Abstract
H19 RNA has been characterized as an oncogenic long non-coding RNA (lncRNA) in breast and colon cancer. However, the role and function of lncRNA H19 in glioma development remain unclear. In this study, we identified that H19/miR-675 signaling was critical for glioma progression. By analyzing glioma gene expression data sets, we found increased H19 in high grade gliomas. H19 depletion via siRNA inhibited invasion in glioma cells. Further, we found H19 positively correlated with its derivate miR-675 expression and reduction of H19 inhibited miR-675 expression. Bioinformatics and luciferase reporter assays showed that miR-675 modulated Cadherin 13 expression by directly targeting the binding site within the 3′ UTR. Finally, introduction of miR-675 abrogated H19 knockdown-induced cell invasion inhibition in glioma cells. To our knowledge, it is first time to demonstrate that H19 regulates glioma development by deriving miR-675 and provide important clues for understanding the key roles of lncRNA-miRNA functional network in glioma.
Introduction
It has been reported that about 98% of the “junk” DNAs are transcribed as non-coding RNAs (ncRNAs) including short ncRNAs (which include, but are not limited to, microRNAs) and long ncRNAs (lncRNAs) [1]. LncRNAs, are defined as endogenous cellular RNAs of more than 200 nucleotides in length and implicated in a myriad of molecular functions, such as modulation of alternative splicing, chromatin remodeling and RNA metabolism [2], [3], [4], [5]. Although lncRNAs are longer than, and functionally as well as structurally distinct from known endogenous small RNAs such as microRNAs, there are some connections between these RNA classes: a small number of lncRNA genes harbor internally encoded small RNAs and certain lncRNAs may acquire functionality by acting as the precursor to small RNAs capable of regulatory function, such as microRNAs[6]. To date, emerging evidence has strongly suggested that aberrant microRNA expression is a feature of human glioblastoma[3]. However, lncRNA-miRNA network in glioma remains unknown and needs further investigation.
LncRNA H19 is produced from imprinted genes H19, whose expression is depending on the parental origin of the chromosome. LncRNA H19 has been considered as an oncogenic lncRNA in hepatocellular and bladder carcinoma [7], [8], [9], [10], [11]. Matouk et al. found that H19 is significantly elevated after exposure to hypoxia, and H19 has pro-tumorigenic properties [12]. Berteaux et al. demonstrated that lncRNA H19 is actively linked to E2F1 (E2F transcription factor 1) to promote cell-cycle progression of breast cancer cells [13]. But the underlying role and mechanism of H19 involved in glioma development remains unclear.
In the current study, we explored the clinical feature, biological function and potential mechanism of lncRNA H19 in glioma. We found that H19 was closely correlated with tumor grade in 3 different glioma data sets. Moreover, as a precursor, H19 derived miR-675 and then regulated Cadherin 13 (CDH13) which is the directly target of miR-675, thereby modulating glioma cell invasion. The oncogenic function of H19/miR-675 signaling may serve as the potential target for glioma therapy.
Materials and Methods
Human tissue samples and cell lines
158 glioma data with mRNA and miRNA expression microarray were downloaded from Chinese Glioma Genome Atlas (CGGA) data portal (http://www.cgga.org.cn/portal.php). The samples comprised 48 astrocytomas (A, WHO Grade II), 13 oligodendrogliomas (O, WHO Grade II), 8 anaplastic astrocytomas (AA, WHO Grade III), 10 anaplastic oligodendrogliomas (AO, WHO Grade III), 15 anaplastic oligoastrocytomas (AOA, WHO Grade III) and 64 GBM (WHO Grade IV). High grade glioma (HGG) including AA, AO, AOA and GBM. Low grade glioma (LGG) including O and A. Glioma gene expression data sets are deposited at Rembrandt data (https://caintegrator.nci.nih.gov/rembrandt/) and the Gene Expression Omnibus Web site (http://www.ncbi.nlm.nih.gov/geo/, accession No. GSE16011). The human U87 and U251 glioblastoma cell lines were purchased from the Chinese Academy of Sciences Cell Bank. The cells were grown in Dulbecco's modified Eagle's medium (DMEM) (Gibco,Los Angeles, CA, USA),supplemented with 10% fetal bovine serum in an atmosphere containing 5% CO2 at 37°C.
