Abstract
Risk factors associated with chronic otitis media (COM) and recurrent otitis media (ROM) have been investigated in previous studies. The objective of this study was to integrate the findings and determine the possible risk factors for COM/ROM based on our meta-analysis. A comprehensive search of electronic bibliographic databases (PubMed, Embase, CNKI and Wanfang database) from 1964 to Dec 2012, as well as a manual search of references of articles, was performed. A total of 2971 articles were searched, and 198 full-text articles were assessed for eligibility; 24 studies were eligible for this meta-analysis. Regarding risk factors for COM/ROM, there were two to nine different studies from which the odds ratios (ORs) could be pooled. The presence of allergy or atopy increased the risk of COM/ROM (OR, 1.36; 95% CI, 1.13–1.64; P = 0.001). An upper respiratory tract infection (URTI) significantly increased the risk of COM/ROM (OR, 6.59; 95% CI, 3.13–13.89; P<0.00001). Snoring appeared to be a significant risk factor for COM/ROM (OR, 1.96; 95% CI, 1.78–2.16; P<0.00001). A patient history of acute otitis media (AOM)/ROM increased the risk of COM/ROM (OR, 11.13; 95% CI, 1.06–116.44; P = 0.04). Passive smoke significantly increased the risk of COM/ROM (OR, 1.39; 95% CI, 1.02–1.89 P = 0.04). Low social status appeared to be a risk factor for COM/ROM (OR, 3.82; 95% CI, 1.11–13.15; P = 0.03). Our meta-analysis identified reliable conclusions that allergy/atopy, URTI, snoring, previous history of AOM/ROM, Second-hand smoke and low social status are important risk factors for COM/ROM. Other unidentified risk factors need to be identified in further studies with critical criteria.
Introduction
Chronic otitis media (COM) and recurrent otitis media (ROM) are two of the most common infectious diseases worldwide. COM and ROM affect diverse cultural and racial groups that are distributed in both developing and industrialized countries. A cross-sectional study conducted in nine countries over three continents revealed that disease prevalence is significant enough to be considered for clinical practice [1]. COM/ROM can cause hearing impairment and speech delay. COM can cause both intracranial and extracranial complications [2]. Effective treatment of the diseases depends on a thorough understanding of the risk factors.
Risk factors associated significantly with COM/ROM include ethnicity [3]–[5], genetic factors [6], gender [7], day-care center attendance [8], breast-feeding [9], and allergy/atopy [10] etc. as reported in previous studies. However, many of the reported studies were difficult to compare because they lacked clear case definitions, standard diagnostic criteria or control groups to evaluate the potential study biases. We conducted a meta-analysis of all available published data and qualified studies that investigated the potential risk factors for COM/ROM to clarify and propose possible means of treatment of the disease.
Materials and Methods
Study Identification
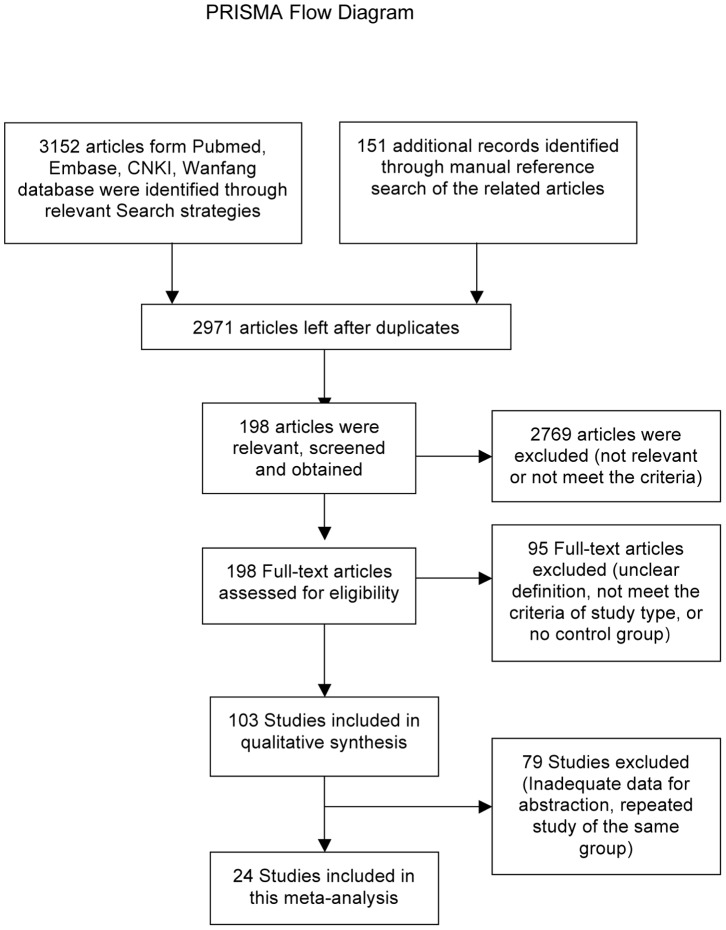
A literature search was conducted manually according to the search strategy (Text S1) to evaluate the risk factors for COM/ROM. We searched for the articles published in Pubmed, Embase, WanFang data (http://www.wanfangdata.com.cn/) and China National Knowledge infrastructure (CNKI) database (http://dlib.edu.cnki.net/kns50/). Articles from 1964 to Dec 2012 were included in the search. The search was limited to humans and performed with no language restrictions. Reference lists of the relevant original and reviewed articles were evaluated to identify additional studies. We used controlled vocabularies (Explosion mapped searches of MeSH terms or Emtree thesaurus terms) and text words for chronic otitis media, recurrent otitis media, middle ear cholesteatoma, and mastoiditis. Concepts related to “Otitis media” with the subheadings of congenital, epidemiology, genetics, immunology, microbiology and virology for all Mesh terms in PubMed were reviewed. Areas of focus that were chosen for otitis media in Embase were genetics, immunology and hematology, microbiology, otorhinolaryngology, pediatrics and public health. Furthermore, terms indicating risks, such as “risk factors”, “probability”, “odds ratio”, “risk assessment”, “causality”, “epidemiologic factors”, “epidemiology”, “epidemiologic studies”, “multivariate analysis”, “logistic models” and their entry terms were also included (see Text S1). Overall, 2547 papers were retrieved from Pubmed, 479 papers were retrieved from Embase, 116 papers were retrieved from CNKI and 10 were retrieved from Wanfang. A total of 151 additional records were retrieved from the manual reference search of the related articles. The workflow of this study follows guidelines by the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Statement [11]( Figure 1 ).
