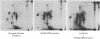Abstract
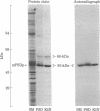
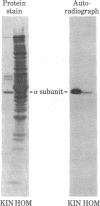
By three criteria, two biochemical and one immunochemical, the major postsynaptic density protein (mPSDp) is indistinguishable from the 50-kilodalton (kDa) alpha subunit of a brain calmodulin-dependent protein kinase. First, the two proteins comigrate on NaDodSO4/polyacrylamide gels. Second, iodinated tryptic peptide maps of the two are identical. Finally, a monoclonal antibody (6G9) that was raised against the protein kinase binds on immunoblots to a single 50 kDa band in crude brain homogenates and to both the alpha subunit of the purified kinase and the mPSDp from postsynaptic density fractions. The purified kinase holoenzyme also contains a 60-kDa subunit termed beta. A comparison of the peptide map of beta with the maps of 60-kDa proteins from the postsynaptic density fraction suggests that beta is present there but is not the only protein present in this molecular weight range. These results indicate that the calmodulin-dependent protein kinase is a major constituent of the postsynaptic density fraction and thus may be a component of type I postsynaptic densities.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akert K., Moor H., Pfenninger K., Sandri C. Contributions of new impregnation methods and freeze etching to the problems of synaptic fine structure. Prog Brain Res. 1969;31:223–240. doi: 10.1016/S0079-6123(08)63241-0. [DOI] [PubMed] [Google Scholar]
- Bennett M. K., Erondu N. E., Kennedy M. B. Purification and characterization of a calmodulin-dependent protein kinase that is highly concentrated in brain. J Biol Chem. 1983 Oct 25;258(20):12735–12744. [PubMed] [Google Scholar]
- Blomberg F., Cohen R. S., Siekevitz P. The structure of postsynaptic densities isolated from dog cerebral cortex. II. Characterization and arrangement of some of the major proteins within the structure. J Cell Biol. 1977 Jul;74(1):204–225. doi: 10.1083/jcb.74.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin R. K., Bartelt D. C., Siekevitz P. Identification of fodrin as a major calmodulin-binding protein in postsynaptic density preparations. J Cell Biol. 1983 Feb;96(2):443–448. doi: 10.1083/jcb.96.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin R. K., Grab D. J., Cohen R. S., Siekevitz P. Isolation and characterization of postsynaptic densities from various brain regions: enrichment of different types of postsynaptic densities. J Cell Biol. 1980 Sep;86(3):831–845. doi: 10.1083/jcb.86.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin R. K., Grab D. J., Siekevitz P. Function of a calmodulin in postsynaptic densities. III. Calmodulin-binding proteins of the postsynaptic density. J Cell Biol. 1981 Jun;89(3):449–455. doi: 10.1083/jcb.89.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Palay V., Palay S. L. Interrelations of basket cell axons and climbing fibers in the cerebellar cortex of the rat. Z Anat Entwicklungsgesch. 1970;132(3):191–227. doi: 10.1007/BF00523377. [DOI] [PubMed] [Google Scholar]
- Cohen R. S., Blomberg F., Berzins K., Siekevitz P. The structure of postsynaptic densities isolated from dog cerebral cortex. I. Overall morphology and protein composition. J Cell Biol. 1977 Jul;74(1):181–203. doi: 10.1083/jcb.74.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonnier M. Synaptic patterns on different cell types in the different laminae of the cat visual cortex. An electron microscope study. Brain Res. 1968 Jul;9(2):268–287. doi: 10.1016/0006-8993(68)90234-5. [DOI] [PubMed] [Google Scholar]