Oligonucleotides and transfection
The 2′-O-methy1 (2′ – OMe-) oligonucleotides were chemically synthesized by GenePharma (Shanghai, China). The sequences are: H19 small interfering RNA (siRNA) 1: sense, 5′-CCC ACA ACA UGA AAG AAA CTT-3′, antisense: 5′-AUU UCU UUC AUG UUG UGG GTT-3′; H19 small interfering RNA (siRNA) 2: sense, 5′-GCU AGA GGA ACC AGA CCU UTT-3′, antisense: 5′-AAG GUC UGG UUC CUC UAG CTT-3′; A siRNA that was unrelated to any human sequence was used as a negative control (NC): sense, 5′-UUC UCC GAA CGU GUC ACG UTT-3′, antisense: 5′-ACG UGA CAC GUU CGG AGA ATT-3′; 2′-OMe-hsa-miR-675 inhibitor: 5′-UGA GCG GUG AGG GCA UAC AG-3′; 2′-OMe-hsa-miR-675 mimics: sense, 5′-CUG UAU GCC CUC ACC GCU CA-3′, antisense: 5′-AGC GGU GAG GGC AUA CAG UU-3′; MircoRNA inhibitor negative control(NC), 5′-CAG UAC UUU UGU GUA GUA CAA-3′. Oligonucleotides (20 µM) were transfected into U87 and U251 glioblastoma cells using Lipofectamine 2000 (Invitrogen) following the manufacturer's instructions.
RNA extraction and Quantitative RT-PCR
RNA was extracted from cells after transfected using TRIzol (Invitrogen) following the manufacturer's protocol. To detect the levels of miR-675 in cells, reverse transcription (RT) was conducted with the Applied Biosystems® TaqMan® MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). The primers for the miR-675 were purchased from Guangzhou Ribo BioCoLTD (Guangzhou, China). U6 was used for normalization. The ABI StepOne Plus (Applied Biosystems, Foster City, CA) was used to perform the amplification reaction. And the date was analyzed by the 2−ΔΔCt method. The PCR reaction for
H19 was performed as previously described [13], [14]. Each experiment was performed in triplicate.
In vitro invasion assay
Cell invasion was determined by the transwell assay and the scratch wound assay. Transwell assay: the related oligonucleotides were transfected into the cells according to the protocol. After incubated for 48 hours, 3×104 cells were transferred on the top of the Matrigel-coated invasion chambers (BD Biosciences, San Jose, USA) in a serum-free DMEM and we add the DMEM containing 10% fetal bovine serum to the lower chamber. After 24 h, non-invasion cells were removed, and the invading cells were fixed with 95% ethanol, stained with0.1% crystal violet, photographed (×100). Tests were repeated via three independent experiments. Scratch wound assay: the related oligonucleotides were transfected into the cells in six-well plates. Cell layers were scratched using a sterile pipette tip to form wound gaps. The wound location in the six-well plates was marked. Cells were photographed to record the wound width (0 h). Twelve (U87) or twenty-four hours later (U251), photographs will be taken again at the marked wound location to measure the cell migration ability.
Luciferase assay
The 3′-UTR of CDH13 containing the putative miR-675 binding sequences was cloned into a firefly luciferase reporter construct. The 3′-UTR of CDH13 without the putative miR-675 bindng sequences was used as mutated controls (Invitrogen). The pGL3-WT-CDH13-3′UTR-Luc/pGL3-MUT-CDH13-3′UTR-Luc and miR-675 mimics/miR-675 inhibitor were co-transfection into the cells. Luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega, USA).
Western blotting
The oligonucleotides were transfected into the cells. Proteins were extracted from cells with RIPA lysis buffer (KenGEN, China) and were quantified using a BCA Protein Assay Kit (Beyotime, China). We add the 30 µg of protein lysates to SDS-PAGE. The electrophoresed proteins were transferred to PVDF membranes membranes (Millipore, USA). The membrane was blocked in 5% nonfat milk and incubated with diluted antibodies against CDH13 (1∶200; Santa Cruz; USA), followed by incubation with a horseradish peroxidase-conjugated secondary antibody (1∶2500; Santa Cruz; USA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a control (1∶1000, CST, USA).
Statistical analysis
All experiments were performed three times. All data are presented as mean ± standard error. Data were analyzed with SPSS 10.0.Statistical. Evaluation of the data was performed by t-test (two-sided) and one-way-ANOVA. P<0.05 was considered statistically significant.