Figure 1. PRISMA Flow Diagram.
Definition of COM/ROM
The diagnosis criteria of COM/ROM was described in individual studies, which included case history, physical examination and other examinations such as tympanogram, microscopic otoscopy or tympanostomy tube insertions ( Table 1 ). The abbreviation COM includes the types of chronic suppurative otitis media (CSOM) and chronic otitis media with effusion (COME). Chronic otitis media with cholesteatoma was not excluded from the COM definition, although no study involving that type was eligible for our meta-analysis.
Table 1. Characteristics of included studies.
| First author | Year of publication | Risk factor | Type of otits meida | Study type | Age, years of participants | Study duration | Number of cases | Number of controls | Total Sample Size | Study Location | Ethnic Group | Diagnostic criteria of COM/ROM |
| Stahlberg, M. R. [37] | 1986 | Day-care center attendance, Passive smoke, Low socioeconomic status | ROM | Case-control | 10–44 months in case group, 14–38 months in control group | March, 1983–Feb, 1984 | 115 | 222 | 337 | Turku, Finland | Inhabitants in Turku, Finland | Three or more episodes of OME |
| Daly, K. [8] | 1988 | Sex, Day-care center attendance, White people, Allergy, Family history of OM | COME | Case-control | 10 months - 8 years of age | Jan, 1982-Sep, 1984 | 177 | 182 | 359 | Minnesota, USA | White people and others unidentified population | MEE persisted in one or both ears at the 3- and 6 week visits, or AOM without resolution of MEE during the 6 weeks |
| Fliss, D. M. [21] | 1991 | History of AOM/ROM, Day-care center attendance, Larger families and more siblings, Sex, Allergy, Sinusitis and recurrent URTI, Breast feeding, Passive smoke | CSOM without Cholesteotoma | Case-control | 2–15 years of age | Jan, 1987-April, 1990 | 88 | 76 | 164 | Southern Israel | Jewish population | Continuous otorrhea ≥2 months |
| Kalm, O. [27] | 1994 | HLA frequency | CSOM | Follow-up | Mean age 16.4 | Follow up 11.1 years | 40 | 1701 for HLA-A and B 438 for HLA-C 102 for HLA-DR | 1741 | Sweden | No comment | Chronic or recurrent mucous middle ear secretion persisting for at least 6 years. |
| Kvaerner, K. J. [29] | 1996 | Birth weight, Gestational age | ROM | Case-control | Before age 7 | Baby born between 1967–1974 | 519 | 5345 | 5864 | Norway | Norwegian twin pairs | Recurrent ear infections |
| Ilicali, O. C. [24] | 1999 | Passive smoke, Sex | ROM | Follow-up | 3–7 years of age | May 1st, 1995– Nov 30th, 1996 | 166 | 166 | 332 | Istanbul, Turkey | Patients from Istanbul School of Medicine | Extensive OM bilateral for at least 3 months or 6 months unilateral. ≥3 episodes of AOM during previous 6 months or minimum 4 episodes during previous 1 year. |
| Juntti, H. [26] | 1999 | Cow's milk allergy | ROM | Case control | 9–11 years of age. Mean age = 10.5±0.6 years | 1986–1987 | 56 | 204 | 260 | Finland | Local residents | 15 episodes of OM in 10 years |
| Engel, J. [19] | 2001 | Sex, Gestational age, Birth weight | COME | Prospective cohort | 2 years of age | 2- year follow-up | 43 | 40 | 83 | Netherland | Newborns from Maastricht University Hospital | Otoscopy and tympanoetry examination to assessed combined with MOMES diagnostic-algorithm |
| Ilicali, O. C. [23] | 2001 | Passive smoke | ROM | Follow-up | 3–8 years of age | Oct, 1996-Apr, 1998 | 114 | 40 | 154 | Istanbul, Turkey | Local residents | OME persisted for ≥3 months bilateral or 6 months unilateral. ≥3 episodes of RAOM during previous6 months or ≥4 episodes during the previous year. |
| Ramet, M. [33] | 2001 | Surfactant protein- A frequencies | ROM | Case-control | 1–10 years of age, mean age = 8.4±5.2 | No comment | 147 | 228 | 375 | Finland | Local patients and residents | At least 5 episodes of AOM |
| Daly, K. A. [17] | 2004 | Support for linkage at chromosomes 10q and 19q, Day-care center attendance, Exclusively formula fed, Passive smoke | COME/ROM | Retrospective cohort | Family members, age not mentioned | 1992–2001 | 371 | 245 | 616 | Minnesota,USA | Families recruited from University of Minnesota | Tympanostomy tube surgery for COME/ROM |
| Keles, B. [28] | 2004 | Pharyngeal reflux, Gastroesophageal reflux | COME | Prospective cohort | 3–7 years, mean age = 6±3.1 | No comment | 25 | 12 | 37 | Konya, Turkey | No comment | COME >3 months |
| Engel, J. A. [20] | 2005 | Breast feeding, Day-care center attendance, Family history of OM, Passive smoke, Snoring, URTI, Mother's smoking during pregnancy, Medication use during pregnancy | ROM | Prospective cohort | 2.1–7.5 years of age | Dec, 1999- Aug, 2003 | 73 | 17 | 90 | Nijmegen and Winterswijk, Netherlands | No comment | MEE at least for 3 months |
| Chantry, C. J. [16] | 2006 | Breast feeding | ROM | Prospective cohort | 6–72 months of age | 1988–1994 | 88 | 271 | 359 | USA | White, black, Mexican American | >3 episodes of OM |
