Ann Intern Med. 1968 Aug;69(2):305–308. doi: 10.7326/0003-4819-69-2-305. [DOI] [PubMed] [Google Scholar]
- 31.Anonymous. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 32-1984. N Engl J Med. 1984;311:388–398. doi: 10.1056/NEJM198408093110608. [DOI] [PubMed] [Google Scholar]
- 32.Anonymous. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 10-1987. A 59-year-old woman with progressive polyneuropathy and monoclonal gammopathy. New England Journal of Medicine. 1987 Mar 5;316(10):606–618. doi: 10.1056/NEJM198703053161008. [DOI] [PubMed] [Google Scholar]
- 33.Donaghy M, Hall P, Gawler J, Gregson NA, Leibowitz S, Jitpimolmard S, et al. Peripheral neuropathy associated with Castleman's disease. J Neurol Sci. 1989 Feb;89(2-3):253–267. doi: 10.1016/0022-510x(89)90027-0. [DOI] [PubMed] [Google Scholar]
- 34.Ganti AK, Pipinos I, Culcea E, Armitage JO, Tarantolo S. Successful hematopoietic stem-cell transplantation in multicentric Castleman disease complicated by POEMS syndrome. Am J Hematol. 2005 Jul;79(3):206–210. doi: 10.1002/ajh.20280. [DOI] [PubMed] [Google Scholar]
- 35.Menke DM, Camoriano JK, Banks PM. Angiofollicular lymph node hyperplasia: a comparison of unicentric, multicentric, hyaline vascular, and plasma cell types of disease by morphometric and clinical analysis. Modern Pathology. 1992 Sep;5(5):525–530. [PubMed] [Google Scholar]
- 36.McCarty MJ, Vukelja SJ, Banks PM, Weiss RB. Angiofollicular lymph node hyperplasia (Castleman's disease) Cancer Treatment Reviews. 1995 Jul;21(4):291–310. doi: 10.1016/0305-7372(95)90034-9. [DOI] [PubMed] [Google Scholar]
- 37.Frizzera G, Peterson BA, Bayrd ED, Goldman A. A systemic lymphoproliferative disorder with morphologic features of Castleman's disease: clinical findings and clinicopathologic correlations in 15 patients. Journal of Clinical Oncology. 1985 Sep;3(9):1202–1216. doi: 10.1200/JCO.1985.3.9.1202. [DOI] [PubMed] [Google Scholar]
- 38.Herrada J, Cabanillas F, Rice L, Manning J, Pugh W. The clinical behavior of localized and multicentric Castleman disease. Annals of Internal Medicine. 1998 Apr 15;128(8):657–662. doi: 10.7326/0003-4819-128-8-199804150-00010. [DOI] [PubMed] [Google Scholar]
- 39.Chronowski GM, Ha CS, Wilder RB, Cabanillas F, Manning J, Cox JD. Treatment of unicentric and multicentric Castleman disease and the role of radiotherapy. Cancer. 2001 Aug 1;92(3):670–676. doi: 10.1002/1097-0142(20010801)92:3<670::aid-cncr1369>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 40.Bowne WB, Lewis JJ, Filippa DA, Niesvizky R, Brooks AD, Burt ME, et al. The management of unicentric and multicentric Castleman's disease: a report of 16 cases and a review of the literature. Cancer. 1999 Feb 1;85(3):706–717. doi: 10.1002/(sici)1097-0142(19990201)85:3<706::aid-cncr21>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 41.Kasantikul V, Panyavoravut V, Benjavongkulchai S, Panichabhongse V. Castleman's disease: a clinicopathologic study of 12 cases. Journal of the Medical Association of Thailand. 1997 Mar;80(3):195–201. [PubMed] [Google Scholar]
- 42.Dispenzieri A. Castleman disease. Cancer Treat Res. 2008;142:293–330. doi: 10.1007/978-0-387-73744-7_13. [DOI] [PubMed] [Google Scholar]
- 43.Talat N, Schulte KM. Castleman's disease: systematic analysis of 416 patients from the literature. Oncologist. 2011;16(9):1316–1324. doi: 10.1634/theoncologist.2011-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frizzera G. Castleman's disease and related disorders. Seminars in Diagnostic Pathology. 1988 Nov;5(4):346–364. [PubMed] [Google Scholar]
- 45.Weisenburger DD. Multicentric angiofollicular lymph node hyperplasia. Pathology of the spleen. American Journal of Surgical Pathology. 1988 Mar;12(3):176–181. doi: 10.1097/00000478-198803000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Shin DY, Jeon YK, Hong YS, Kim TM, Lee SH, Kim DW, et al. Clinical dissection of multicentric Castleman disease. Leuk Lymphoma. 2011 Aug;52(8):1517–1522. doi: 10.3109/10428194.2011.574759. [DOI] [PubMed] [Google Scholar]
- 47.Weisenburger DD. Membranous nephropathy. Its association with multicentric angiofollicular lymph node hyperplasia. Arch Pathol Lab Med. 1979 Oct;103(11):591–594. [PubMed] [Google Scholar]
- 48.Nishimoto N, Sasai M, Shima Y, Nakagawa M, Matsumoto T, Shirai T, et al. Improvement in Castleman's disease by humanized anti-interleukin-6 receptor antibody therapy. Blood. 2000 Jan 1;95(1):56–61. [PubMed] [Google Scholar]
- 49.Marti S, Pahissa A, Guardia J, Moragas A, Bacardi R. Multicentric giant follicular lymph node hyperplasia. Favorable response to radiotherapy. Cancer. 1983 Mar 1;51(5):808–810. doi: 10.1002/1097-0142(19830301)51:5<808::aid-cncr2820510510>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 50.Sethi T, Joshi K, Sharma SC, Gupta BD. Radiation therapy in the management of giant lymph node hyperplasia. British Journal of Radiology. 1990 Aug;63(752):648–650. doi: 10.1259/0007-1285-63-752-648. [DOI] [PubMed] [Google Scholar]
- 51.Lerza R, Castello G, Truini M, Ballarino P, Tredici S, Cavallini D, et al. Splenectomy induced complete remission in a patient with multicentric Castleman's disease and autoimmune hemolytic anemia. Annals of Hematology. 1999 Apr;78(4):193–196. doi: 10.1007/s002770050500. [DOI] [PubMed] [Google Scholar]
- 52.Tal Y, Haber G, Cohen MJ, Phillips M, Amir G, Ben-Yehuda D, et al. Autologous stem cell transplantation in a rare multicentric Castleman disease of the plasma cell variant. Int J Hematol. 2011 May;93(5):677–680. doi: 10.1007/s12185-011-0812-0. [DOI] [PubMed] [Google Scholar]