Results
H19 expression correlates with glioma grade
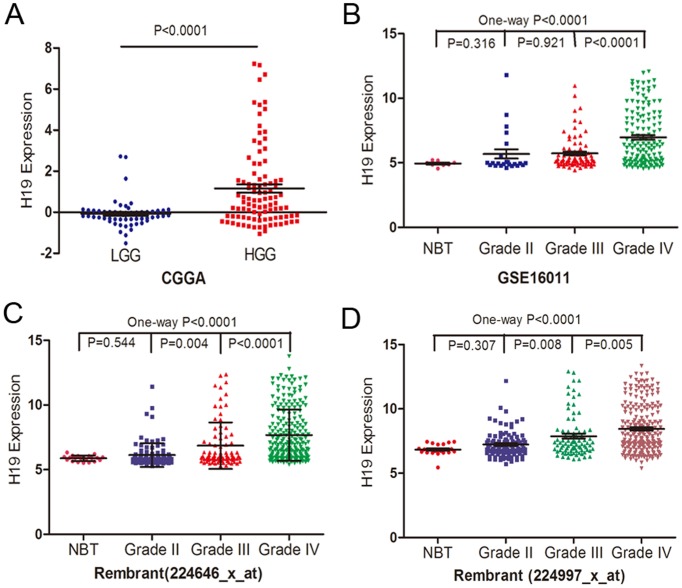
We initially analyzed H19 expression pattern in whole genome gene profiling of 158 glioma tissues in CGGA data and found that, as shown in Fig. 1A, H19 expression was significant higher in HGG tissues than in LGG ones (P<0.0001). Further, two independent glioma gene expression data sets (Rembrandt data and GSE16011 data) were employed to examine the association between H19 expression levels and glioma grade (Fig. 1 B–D). One-way ANOVA analysis showed that H19 expression levels were significantly associated with tumor grade (P<0.0001 for both Rembrandt data and GSE16011 data), which was similar with the CGGA data. These findings demonstrate that H19 may play an important role in glioma progress.
Figure 1. H19 expression in glioma tissues.
(A) H19 levels were analyzed in glioma tissues of the CGGA glioma datasets (61 cases of grade II, 33 cases of grade III and 64 cases of grade IV). (B) The expression of H19 was analyzed in glioma tissues of the GSE16011 glioma datasets. (C–D) H19 expression with two probes was analyzed in glioma tissues of the Rembrant glioma datasets.
H19 induced invasion of glioma cell
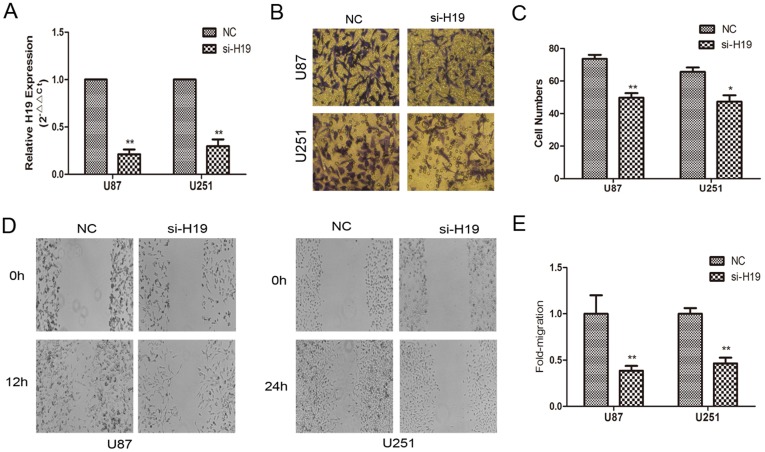
To explore the effect of H19 RNA on cell invasion in glioma, H19 was down-regulated by si-RNA (Fig. 2 A). The effects of H19 RNA on the invasiveness and migration of glioma cells were checked by Transwell and wound healing assays. An in vitro Matrigel invasion assay revealed that invasiveness of U87 and U251 cells transfected with si-H19 were suppressed compared with negative control (Fig. 2 B and C). The results of in vitro wound healing assay displayed that the levels of H19 was decreased, the migration of U87 and U251 cells was significantly attenuated compared with the control cells (Fig. 2 D and E).
Figure 2. Evaluation of biological functions of H19 in U87 and U251 cells.