| Gozal, D. [22] | 2008 | Snoring, African American, Chronic nasal obstruction, Allergy, Passive smoke | ROM | Retrospective corhort | 5–7 years of age | 1999–2004 | 5074 | 11247 | 16321 | Louisville, USA | African American and other unclassified ethnic groups | History of ROM and insertion of tympanostomy tubes |
| Lasisi, A. O. [30] | 2009 | Serum retinol level | CSOM | Follow-up | 6 months–7 years, mean age = 7.8 years | No comment | 116 | 52 | 168 | Ibadan, Nigeria | No comment | Persistence of otorrhoea ≥3 months |
| Lasisi, A. O. [31] | 2007 | URTI, Indoor- cooking, Allergy, Low social status group, Passive smoke, Breast- feeding, Day-care center attendance | COME | Case-Control | 30 days-14 years of age | No comment | 189 | 100 | 289 | Nigeria | No comment | ≥3 episodes of OM in 1 year |
| Schejbel, L [36] | 2009 | Properdin deficiency | ROM | Retrospective cohort | All age from three generations of a family | No comment | 4 | 21 | 25 | Denmark | Indian | Several episodes of OM |
| Bakhshaee, M. [15] | 2011 | Allergy | CSOM | Prospective cohort | 10–50 years, mean age = 30 years) | No comment | 68 | 184 | 252 | Mashad, Iran | No comment | CSOM diagnosed for at least 1 year |
| Elemraid, M. A. [18] | 2011 | Nutritional factors | CSOM | Case-control study | 0.6–15 years (mean = 6.0) in case group 0.9–15 years (mean = 8.2) in control group | March to May 2007 | 75 | 74 | 149 | Sana’a, Yemen | Local children | Diagnosis of CSOM and history of persistent discharging ear(s) for at least 2 weeks |
| Jensen, R. G. [25] | 2011 | Sex, Ethnicity, Low education of mother, Family history of COM, Breast feeding | CSOM | Follow-up | 11–15 years | 1996–2008 | 45 | 191 | 236 | Nuuk and Sisimiut, Greenland | Inuit, Danish, Mixed | ≥2 weeks of otorrhea for ≥3 months |
| Nelson, H. M. [32] | 2011 | Overweight in toddlers | ROM | Prospective cohort | 1 month- 27 months. Mean age = 24.1 months | 1991–1996 | 203 | 227 | 430 | Minneapolis, USA | Local toddlers | ROM treated with tympanostomy tubes |
| Sale, M. M. [35] | 2011 | Day-care center attendance, Breast feeding, Allergy | COME/ROM | Case-Control | Mean age = 5.9 in case group. 5.4 in control group | Oct, 1996 - Apr, 1998 | 380 | 238 | 618 | Istanbul, Turkey | Local residents | OME or ROM treated with ventilation tubes |
Abbreviation: OM: Otitis media. OME: Otitis media with effusion. URTI: upper respiratory tract infection CSOM: Chronic suppurative otitis media. COME: Chronic otitis media with effusion. MEE: Middle ear effusion. ROM: Recurrent otitis media. RAOM: Recurrent acute otitis media MOMES: Maastricht Otitis Media with Effusion Study. TM: Tympanic membrane.
Study Selection and Quality
We included the study of the prospective cohort, case-cohort, and nested case-control design, case control or nested case-control, retrospective case-control, and cross-sectional studies. The publications included were required to meet the following criteria:
Inclusion of human subjects
Clear definitions of COM/ROM and estimation of the association of the relative risks (hazard ration, risk factors) of COM/ROM;
The numbers for both controls and COM/ROM cases;
Sufficient data are to determine the odds ratio (OR) with 95% confidence intervals (CIs).
We excluded descriptive studies, case reports, case series, reviews, letters, commentaries, and studies on the pathogenesis and treatment of COM/ROM. We excluded repeated reports with a small number of participants and these data were included in large studies mentioned above. We excluded the studies of recurrent acute otitis media, congenital cholesteatoma and unclassified OM. Inclusion discrepancy was resolved in joint discussions by the investigators. We appraised the quality of the studies, focusing on the selection of cohorts and assessment of the outcomes.
Data Extraction
Two investigators, (Yan Zhang and Jin Zhang) independently extracted and registered the data from the eligible publications. The following data from each article was extracted: author, year of publication, risk factor, type of otitis media, study type, age/years of participants, study duration, number of cases, number of controls, total sample size, study location, ethnic group and diagnostic criteria for COM/ROM. All disagreements were resolved through group discussion.
Statistical Analysis
The meta-analysis was processed using Review Manager 5.1, version: 5.1.6. We estimated the odds ratios (ORs) and 95% confidence intervals (CIs), and the statistical heterogeneity of the studies was assessed before combining the results. Estimates of the risk factors were pooled using a random effects model [12]. Inconsistency of the studies was quantified by using the I2 statistic, which describes heterogeneity across studies. I2 values of <25% and >50% reflects low and high heterogeneity, respectively [13]. A sensitivity analysis was performed by calculating the outcomes after a single study was omitted in each turn. Finally, publication bias was assessed by performing funnel plots [14] (see Figure S1).