- Cotman C. W., Banker G., Churchill L., Taylor D. Isolation of postsynaptic densities from rat brain. J Cell Biol. 1974 Nov;63(2 Pt 1):441–455. doi: 10.1083/jcb.63.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P., Harris S. M., Jr, Huttner W. B., Greengard P. Synapsin I (Protein I), a nerve terminal-specific phosphoprotein. II. Its specific association with synaptic vesicles demonstrated by immunocytochemistry in agarose-embedded synaptosomes. J Cell Biol. 1983 May;96(5):1355–1373. doi: 10.1083/jcb.96.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorenzo R. J., Freedman S. D., Yohe W. B., Maurer S. C. Stimulation of Ca2+-dependent neurotransmitter release and presynaptic nerve terminal protein phosphorylation by calmodulin and a calmodulin-like protein isolated from synaptic vesicles. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1838–1842. doi: 10.1073/pnas.76.4.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Flanagan S. D., Yost B., Crawford G. Putative 51,000-Mr protein marker for postsynaptic densities is virtually absent in cerebellum. J Cell Biol. 1982 Sep;94(3):743–748. doi: 10.1083/jcb.94.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florendo N. T., Barrnett R. J., Greengard P. Cyclic 3',5'-nucleotide phosphodiesterase: cytochemical localization in cerebral cortex. Science. 1971 Aug 20;173(3998):745–747. doi: 10.1126/science.173.3998.745. [DOI] [PubMed] [Google Scholar]
- GRAY E. G. Axo-somatic and axo-dendritic synapses of the cerebral cortex: an electron microscope study. J Anat. 1959 Oct;93:420–433. [PMC free article] [PubMed] [Google Scholar]
- Gottlieb D. I., Cowan W. M. On the distribution of axonal terminals containing spheroidal and flattened synaptic vesicles in the hippocampus and dentate gyrus of the rat and cat. Z Zellforsch Mikrosk Anat. 1972;129(3):413–429. doi: 10.1007/BF00307297. [DOI] [PubMed] [Google Scholar]
- Grab D. J., Berzins K., Cohen R. S., Siekevitz P. Presence of calmodulin in postsynaptic densities isolated from canine cerebral cortex. J Biol Chem. 1979 Sep 10;254(17):8690–8696. [PubMed] [Google Scholar]
- Grab D. J., Carlin R. K., Siekevitz P. Function of a calmodulin in postsynaptic densities. II. Presence of a calmodulin-activatable protein kinase activity. J Cell Biol. 1981 Jun;89(3):440–448. doi: 10.1083/jcb.89.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grab D. J., Carlin R. K., Siekevitz P. Function of calmodulin in postsynaptic densities. I. Presence of a calmodulin-activatable cyclic nucleotide phosphodiesterase activity. J Cell Biol. 1981 Jun;89(3):433–439. doi: 10.1083/jcb.89.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grab D. J., Carlin R. K., Siekevitz P. The presence and functions of calmodulin in the postsynaptic density. Ann N Y Acad Sci. 1980;356:55–72. doi: 10.1111/j.1749-6632.1980.tb29599.x. [DOI] [PubMed] [Google Scholar]
- Gray E. G. Electron microscopy of excitatory and inhibitory synapses: a brief review. Prog Brain Res. 1969;31:141–155. doi: 10.1016/S0079-6123(08)63235-5. [DOI] [PubMed] [Google Scholar]
- Huttner W. B., Schiebler W., Greengard P., De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983 May;96(5):1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P. T., Cotman C. W. Identification of glycoproteins and proteins at synapses in the central nervous system. J Biol Chem. 1977 Jan 25;252(2):786–793. [PubMed] [Google Scholar]
- Kelly P. T., Cotman C. W. Intermolecular disulfide bonds at central nervous system synaptic junctions. Biochem Biophys Res Commun. 1976 Dec 20;73(4):858–864. doi: 10.1016/0006-291x(76)90200-x. [DOI] [PubMed] [Google Scholar]
- Kelly P. T., Cotman C. W. Synaptic proteins. Characterization of tubulin and actin and identification of a distinct postsynaptic density polypeptide. J Cell Biol. 1978 Oct;79(1):173–183. doi: 10.1083/jcb.79.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P. T., Montgomery P. R. Subcellular localization of the 52,000 molecular weight major postsynaptic density protein. Brain Res. 1982 Feb 11;233(2):265–286. doi: 10.1016/0006-8993(82)91202-1. [DOI] [PubMed] [Google Scholar]