(A) Transfection efficiency of si-H19 in U87 and U251 cells was indicated by PCR. (B–C) si-H19 inhibited the invasiveness of U87 and U251 cells. Cells were examined for cell invasion in 24-well plates with transwell chambers. Migrated cells were stained with crystal violet. The invasiveness of U87 and 251 cells was attenuated with the decreased expression of H19. (D–E) Wound healing assay of glioma cells transfected with either control or the si-H19, respectively. The wound healing was photographed at different time points and wounded gaps were analyzed by measuring the distance of migrating cells for 3 different areas for each wound. *P<0.05, **P<0.01.
H19 is a developmental reservoir of miR-675 in promote cell invasion
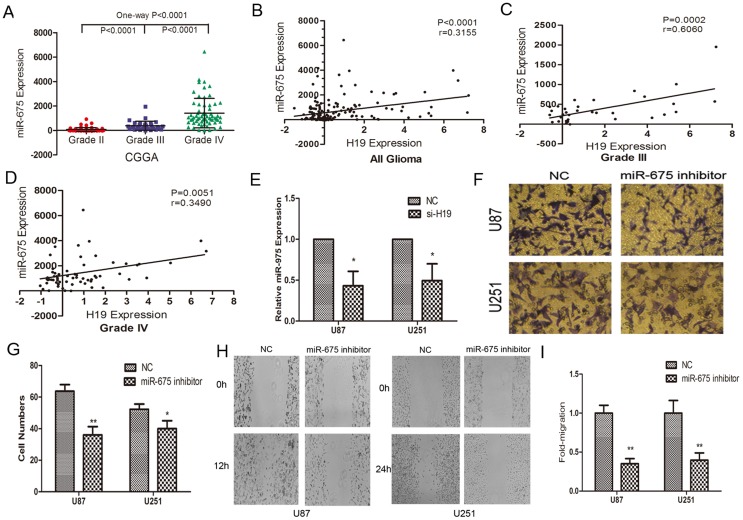
H19 is reported to be the primary precursor of two distinct miRNAs (miR-675-5p and miR-675-3p), in which transfection with H19 complementary DNA containing the pri-miR-675 hairpin increased the expression of mature miR-675 in human kidney 293T cells and it has been suggested that it may be these miRNAs that confer functionality on H19 [15], [16], [17]. Thus, we analyzed miR-675 expression level in 158 glioma tissues in CGGA data. One-way ANOVA analysis showed that miR-675 was significantly associated with tumor grade and miR-675 expression was significant higher in high grade glioma than in low grade glioma (Fig. 3 A). To evaluate the potential correlativity in aberrant expression of H19 and miR-675 expression values of 158 glioma specimens in CGGA data, Pearson correlation assay revealed a significant and positive correlation between H19 and miR-675 (Fig. 3 B–D). We then found that H19 knockdown remarkably reduced miR-675 expression in U87 and U251 cells (Fig. 3 E). Furthermore, we also found that miR-675 inhibition significantly suppressed cell invasion in U87 and U251 glioma cells (Fig. 3 F–I).
Figure 3. H19 regulates the expression of miR-675 which is associated with cell invasion.
(A) MiR-675 expression in 158 glioma tissues of the CGGA glioma datasets. (B) The data shows that miR-675 positively correlated with H19, in 158 glioma samples. (C–D) MiR-675 positively correlated with H19 in HGG of 158 glioma samples. (E) si-H19 decreases miR-675 expression compared with NC in U87 and U251 cells. (F–G) The effects of miR-675 inhibitor on the invasion of U87 and U251 cells were assessed by transwell invasion assay. (H–I) The results of in vitro scratch wound assay showed that knockdown miR-675 inhibit the migration of glioma cells. *P<0.05, **P<0.01.