Results
Literature Search and Study Selection
Of the total 2971 relevant references identified, 198 articles were considered potentially relevant. The excluded references that were considered irrelevant included reviews, letters, commentaries, studies on pathogenesis, pathologies, and treatment, and microbiological studies. A total of 103 case control or cohort studies examined the risk factors of COM/ROM, and 79 studies failed to meet the inclusion criteria for the following reasons: unclear definition of COM/ROM, no classification of OM, no control groups, and inadequate data for abstraction. For repeated studies, we retained the one with the larger sample size. Figure 1 shows the selection flow for this meta-analysis; 24 independent studies met all of the inclusion criteria [8], [15]–[38]. The characteristics of the included studies are summarized in Table 1 .
Pooled Analysis of Risk Factors
Pooled data from 7 studies indicated the presence of allergy or atopy and increased the risk of COM/ROM (OR, 1.36; 95% CI, 1.13–1.64; P = 0.001). A total of four studies investigated the association between upper respiratory tract infection (URTI) and COM/ROM, which includes the presence of cough or rhinorrhea or nasal stuffiness or sore throat or adenoiditis/adenoid hypertrophy. Pooled data from these showed that URTI significantly increased the risk of COM/ROM (OR, 6.59; 95% CI, 3.13–13.89; P<0.00001). A total of two studies showed that snoring appeared to be a significant risk factor for COM/ROM (OR, 1.96; 95% CI, 1.78–2.16; P<0.00001). Pooled data from two studies revealed that a patient history of AOM/ROM increased the risk of COM/ROM (OR, 11.13; 95% CI, 1.06–116.44; P = 0.04); nine studies investigated parental smoking, exposure to smoking at home and other smokers residing in the same household of frequent visitors. Pooled data showed Second-hand smoke, including the conditions above, increased the risk of COM/ROM (OR, 1.39; 95% CI, 1.02–1.89 P = 0.04). Pooled data from two studies showed low social status as an increased risk factor of COM/ROM (OR, 3.82; 95% CI, 1.11–13.15; P = 0.03).
The factors that were determined to not be significantly associated with increased risk included chronic nasal obstruction (OR, 1.19; 95% CI, 0.84–1.69; P = 0.34), male sex (OR, 1.24; 95% CI, 0.99–1.54; P = 0.06), attending day-care centers (OR, 1.70; 95% CI, 0.95–3.05; P = 0.07), family history of otitis media (OR, 1.40; 95% CI, 0.86–2.28; P = 0.18), low education of the mother (OR, 1.68; 95% CI, 0.32–8.68; P = 0.54), mother's smoking during pregnancy (OR, 2.34; 95% CI, 0.64–8.54; P = 0.20), larger families and more siblings (OR, 1.57; 95% CI, 0.93–2.63; P = 0.09). Pooled data revealed that an association between breast-feeding >6 months and COM/ROM was not statistically significant (OR, 0.57; 95% CI, 0.17–1.93; P = 0.36), neither was an association between breast feeding (yes/no) and COM/ROM (OR, 0.91; 95% CI, 0.47–1.79; P = 0.79). Pooled risk factors for COM/ROM are summarized in Table 2 and Figure S2.
Table 2. Pooled analysis of risk factors.
| Risk factor | No. of studies[references] | No. of subjects | OR | 95% CI | P value | I2 (%) |
| Allergy/Atopy | 7 [8], [15], [21], [22], [26], [31], [35] | 18263 | 1.36 | [1.13, 1.64] | 0.001 | 26 |
| Upper respiratory tract infections | 4 [20], [21], [31], [38] | 865 | 6.59 | [3.13, 13.89] | <0.00001 | 65 |
| Chronic nasal obstruction | 2 [22], [31] | 16610 | 1.19 | [0.84, 1.69] | 0.34 | 54 |
| Snoring | 2 [20], [22] | 16411 | 1.96 | [1.78, 2.16] | <0.00001 | 0 |
| Sex (male) | 6 [8], [19], [21], [24], [25], [38] | 1435 | 1.24 | [0.99, 1.54] | 0.06 | 0 |
| Attending day-care centers | 7 [8], [17], [20], [21], [31], [35], [37] | 2454 | 1.70 | [0.95, 3.05] | 0.07 | 89 |
| Family history of otitis media | 4 [8], [19], [25], [38] | 1166 | 1.40 | [0.86, 2.28] | 0.18 | 52 |
| Patient history of AOM/ROM | 2 [21], [38] | 425 | 11.13 | [1.06,116.44] | 0.04 | 94 |
| Passive Smoke | 9[17], [20]–[24], [31], [37], [38] | 18876 | 1.39 | [1.02, 1.89] | 0.04 | 80 |
| Low social status group | 2 [31], [37] | 600 | 3.82 | [1.11, 13.15] | 0.03 | 82 |
| Low education level of mother | 2 [25], [38] | 495 | 1.68 | [0.32, 8.68] | 0.54 | 90 |
| Mother's smoking during pregnancy | 2 [20], [24] | 422 | 2.34 | [0.64, 8.54] | 0.20 | 70 |
| Larger families and more siblings | 2 [21], [[38]] | 425 | 1.57 | [0.93, 2.63] | 0.09 | 5 |
| Breast feeding >6 months | 2 [16], [25] | 912 | 0.57 | [0.17, 1.93] | 0.36 | 88 |
| Breast feeding (yes/no) | 3 [17], [21], [35] | 1363 | 0.91 | [0.47, 1.79] | 0.79 | 86 |
OR: Odds ratio. 95% CI: 95% confidential intervals. I2 describes heterogeneity across studies.
Other risk factor investigations for COM/ROM included in our eligible studies included HLA frequencies [27], nutritional factors [18], medication use during pregnancy [20], ethnicities of Greenland [25], White [8], African American [22], properdin deficiency [36], indoor cooking [31], pharyngeal reflux [28], overweight status [32], older siblings [38], dietary history [18], serum retinol [30], genome scan for loci of 10q and 19q [17], and?Surfactant protein-A gene locus [33]. Unfortunately, only one research group reported each risk factor above, which made the data unavailable. There was an association between gestational age and COM/ROM from two research groups [19], [29], but birth weight and COM/ROM from these groups applied different criteria and made it impossible to combine the data.