- Kennedy M. B., Greengard P. Two calcium/calmodulin-dependent protein kinases, which are highly concentrated in brain, phosphorylate protein I at distinct sites. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1293–1297. doi: 10.1073/pnas.78.2.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. B., McGuinness T., Greengard P. A calcium/calmodulin-dependent protein kinase from mammalian brain that phosphorylates Synapsin I: partial purification and characterization. J Neurosci. 1983 Apr;3(4):818–831. doi: 10.1523/JNEUROSCI.03-04-00818.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mahler H. R., Kleine L. P., Ratner N., Sorensen R. G. Identification and topography of synaptic phosphoproteins. Prog Brain Res. 1982;56:27–48. doi: 10.1016/S0079-6123(08)63767-X. [DOI] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Matus A. I., Taff-Jones D. H. Morphology and molecular composition of isolated postsynaptic junctional structures. Proc R Soc Lond B Biol Sci. 1978 Dec 4;203(1151):135–151. doi: 10.1098/rspb.1978.0097. [DOI] [PubMed] [Google Scholar]
- Matus A. I., Walters B. B. Ultrastructure of the synaptic junctional lattice isolated from mammalian brain. J Neurocytol. 1975 Jun;4(3):369–375. doi: 10.1007/BF01102119. [DOI] [PubMed] [Google Scholar]
- Moore H. P., Fritz L. C., Raftery M. A., Brockes J. P. Isolation and characterization of a monoclonal antibody against the saxitoxin-binding component from the electric organ of the eel Electrophorus electricus. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1673–1677. doi: 10.1073/pnas.79.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng M., Matus A. Protein phosphorylation in isolated plasma membranes and postsynaptic junctional structures from brain synapses. Neuroscience. 1979;4(1):169–180. doi: 10.1016/0306-4522(79)90226-4. [DOI] [PubMed] [Google Scholar]
- O'Callaghan J. P., Dunn L. A., Lovenberg W. Calcium-regulated phosphorylation in synaptosomal cytosol: dependence on calmodulin. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5812–5816. doi: 10.1073/pnas.77.10.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palfrey H. C., Rothlein J. E., Greengard P. Calmodulin-dependent protein kinase and associated substrates in Torpedo electric organ. J Biol Chem. 1983 Aug 10;258(15):9496–9503. [PubMed] [Google Scholar]
- Ratner N., Mahler H. R. Structural organization of filamentous proteins in postsynaptic density. Biochemistry. 1983 May 10;22(10):2446–2453. doi: 10.1021/bi00279a022. [DOI] [PubMed] [Google Scholar]
- Sampedro M. N., Bussineau C. M., Cotman C. W. Postsynaptic density antigens: preparation and characterization of an antiserum against postsynaptic densities. J Cell Biol. 1981 Sep;90(3):675–686. doi: 10.1083/jcb.90.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman H., Greengard P. Ca2+-dependent protein phosphorylation system in membranes from various tissues, and its activation by "calcium-dependent regulator". Proc Natl Acad Sci U S A. 1978 Nov;75(11):5432–5436. doi: 10.1073/pnas.75.11.5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A. A., Ingebritsen T. S., Manalan A., Klee C. B., Cohen P. Discovery of a Ca2+- and calmodulin-dependent protein phosphatase: probable identity with calcineurin (CaM-BP80). FEBS Lett. 1982 Jan 11;137(1):80–84. doi: 10.1016/0014-5793(82)80319-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T., Greengard P. Adenosine 3':5'-monophosphate-regulated phosphoprotein system of neuronal membranes. I. Solubilization, purification, and some properties of an endogenous phosphoprotein. J Biol Chem. 1977 Jul 25;252(14):5155–5163. [PubMed] [Google Scholar]
- Ueda T., Greengard P., Berzins K., Cohen R. S., Blomberg F., Grab D. J., Siekevitz P. Subcellular distribution in cerebral cortex of two proteins phosphorylated by a cAMP-dependent protein kinase. J Cell Biol. 1979 Nov;83(2 Pt 1):308–319. doi: 10.1083/jcb.83.2.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters B. B., Matus A. I. Tubulin in postynaptic junctional lattice. Nature. 1975 Oct 9;257(5526):496–498. doi: 10.1038/257496a0. [DOI] [PubMed] [Google Scholar]
- Wood J. G., Wallace R. W., Whitaker J. N., Cheung W. Y. Immunocytochemical localization of calmodulin and a heat-labile calmodulin-binding protein (CaM-BP80) in basal ganglia of mouse brain. J Cell Biol. 1980 Jan;84(1):66–76. doi: 10.1083/jcb.84.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]