Cadherin 13 is a direct target of miR-675
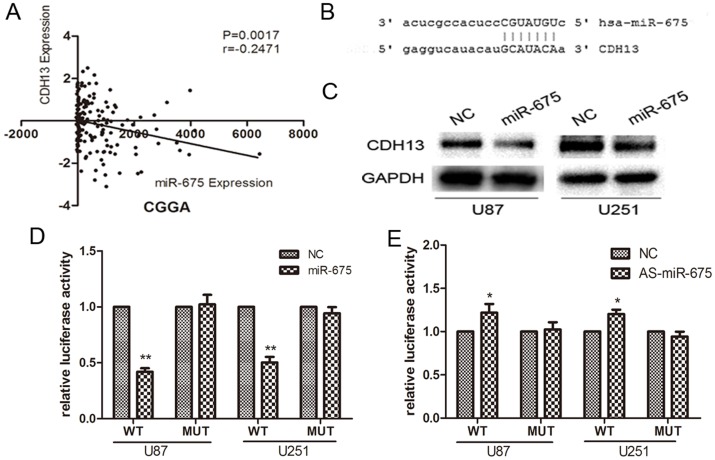
To investigate the mechanism of action of miR-675 in glioma cell invasion, we performed bioinformatic analysis, using miRanda and Pictar alghorithms, and found that the “seed sequence” of miR-675 matched the 3′ UTR of the CDH 13 gene (Fig 4A). We used Pearson correlations to analyze the relationship between miR-675 and CDH13 in 158 glioma specimens from CGGA date and determined the expression of miR-675 was correlated with CDH13 in glioma (P = 0.0017; Fig. 4B). Further, Western blot analysis showed that increasing miR-675 expression in cells led to a decrease in CDH13 protein (Fig. 4C). To indicate the direct interaction between miR-675 and its binding site within 3′ UTR of CDH13, we created pGL3-WT-CDH13-3′UTR plasmids and pGL3-MUT-CDH13-3′UTR. Luciferase reporter assays showed that over-expression of miR-675(supplementary Fig.S1) led to a marked decrease of luciferase activity of PGL3-WT-CDH13-3′UTR and knock-down miR-675(supplementary Fig. S2) led to an increase of the luciferase activity in U87 and U251 cells without change in luciferase activity of PGL3-MUT-CDH13-3′UTR (Fig. 4 D and E). These results indicate that miR-675 directly modulate CDH13 expression by binding 3′ UTR of CDH13.
Figure 4. CDH13 is a directly target of miR-675.
(A) Correlations of miR-675 with CDH13 in glioma tissues. (B) Putative binding sites of miR-675 within theCDH13 3′UTR, as predicted by miRanda and Pictar alghorithms. (C) CDH13 protein levels were measured in U87 and U251 cells at 48 h post-transfection. (D) MiR-675 down-regulated luciferase activities controlled by wild-type CDH13 3′UTR, but did not affect luciferase activity controlled by mutant CDH13 3′UTR. The luciferase activity was measured by dual-luciferase reporter assay (Promega) and was normalized to Renilla luciferase activity. *P<0.05, **P<0.01.
Expression of miR-675 overrides si-H19-induced modulation of invasion in glioma
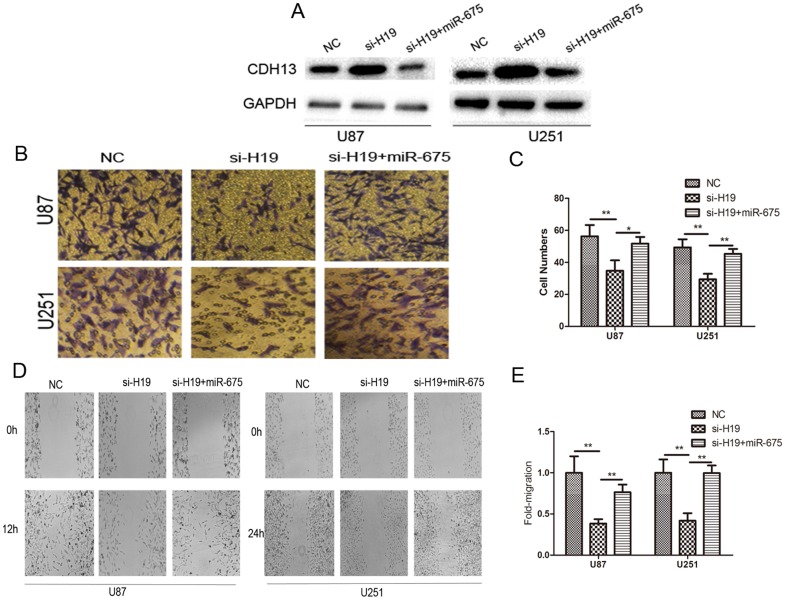
Having demonstrated the relationship between lncRNA H19 and miR-675 by our present data, the importance of miR-675 in lncRNA H19-mediated cell invasion is still unclear in glioma cells. Western blot analysis showed that miR-675 largely abrogated the effect of si-H19 on elevating the expression of CDH13 (Figure 5 A). As shown in Fig. 5 B and C, increased miR-675 in lncRNA H19-depleted cells rescued the invasion phenotype induced by si-H19 transfection. Moreover, expression of miR-675 largely rescued the effect of si-H19 on cell migration (Fig. 5 D and E). Our results strongly indicated that miR-675 is a critical participant of H19 involved in cell invasion.