Discussion
COM/ROM is a disease with different possible etiologies. Using a meta-analysis design applying strict diagnostic and inclusion criteria, we performed a reliable study to investigate the risk factors associated with the disease. This study is to the best of our knowledge the first meta-analysis investigating the risk factors for COM/ROM. There are two published studies on the risk factors and etiology of AOM [39], [40].
Allergy or atopy is a significant risk factor for COM/ROM. Indoor allergens and respiratory allergies such as allergic rhinitis contribute to the onset of COM/ROM. The prevalence of atopic conditions, including allergic rhinitis in patients with COM/ROM ranges from 24% to 89% [41]. New evidence from cellular biology and immunology explained allergy as a cause for Eustachian tube (ET) obstruction [42]. People with allergic or atopic conditions are more likely to suffer from COM/ROM.
Upper respiratory tract infection (URTI), which includes the presence of cough or rhinorrhea or sore throat, was indicated as a significant prognostic factor for COM/ROM. Studies support that the mucosal condition of ET could be affected by URTI [43]. A preceding or concurrent viral URTI, as well as a poly-microbial disease is considered one of the risk factors for the onset of OM. Viral URTI promotes the replication of the bacterial infection and increases inflammation in the nasopharynx and ET [44].
Snoring, defined as the presence of loud snoring at least three times per week, is a common symptom in children and is highly prevalent in children [45]. Eligible studies in this meta-analysis suggested that the risk for COM/ROM appeared to be related to the presence of snoring. Snoring is pathophysiologically determined by the size of the upper airway lymphadeniod tissue size [46]. The mechanism underlying snoring and COM/ROM appears to be increasing upper airway resistance as well as Eustachian tube dysfunction [22]. Early evaluation and intervention in children with loud snoring may prevent them from developing middle ear disease.
Previous history of AOM/ROM was studied as a predictive factor for COM/ROM. Subjects who experience episodes of AOM/ROM have an increased risk of developing chronic and recurrent middle ear infections.
Second-hand smoke has been reported to be associated with increased prevalence of middle ear disease [47]. In the meta-analysis of risk factors for acute otitis media, it was concluded that parental smoking increased the onset of acute middle ear infectious disease in children [39]. Our study drew the same conclusion about Second-hand smoking as a remarkable causative factor that contributes to the morbidity of COM/ROM. Several studies suggest that nicotine and other smoking products could make subjects more susceptible to ear infections and enhance the possibility of microorganism invasion to the middle ear. Smoke exposure could impair the mucociliary function of the ET, resulting in blockage of the nasopharyngeal airway [48]. Microorganism adherence to the epithelial cell surface and depression of local immune function were both investigated as the pathogenetic mechanism of the onset of middle ear disease caused by Second-hand smoking [49]. Effective methods should be urgently taken to decrease the prevalence of the smoke exposure.
The possibility that COM/ROM is associated with low social status has been debated for a long period of time [50]. Our data from two eligible studies considered the social prestige of professions and occupations, as well as income earnings of the parents. The statistical data revealed that patients with COM/ROM were more often belonged to low socioeconomic conditions than the controls. Various reports concerning this hazard originated from poor housing, environmental and occupational conditions [51], [52].
Sex difference in otitis media risk has been estimated in various studies. Other than a conclusion that the male sex was more likely to suffer from acute otitis media in children [39], our study failed to find any significance in the difference between male and female morbidity of COM/ROM.
Breast-feeding is believed to provide antimicrobial, anti-inflammatory, and immunomodulatory agents that contribute to an optimal immune system [53]. The relative contribution of breast-feeding to preventing middle ear infection otitis media risk has been reported in numerous studies [54]–[56]. It is reported that breast-feeding, even for only 3 months, could decrease the risk for acute otitis media in children [39]. However, patients with COM/ROM did not differ from the control group in this respect in our study. The study for preventative effects of breast-feeding over 6 months failed to find statistical significance within the control group. Even without any breast-feeding, the impact on the incidence of COM/ROM appeared to be unremarkable in our meta-analysis.
Day-care center attendance could increase the risk of children’s exposure to respiratory pathogens. It has been reported to be a significant risk factor for acute respiratory infectious disease in children [39], [52], [57]. However, this was not consistent with some other studies [58]. In this meta-analysis, no association was found between COM/ROM and day-care center attendance.
The causal relationship between other factors, which include chronic nasal obstruction, family history of otitis media, mother's smoking during pregnancy and COM/ROM is not completely established. Association between larger families and more siblings with COM/ROM was not statistically significant.
Genetic predisposition is considered to be an important prognostic factor that could influence the risk of otitis media. Previous candidate gene studies associated a number of immune system genes with otitis media, which included TNF-α, IL-6, IL-10, Tlr4, surfactant, CD14, FcγRIIa, IFNγ, Eya4, p73, MyD88, Fas, E2f4, Plg, Fbxo11, and Evi1 [59]. Other genetic predispositions include HLA frequencies and properdin deficiency. Unfortunately, eligible studies included in our meta-analysis investigated single gene defects in each study, which made it impossible to pool the data and make a conclusion.
Similar to risk factors for genetic predisposition, other risk factors for COM/ROM prevented the data from being pooled in our eligible studies; these include nutritional factors, medication use during pregnancy, ethnicities of Greenland, White, African American, indoor cooking, pharyngeal reflux, and overweight status.
The association between gestational age and COM/ROM from the two groups [19], [29], birth weight and COM/ROM from the same groups applied different criteria and thus made it impossible to combine the data.
We noticed that the risk factors of sex, attending day-care centers, large families and more siblings have p-values of 0.06, 0.07 and 0.09, respectively. With the application of the 0.05 p-value, which is the conventionally used criterion, no significance was found. Using a cut-off of 10% for significance may ameliorate this problem but could increase the risk of drawing a false positive conclusion (type I error) [13], [60]. However, these three risk factors should be at least considered as constituting a strong trend of risk factors for COM/ROM.