Figure 5. Effect of H19/miR-675 signaling on glioma cell biology.
(A) CDH13 expression changes following transfection with si-H19 and miR-675 identified by Western blots. (B–C) Effect of si-H19 and miR-675 on glioma cell invasion, after differential treatment in the in vitro transwell invasion assay. (D–E) Cell migration change was analyzed by scratch wound assay. *P<0.05, **P<0.01.
Discussion
H19 is transcribed in an untranslated RNA molecule (lncRNA H19) [18] that lacks conserved open reading frames but does have a conserved secondary RNA structure [19], which accumulates in the human placenta and several fetal tissues, and probably plays a pivotal role in embryogenesis and fetal growth and development [20]. H19 is located on chromosome 11 p 15.5 and lies within 200 kbp downstream of the IGF-2 gene. These two genes are imprinted in opposite directions, so that the paternal IGF-2 and the maternal H19 alleles are selectively expressed [21], [22]. Extensive deletions and/or point mutations in the 5′-long untranslated region of an ectopic human H19 RNA enable 26-kDa protein translation, but no endogenous translation product has so far been identified[23], [24]. Therefore, it was proposed that H19 functions as a riboregulator [18]. Since the first mention of H19 in 1984 by Pachnis et al[25], its functions remain enigmatic. It has been suggested that H19 functions as a tumor suppressor in some Wilms' tumors, embryonic rhabdomyosarcoma, and the Beckwith-Wiedmann cancer predisposing syndrome [26], [27], [28]. Furthermore, ectopic expression of the H19 gene in human embryonic tumour cell lines leads to loss of clonogenicity and reduced tumourigenicity in nude mice[6], [29]. However, several other studies [29] have shown that H19 characterized as oncogenic factor in breast adenocarcinoma, bladder tumor and choriocarcinoma. Our study also demonstrated that H19 play a key role in contributing tumorigenesis. H19 was associated with tumor grade, and H19 repression inhibited glioma cell invasion.
Smits et al. [30] showed a high conservation of H19 over 148 Ma of mammalian evolution and suggested that the H19 transcript have a dual role: first as the full-length transcript based on the conservation of exon-intron structure and second as the miR-675 precursor based on the sequence similarity. Recently, H19 was reported to be the primary miRNA precursor of miR-675 in both human and mice [16]. In our in this study, we showed that both miR-675 and its precursor H19 expressions are elevated in HGG tissues compared with LGG ones. In addition, miR-675 was found to positively correlate with H19 expression in CGGA microarray data. In vitro experiment showed that deprivation of H19 expression remarkably reduced miR-675 expression in glioma cells. Such correlation is in agreement with the findings from the present study showing that H19 is the precursor of miR-675 in glioma. Furthermore, our results proved that the effect of miR-675 on invasion in glioma cells by directly targeting CDH13. Moreover, further study implies miR-675 largely abrogated the effect of si-H19 on elevating the invasion of glioma cells. In short, the present study confirmed the important role of the miR-675 pathway in the biological function of H19.
According to our data, the oncogenic function of H19/miR-675 is featured by targeting the nonclassical cadherin CDH13. In recent years, the associations of CDH13 with human cancers have been proposed. Current studies have highlighted the role of CDH13 as a tumor suppressor in lung cancer, breast cancer and malignant melanoma, and so on[31]. But the effect of CDH13 on glioma is still poorly understood. In the present study, we firstly showed the role of the H19/miR-675/CDH13 pathway in glioma development. However, more function of H19 besides provide miR-675 in glioma need further investigation.
In summary, H19 and its derivate miR-675 were positively correlated with glioma grade. And H19 regulated glioma cell invasion by deriving miR-675 and inhibited CDH13. Here, we find lncRNA directly regulates miRNA expression, and plays a “trigger” role in inducing invasion in glioma by deriving miRNA. To our knowledge, this is the first study to show the role and function of H19 in glioma. Therefore, understanding the key role of “lncRNA-miRNA” module in glioma will lead to the identification of new therapeutic targets for treating glioma and warrants further investigation.