In judging the inconsistency of the studies, I2 was applied to test heterogeneity. In our studies of no inconsistency (I2 = 0) or low heterogeneity (I2<25%), using either fixed or random effect models produced identical results and the same direction of effect. The random effect model was the standard approach for the studies of moderate to high I2 values. Some analysts might try to reduce the heterogeneity by limiting the meta-analysis to a smaller more homogeneous study group. However, this could probably result in misleading conclusions if not performed with care or may limit the scope of the meta-analysis and essentially eliminates any useful information [61]. The random effect model, which was the available model to incorporate and evaluate sources of heterogeneity [12], was applied in our study. In our meta-analysis, a limited number of included studies confined our attempts to divide the studies into subgroups. Sources of between-study heterogeneity could probably originate from different study designs, sample size in each individual study, incidence rates among unexposed, length of follow-up, and/or study qualities. In our sensitivity analysis, the observed directions and magnitudes of effects weren’t changed significantly after a single study was randomly omitted in each turn.
A full understanding of the etiologic factors for COM/ROM could be beneficial for the treatment and prevention of the disease. Our study evaluates the risk factors by an objective scientific procedure, meta-analysis, to provide precise causal prophylaxis evidence. Meta-analysis is widely used in medical studies of randomized clinical trials, as well as etiologic factors of the disease. The controversy of meta-analysis is in the homogeneity of the studies. Dickersin and others noted that heterogeneity is not all that bad [62], [63]. It improves the generalizability of the meta-analysis results. The pooled estimates of odds ratios are valuable and important indicators for assessing the risk factors of a disease. The heterogeneity of risk factors is carefully estimated, and the results are cautiously interpreted in our study.
Conclusions
The risk factors for COM/ROM are closely interrelated. Our meta-analysis identified reliable conclusions that allergy/atopy, upper respiratory tract infection, snoring, previous history of AOM/ROM, Second-hand smoke, low social status are important risk factors for COM/ROM. Other unidentified risk factors investigated in single studies need possible repeated studies with critical criteria to be estimated properly. We suggest that the above COM/ROM risk factors be interfered effectively to prevent and decrease the onset of the disease.
Supporting Information
Funnel plot. Symmetric inverted funnel shape indicates unlikely publication bias.
(TIFF)
Risk factors for COM/ROM. Pooled odds ratios from eligible studies analyzed in the meta-analysis of risk factors for COM/ROM
(TIFF)
PRISMA checklist.
(DOC)
Search strategy.
(DOC)
Acknowledgments
We thank Qian Wu for statistical analysis and Sherrie Irwin for language editing.
Funding Statement
This work was supported by the National Natural Science Foundation of China [30973300 to M.X.] and Key Clinical Program of Second Hospital Second Hospital, Xi’an Jiaotong University [YJ(ZDJH)201103 to M.X.]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Arguedas A, Kvaerner K, Liese J, Schilder AG, Pelton SI (2010) Otitis media across nine countries: disease burden and management. Int J Pediatr Otorhinolaryngol 74: 1419–1424. [DOI] [PubMed] [Google Scholar]
- 2. Osma U, Cureoglu S, Hosoglu S (2000) The complications of chronic otitis media: report of 93 cases. J Laryngol Otol 114: 97–100. [DOI] [PubMed] [Google Scholar]
- 3. Vernacchio L, Lesko SM, Vezina RM, Corwin MJ, Hunt CE, et al. (2004) Racial/ethnic disparities in the diagnosis of otitis media in infancy. Int J Pediatr Otorhinolaryngol 68: 795–804. [DOI] [PubMed] [Google Scholar]
- 4. Amusa YB, Ijadunola IK, Onayade OO (2005) Epidemiology of otitis media in a local tropical African population. West Afr J Med 24: 227–230. [DOI] [PubMed] [Google Scholar]
- 5. Morris PS, Leach AJ, Silberberg P, Mellon G, Wilson C, et al. (2005) Otitis media in young Aboriginal children from remote communities in Northern and Central Australia: a cross-sectional survey. BMC Pediatr 5: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Casselbrant ML, Mandel EM, Rockette HE, Kurs-Lasky M, Fall PA, et al. (2004) The genetic component of middle ear disease in the first 5 years of life. Arch Otolaryngol Head Neck Surg 130: 273–278. [DOI] [PubMed] [Google Scholar]
- 7. Lanphear BP, Byrd RS, Auinger P, Hall CB (1997) Increasing prevalence of recurrent otitis media among children in the United States. Pediatrics 99: E1. [DOI] [PubMed] [Google Scholar]
- 8. Daly K, Giebink GS, Le CT, Lindgren B, Batalden PB, et al. (1988) Determining risk for chronic otitis media with effusion. Pediatr Infect Dis J 7: 471–475. [DOI] [PubMed] [Google Scholar]
- 9. Duncan B, Ey J, Holberg CJ, Wright AL, Martinez FD, et al. (1993) Exclusive breast-feeding for at least 4 months protects against otitis media. Pediatrics 91: 867–872. [PubMed] [Google Scholar]
- 10. Martines F, Bentivegna D, Maira E, Sciacca V, Martines E (2011) Risk factors for otitis media with effusion: case-control study in Sicilian schoolchildren. Int J Pediatr Otorhinolaryngol 75: 754–759. [DOI] [PubMed] [Google Scholar]
- 11. Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 12. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 13. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bakhshaee M, Rajati M, Fereidouni M, Khadivi E, Varasteh A (2011) Allergic rhinitis and chronic suppurative otitis media. Eur Arch Otorhinolaryngol 268: 87–91. [DOI] [PubMed] [Google Scholar]
- 16. Chantry CJ, Howard CR, Auinger P (2006) Full breastfeeding duration and associated decrease in respiratory tract infection in US children. Pediatrics 117: 425–432. [DOI] [PubMed] [Google Scholar]
- 17. Daly KA, Brown WM, Segade F, Bowden DW, Keats BJ, et al. (2004) Chronic and recurrent otitis media: a genome scan for susceptibility loci. Am J Hum Genet 75: 988–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Elemraid MA, Mackenzie IJ, Fraser WD, Harper G, Faragher B, et al. (2011) A case-control study of nutritional factors associated with chronic suppurative otitis media in Yemeni children. Eur J Clin Nutr 65: 895–902. [DOI] [PubMed] [Google Scholar]
- 19. Engel J, Mahler E, Anteunis L, Marres E, Zielhuis G (2001) Why are NICU infants at risk for chronic otitis media with effusion? Int J Pediatr Otorhinolaryngol 57: 137–144. [DOI] [PubMed] [Google Scholar]
- 20. Engel JA, Straetemans M, Zielhuis GA (2005) Birth characteristics and recurrent otitis media with effusion in young children. Int J Pediatr Otorhinolaryngol 69: 533–540. [DOI] [PubMed] [Google Scholar]
- 21. Fliss DM, Shoham I, Leiberman A, Dagan R (1991) Chronic suppurative otitis media without cholesteatoma in children in Southern Israel: Incidence and risk factors. Pediatric Infectious Disease Journal 10: 895–899. [DOI] [PubMed] [Google Scholar]
- 22. Gozal D, Kheirandish-Gozal L, Capdevila OS, Dayyat E, Kheirandish E (2008) Prevalence of recurrent otitis media in habitually snoring school-aged children. Sleep Medicine 9: 549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ilicali OC, Keles N, De er K, Sa un OF, Guldiken Y (2001) Evaluation of the effect of passive smoking on otitis media in children by an objective method: urinary cotinine analysis. Laryngoscope 111: 163–167. [DOI] [PubMed] [Google Scholar]
- 24. Ilicali OC, Keles N, Deger K, Savas I (1999) Relationship of passive cigarette smoking to otitis media. Arch Otolaryngol Head Neck Surg 125: 758–762. [DOI] [PubMed] [Google Scholar]
- 25. Jensen RG, Homoe P, Andersson M, Koch A (2011) Long-term follow-up of chronic suppurative otitis media in a high-risk children cohort. International Journal of Pediatric Otorhinolaryngology 75: 948–954. [DOI] [PubMed] [Google Scholar]
- 26. Juntti H, Tikkanen S, Kokkonen J, Alho OP, Niinimaki A (1999) Cow's milk allergy is associated with recurrent otitis media during childhood. Acta Otolaryngol 119: 867–873. [DOI] [PubMed] [Google Scholar]
- 27. Kalm O, Johnson U, Prellner K (1994) HLA frequency in patients with chronic secretory otitis media. Int J Pediatr Otorhinolaryngol 30: 151–157. [DOI] [PubMed] [Google Scholar]
- 28. Keles B, Ozturk K, Gunel E, Arbag H, Ozer B (2004) Pharyngeal reflux in children with chronic otitis media with effusion. Acta Otolaryngol 124: 1178–1181. [DOI] [PubMed] [Google Scholar]
- 29. Kvaerner KJ, Tambs K, Harris JR, Magnus P (1996) The relationship between otitis media and intrauterine growth: a co-twin control study. Int J Pediatr Otorhinolaryngol 37: 217–225. [DOI] [PubMed] [Google Scholar]
- 30. Lasisi AO (2009) The role of retinol in the etiology and outcome of suppurative otitis media. Eur Arch Otorhinolaryngol 266: 647–652. [DOI] [PubMed] [Google Scholar]
- 31. Lasisi AO, Olaniyan FA, Muibi SA, Azeez IA, Abdulwasiu KG, et al. (2007) Clinical and demographic risk factors associated with chronic suppurative otitis media. International Journal of Pediatric Otorhinolaryngology 71: 1549–1554. [DOI] [PubMed] [Google Scholar]
- 32. Nelson HM, Daly KA, Davey CS, Himes JH, Synder DJ, et al. (2011) Otitis media and associations with overweight status in toddlers. Physiol Behav 102: 511–517. [DOI] [PubMed] [Google Scholar]
- 33. Ramet M, Lofgren J, Alho OP, Hallman M (2001) Surfactant protein-A gene locus associated with recurrent otitis media. J Pediatr 138: 266–268. [DOI] [PubMed] [Google Scholar]
- 34. Rye MS, Warrington NM, Scaman ES, Vijayasekaran S, Coates HL, et al. (2012) Genome-wide association study to identify the genetic determinants of otitis media susceptibility in childhood. PLoS ONE 7: e48215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sale MM, Chen WM, Weeks DE, Mychaleckyj JC, Hou X, et al. (2011) Evaluation of 15 functional candidate genes for association with chronic otitis media with effusion and/or recurrent otitis media (COME/ROM). PLoS ONE 6. [DOI] [PMC free article] [PubMed]
- 36. Schejbel L, Rosenfeldt V, Marquart H, Valerius NH, Garred P (2009) Properdin deficiency associated with recurrent otitis media and pneumonia, and identification of male carrier with Klinefelter syndrome. Clinical Immunology 131: 456–462. [DOI] [PubMed] [Google Scholar]
- 37. Stahlberg MR, Ruuskanen O, Virolainen E (1986) Risk factors for recurrent otitis media. Pediatr Infect Dis 5: 30–32. [DOI] [PubMed] [Google Scholar]
- 38. van der Veen EL, Schilder AG, van Heerbeek N, Verhoeff M, Zielhuis GA, et al. (2006) Predictors of chronic suppurative otitis media in children. Arch Otolaryngol Head Neck Surg 132: 1115–1118. [DOI] [PubMed] [Google Scholar]