Supporting Information
Transfection efficiency of miR-675 mimics in glioma cells. The expression of miR-675 was up-regulated by miR-675 mimics in U87 and U251 cells and the levels of miR-675 were indicated by PCR. *P<0.05, **P<0.01.
(TIF)
Transfection efficiency of AS-miR-675 in U87 and U251 cells. MiR-675 was down-regulated by AS-miR-675 and the levels of miR-675 were indicated by PCR. *P<0.05, **P<0.01.
(TIF)
Acknowledgments
We thank Chinese Glioma Cooperative Group (CGCG) for human tissue samples data assistance.
Funding Statement
This work is supported by National High Technology Research and Development Program 863 (2012AA02A508), China National Natural Scientific Fund (81072078, 81172389 and 81101901), Jiangsu Province's Natural Science Foundation (BK2010580 and 2011847), Jiangsu Province's Key Discipline of Medicine (XK201117), Jiangsu Province's Medical Major Talent program (RC2011051), Program for Development of Innovative Research Team in the First Affiliated Hospital of NJMU and Provincial Initiative Program for Excellency Disciplines, Jiangsu Province. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, et al. (2001) Initial sequencing and analysis of the human genome. Nature 409: 860–921. [DOI] [PubMed] [Google Scholar]
- 2. Gutschner T, Diederichs S (2012) The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol 9: 703–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Calin GA, Croce CM (2006) MicroRNA signatures in human cancers. Nat Rev Cancer 6: 857–866. [DOI] [PubMed] [Google Scholar]
- 4. Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, et al. (2010) Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 464: 1071–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kotake Y, Nakagawa T, Kitagawa K, Suzuki S, Liu N, et al. (2011) Long non-coding RNA ANRIL is required for the PRC2 recruitment to and silencing of p15(INK4B) tumor suppressor gene. Oncogene 30: 1956–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hao Y, Crenshaw T, Moulton T, Newcomb E, Tycko B (1993) Tumour-suppressor activity of H19 RNA. Nature 365: 764–767. [DOI] [PubMed] [Google Scholar]
- 7. Adriaenssens E, Dumont L, Lottin S, Bolle D, Lepretre A, et al. (1998) H19 overexpression in breast adenocarcinoma stromal cells is associated with tumor values and steroid receptor status but independent of p53 and Ki-67 expression. Am J Pathol 153: 1597–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lottin S, Adriaenssens E, Dupressoir T, Berteaux N, Montpellier C, et al. (2002) Overexpression of an ectopic H19 gene enhances the tumorigenic properties of breast cancer cells. Carcinogenesis 23: 1885–1895. [DOI] [PubMed] [Google Scholar]
- 9. Lustig-Yariv O, Schulze E, Komitowski D, Erdmann V, Schneider T, et al. (1997) The expression of the imprinted genes H19 and IGF-2 in choriocarcinoma cell lines. Is H19 a tumor suppressor gene? Oncogene 15: 169–177. [DOI] [PubMed] [Google Scholar]
- 10. Biran H, Ariel I, de Groot N, Shani A, Hochberg A (1994) Human imprinted genes as oncodevelopmental markers. Tumour Biol 15: 123–134. [DOI] [PubMed] [Google Scholar]
- 11. Cooper MJ, Fischer M, Komitowski D, Shevelev A, Schulze E, et al. (1996) Developmentally imprinted genes as markers for bladder tumor progression. J Urol 155: 2120–2127. [PubMed] [Google Scholar]
- 12. Matouk IJ, DeGroot N, Mezan S, Ayesh S, Abu-lail R, et al. (2007) The H19 non-coding RNA is essential for human tumor growth. PLoS One 2: e845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berteaux N, Lottin S, Monte D, Pinte S, Quatannens B, et al. (2005) H19 mRNA-like noncoding RNA promotes breast cancer cell proliferation through positive control by E2F1. J Biol Chem 280: 29625–29636. [DOI] [PubMed] [Google Scholar]
- 14. Tsang WP, Ng EK, Ng SS, Jin H, Yu J, et al. (2010) Oncofetal H19-derived miR-675 regulates tumor suppressor RB in human colorectal cancer. Carcinogenesis 31: 350–358. [DOI] [PubMed] [Google Scholar]