- 39. Uhari M, Mantysaari K, Niemela M (1996) A meta-analytic review of the risk factors for acute otitis media. Clin Infect Dis 22: 1079–1083. [DOI] [PubMed] [Google Scholar]
- 40. Bardach A, Ciapponi A, Garcia-Marti S, Glujovsky D, Mazzoni A, et al. (2011) Epidemiology of acute otitis media in children of Latin America and the Caribbean: a systematic review and meta-analysis. Int J Pediatr Otorhinolaryngol 75: 1062–1070. [DOI] [PubMed] [Google Scholar]
- 41. Lack G, Caulfield H, Penagos M (2011) The link between otitis media with effusion and allergy: a potential role for intranasal corticosteroids. Pediatr Allergy Immunol 22: 258–266. [DOI] [PubMed] [Google Scholar]
- 42.Hurst DS (2011) The role of allergy in otitis media with effusion. Otolaryngol Clin North Am 44: 637–654, viii-ix. [DOI] [PubMed]
- 43. Miura M, Takahashi H, Honjo I, Hasebe S, Tanabe M (1997) Influence of the upper respiratory tract infection on tubal compliance in children with otitis media with effusion. Acta Otolaryngol 117: 574–577. [DOI] [PubMed] [Google Scholar]
- 44. Bakaletz LO (2010) Immunopathogenesis of polymicrobial otitis media. J Leukoc Biol 87: 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. O'Brien LM, Holbrook CR, Mervis CB, Klaus CJ, Bruner JL, et al. (2003) Sleep and neurobehavioral characteristics of 5- to 7-year-old children with parentally reported symptoms of attention-deficit/hyperactivity disorder. Pediatrics 111: 554–563. [DOI] [PubMed] [Google Scholar]
- 46. Arens R, Marcus CL (2004) Pathophysiology of upper airway obstruction: a developmental perspective. Sleep 27: 997–1019. [DOI] [PubMed] [Google Scholar]
- 47. Kum-Nji P, Meloy L, Herrod HG (2006) Environmental tobacco smoke exposure: prevalence and mechanisms of causation of infections in children. Pediatrics 117: 1745–1754. [DOI] [PubMed] [Google Scholar]
- 48. Fukuma M, Seto Y, Fukushima K, Sakurai T, Dan K, et al. (1986) The effect of food dye and other environmental substances on the host defense reaction in mice in relation to virus infection. J Toxicol Sci 11: 169–177. [DOI] [PubMed] [Google Scholar]
- 49. Holt PG (1987) Immune and inflammatory function in cigarette smokers. Thorax 42: 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bluestone CD, Carder HM, Coffey JD Jr, Kenna MA, Pelton SI, et al. (1985) Consensus: management of the child with a chronic draining ear. Pediatr Infect Dis 4: 607–612. [DOI] [PubMed] [Google Scholar]
- 51. Daly KA, Pirie PL, Rhodes KL, Hunter LL, Davey CS (2007) Early otitis media among Minnesota American Indians: the Little Ears Study. Am J Public Health 97: 317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Daly KA, Selvius RE, Lindgren B (1997) Knowledge and attitudes about otitis media risk: implications for prevention. Pediatrics 100: 931–936. [DOI] [PubMed] [Google Scholar]
- 53. Labbok MH, Clark D, Goldman AS (2004) Breastfeeding: maintaining an irreplaceable immunological resource. Nat Rev Immunol 4: 565–572. [DOI] [PubMed] [Google Scholar]
- 54. Sabirov A, Casey JR, Murphy TF, Pichichero ME (2009) Breast-feeding is associated with a reduced frequency of acute otitis media and high serum antibody levels against NTHi and outer membrane protein vaccine antigen candidate P6. Pediatr Res 66: 565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mew JR, Meredith GW (1992) Middle ear effusion: an orthodontic perspective. J Laryngol Otol 106: 7–13. [DOI] [PubMed] [Google Scholar]
- 56. McNiel ME, Labbok MH, Abrahams SW (2010) What are the risks associated with formula feeding? A re-analysis and review. Breastfeed Rev 18: 25–32. [PubMed] [Google Scholar]
- 57. Kvaerner KJ, Nafstad P, Hagen JA, Mair IW, Jaakkola JJ (1996) Early acute otitis media and siblings' attendance at nursery. Arch Dis Child 75: 338–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Paradise JL, Rockette HE, Colborn DK, Bernard BS, Smith CG, et al. (1997) Otitis media in 2253 Pittsburgh-area infants: prevalence and risk factors during the first two years of life. Pediatrics 99: 318–333. [DOI] [PubMed] [Google Scholar]
- 59. Rye MS, Bhutta MF, Cheeseman MT, Burgner D, Blackwell JM, et al. (2011) Unraveling the genetics of otitis media: from mouse to human and back again. Mamm Genome 22: 66–82. [DOI] [PubMed] [Google Scholar]
- 60. Hardy RJ, Thompson SG (1998) Detecting and describing heterogeneity in meta-analysis. Stat Med 17: 841–856. [DOI] [PubMed] [Google Scholar]
- 61. Berman NG, Parker RA (2002) Meta-analysis: neither quick nor easy. BMC Med Res Methodol 2: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dickersin K, Min YI, Meinert CL (1992) Factors influencing publication of research results. Follow-up of applications submitted to two institutional review boards. JAMA 267: 374–378. [PubMed] [Google Scholar]
- 63. Biggerstaff BJ, Tweedie RL (1997) Incorporating variability in estimates of heterogeneity in the random effects model in meta-analysis. Stat Med 16: 753–768. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Funnel plot. Symmetric inverted funnel shape indicates unlikely publication bias.
(TIFF)
Risk factors for COM/ROM. Pooled odds ratios from eligible studies analyzed in the meta-analysis of risk factors for COM/ROM
(TIFF)
PRISMA checklist.
(DOC)
Search strategy.
(DOC)