- 15. Gabory A, Jammes H, Dandolo L (2010) The H19 locus: role of an imprinted non-coding RNA in growth and development. Bioessays 32: 473–480. [DOI] [PubMed] [Google Scholar]
- 16. Cai X, Cullen BR (2007) The imprinted H19 noncoding RNA is a primary microRNA precursor. RNA 13: 313–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dudek KA, Lafont JE, Martinez-Sanchez A, Murphy CL (2010) Type II collagen expression is regulated by tissue-specific miR-675 in human articular chondrocytes. J Biol Chem 285: 24381–24387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brannan CI, Dees EC, Ingram RS, Tilghman SM (1990) The product of the H19 gene may function as an RNA. Mol Cell Biol 10: 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Juan V, Crain C, Wilson C (2000) Evidence for evolutionarily conserved secondary structure in the H19 tumor suppressor RNA. Nucleic Acids Res 28: 1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ariel I, de Groot N, Hochberg A (2000) Imprinted H19 gene expression in embryogenesis and human cancer: the oncofetal connection. Am J Med Genet 91: 46–50. [DOI] [PubMed] [Google Scholar]
- 21. Giannoukakis N, Deal C, Paquette J, Goodyer CG, Polychronakos C (1993) Parental genomic imprinting of the human IGF2 gene. Nat Genet 4: 98–101. [DOI] [PubMed] [Google Scholar]
- 22. Zhang Y, Tycko B (1992) Monoallelic expression of the human H19 gene. Nat Genet 1: 40–44. [DOI] [PubMed] [Google Scholar]
- 23. Joubel A, Curgy JJ, Pelczar H, Begue A, Lagrou C, et al. (1996) The 5′ part of the human H19 RNA contains cis-acting elements hampering its translatability. Cell Mol Biol (Noisy-le-grand) 42: 1159–1172. [PubMed] [Google Scholar]
- 24. Pachnis V, Belayew A, Tilghman SM (1984) Locus unlinked to alpha-fetoprotein under the control of the murine raf and Rif genes. Proc Natl Acad Sci U S A 81: 5523–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pachnis V, Brannan CI, Tilghman SM (1988) The structure and expression of a novel gene activated in early mouse embryogenesis. EMBO J 7: 673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Okamoto K, Morison IM, Taniguchi T, Reeve AE (1997) Epigenetic changes at the insulin-like growth factor II/H19 locus in developing kidney is an early event in Wilms tumorigenesis. Proc Natl Acad Sci U S A 94: 5367–5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Scrable HJ, Sapienza C, Cavenee WK (1990) Genetic and epigenetic losses of heterozygosity in cancer predisposition and progression. Adv Cancer Res 54: 25–62. [DOI] [PubMed] [Google Scholar]
- 28. Steenman MJ, Rainier S, Dobry CJ, Grundy P, Horon IL, et al. (1994) Loss of imprinting of IGF2 is linked to reduced expression and abnormal methylation of H19 in Wilms' tumour. Nat Genet 7: 433–439. [DOI] [PubMed] [Google Scholar]
- 29. Rachmilewitz J, Elkin M, Rosensaft J, Gelman-Kohan Z, Ariel I, et al. (1995) H19 expression and tumorigenicity of choriocarcinoma derived cell lines. Oncogene 11: 863–870. [PubMed] [Google Scholar]
- 30. Smits G, Mungall AJ, Griffiths-Jones S, Smith P, Beury D, et al. (2008) Conservation of the H19 noncoding RNA and H19-IGF2 imprinting mechanism in therians. Nat Genet 40: 971–976. [DOI] [PubMed] [Google Scholar]
- 31. Andreeva AV, Kutuzov MA (2010) Cadherin 13 in cancer. Genes Chromosomes Cancer 49: 775–790. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transfection efficiency of miR-675 mimics in glioma cells. The expression of miR-675 was up-regulated by miR-675 mimics in U87 and U251 cells and the levels of miR-675 were indicated by PCR. *P<0.05, **P<0.01.
(TIF)
Transfection efficiency of AS-miR-675 in U87 and U251 cells. MiR-675 was down-regulated by AS-miR-675 and the levels of miR-675 were indicated by PCR. *P<0.05, **P<0.01.
(TIF)